Molecular and Genetic Bases of Fruit Firmness Variation in Blueberry—A Review
Abstract
:1. Introduction
2. Physiological, Cellular, and Molecular Changes Affecting Fruit Firmness
2.1. Fruit Anatomy and Growth during Ripening
2.2. Ripening-Associated Physiological Changes
2.3. Molecular and Architectural Changes in Plant Cell during Fruit Ripening
2.4. Fruit Tissue and Cellular Differences Underlying Firmness Variation
3. Genetics and Breeding of Blueberry Firmness
3.1. Measuring Firmness
3.2. Phenotypic Variation and Breeding Improvement in Fruit Firmness
3.3. Quantitative Genetics of Firmness Trait in SHB Blueberry Breeding
3.4. Genomics Tools for Fruit Firmness Improvement
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Szajdek, A.; Borowska, E.J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Cifuentes-Gomez, T.; Spencer, J.P.E. Bioavailability of wild blueberry (poly)phenols at different levels of intake. J. Berry Res. 2016, 6, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations -FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 17 May 2018).
- Gallardo, R.K.; Stafne, E.T.; DeVetter, L.W.; Zhang, Q.; Li, C.; Takeda, F.; Williamson, J.; Yang, W.Q.; Cline, W.O.; Beaudry, R. Blueberry Producers’ Attitudes toward Harvest Mechanization for Fresh Market. Horttechnology 2018, 28, 10–16. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Martin, R.B. A survey of fruit firmness in highbush blueberry and species-introgressed blueberry cultivars. HortScience 2002, 37, 386–389. [Google Scholar]
- Olmstead, J.W.; Finn, C.E. Breeding highbush blueberry cultivars adapted to machine harvest for the fresh market. Horttechnology 2014, 24, 290–294. [Google Scholar]
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.; Chen, P.M.; Spotts, R.A. Changes in Ripening Behaviors of 1-MCP-Treated ‘d’ Anjou’ Pears After Storage Changes in Ripening Behaviors of 1-MCP-Treated ‘d’ Anjou’ Pears After Storage. Int. J. Fruit Sci. 2005, 5, 3–18. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; González, G.; Lobos, G.A. Firmness at Harvest Impacts Postharvest Fruit Softening and Internal Browning Development in Mechanically Damaged and Non-damaged Highbush Blueberries (Vaccinium corymbosum L.). Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Mehra, L.K.; MacLean, D.D.; Savelle, A.T.; Scherm, H. Postharvest Disease Development on Southern Highbush Blueberry Fruit in Relation to Berry Flesh Type and Harvest Method. Plant Dis. 2013, 97, 213–221. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Guthart, M.J.; Gezan, S.A.; Pisaroglo de Carvalho, M.; Schwieterman, M.L.; Colquhoun, T.A.; Bartoshuk, L.M.; Sims, C.A.; Clark, D.G.; Olmstead, J.W. Identifying Breeding Priorities for Blueberry Flavor Using Biochemical, Sensory, and Genotype by Environment Analyses. PLoS ONE 2015, 10, e0138494. [Google Scholar] [CrossRef] [PubMed]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Instrumental and sensory quality characteristics of blueberry fruit from twelve cultivars. Postharvest Biol. Technol. 2008, 49, 19–26. [Google Scholar] [CrossRef]
- Blaker, K.M.; Olmstead, J.W. Stone cell frequency and cell area variation of crisp and standard texture southern highbush blueberry fruit. J. Am. Soc. Hortic. Sci. 2014, 139, 553–557. [Google Scholar]
- Forney, C.F.; Kalt, W.; Jordan, M.A.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Blueberry and cranberry fruit composition during development. J. Berry Res. 2012, 2, 169–177. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Sci. Hortic. (Amsterdam) 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cellon, C.; Amadeu, R.R.; Olmstead, J.W.; Mattia, M.R.; Ferrao, L.F.V.; Munoz, P.R. Estimation of genetic parameters and prediction of breeding values in an autotetraploid blueberry breeding population with extensive pedigree data. Euphytica 2018, 214, 87. [Google Scholar] [CrossRef]
- Bell, H.P.; Burchill, J. Flower development in the lowbush blueberry. Can. J. Bot. 1955, 33, 251–258. [Google Scholar] [CrossRef]
- Gough, R.E. The Highbush Blueberry and Its Management; Food Products Press: Binghamton, NY, USA, 1994. [Google Scholar]
- Chu, W.; Gao, H.; Chen, H.; Wu, W.; Fang, X. Changes in Cuticular Wax Composition of Two Blueberry Cultivars during Fruit Ripening and Postharvest Cold Storage. J. Agric. Food Chem. 2018, 66, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.; Monterubbianesi, G.; Tognetti, J. Analysis of highbush blueberry (Vaccinium corymbosum L.) fruit growth with exponential mixed models. Sci. Hortic. (Amsterdam) 2008, 115, 368–376. [Google Scholar] [CrossRef]
- Jorquera-Fontena, E.; Génard, M.; Franck, N. Analysis of blueberry (Vaccinium corymbosum L.) fruit water dynamics during growth using an ecophysiological model. J. Hortic. Sci. Biotechnol. 2017, 92, 646–659. [Google Scholar] [CrossRef]
- Janick, J.; Paull, R.E. The Encyclopedia of Fruit and Nuts; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.-P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2013, 65, 4561–4575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haji, T.; Yaegaki, H.; Yamaguchi, M. Softening of stony hard peach by ethylene and the induction of endogenous ethylene by 1-aminocyclopropane-1-carboxylic acid (ACC). J. Jpn. Soc. Hortic. Sci. 2003, 72, 212–217. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; Van Der Plas, L.H.W.; Woltering, E.J. Ethylene perception is required for the expression of tomato ripening-related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biol. Technol. 2002, 26, 125–133. [Google Scholar] [CrossRef]
- Suzuki, A.; Kikuchi, T.; Aoba, K. Changes of ethylene evolution, ACC content, ethylene forming enzyme activity and respiration in fruits of highbush blueberry. J. Jpn. Soc. Hortic. Sci. 1997, 66, 23–27. [Google Scholar] [CrossRef]
- Ban, T.; Kugishima, M.; Ogata, T.; Shiozaki, S.; Horiuchi, S.; Ueda, H. Effect of ethephon (2-chloroethylphosphonic acid) on the fruit ripening characters of rabbiteye blueberry. Sci. Hortic. (Amsterdam) 2007, 112, 278–281. [Google Scholar] [CrossRef]
- Costa, D.V.T.A.; Pintado, M.; Almeida, D.P.F. Postharvest ethylene application affects anthocyanin content and antioxidant activity of blueberry cultivars. ISHS Acta Hortic. 2014, 1017, 525–530. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Shelf-life extension of highbush blueberry using 1-methylcyclopropene stored under air and controlled atmosphere. Food Chem. 2011, 126, 1812–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhou, Q.; Zhou, X.; Wei, B.; Ji, S. The effect of ethylene absorbent treatment on the softening of blueberry fruit. Food Chem. 2018, 246, 286–294. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.M.; Prange, R.K.; Bishop, C.; Harrison, P.A.; Ryan, D.A.J. The influence of 1-MCP on shelf-life quality of highbush blueberry. HortScience 2003, 38, 417–418. [Google Scholar]
- Deng, J.; Shi, Z.; Li, X.; Liu, H. Effects of cold storage and 1-methylcyclopropene treatments on ripening and cell wall degrading in rabbiteye blueberry (Vaccinium ashei) fruit. Food Sci. Technol. Int. 2014, 20, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Blaker, K.M.; Olmstead, J.W. Effects of Preharvest Applications of 1-Methylcyclopropene on Fruit Firmness in Southern Highbush Blueberry. ISHS Acta Hortic. 2014, 1017, 71–75. [Google Scholar] [CrossRef]
- MacLean, D.D.; NeSmith, D.S. Rabbiteye blueberry postharvest fruit quality and stimulation of ethylene production by 1-methylcyclopropene. HortScience 2011, 46, 1278–1281. [Google Scholar]
- Sun, Y.; Hou, Z.; Su, S.; Yuan, J. Effects of ABA, GA3 and NAA on fruit development and anthocyanin accumulation in blueberry. J. South China Agric. Univ. 2013, 34, 6–11. [Google Scholar]
- Buran, T.J.; Sandhu, A.K.; Azeredo, A.M.; Bent, A.H.; Williamson, J.G.; Gu, L. Effects of exogenous abscisic acid on fruit quality, antioxidant capacities, and phytochemical contents of southern high bush blueberries. Food Chem. 2012, 132, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103. [Google Scholar] [CrossRef]
- Cell, P.; Sørensen, I.; Willats, W.G.T. The Plant Cell Wall; Humana Press: Totowa, NJ, USA, 2011; Volume 715, pp. 115–121. ISBN 978-1-61779-007-2. [Google Scholar]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.J. Polyuronide degradation and hemicellulose modifications in ripening tomato fruit. J. Am. Soc. Hortic. Sci. 1983, 108, 405–409. [Google Scholar]
- Cutillas-Iturralde, A.; Zarra, I.; Fry, S.C.; Lorences, E.P. Implication of persimmon fruit hemicellulose metabolism in the softening process. Importance of xyloglucan endotransglycosylase. Physiol. Plant. 1994, 91, 169–176. [Google Scholar] [CrossRef]
- Brummell, D.A.; Schröder, R. Xylan metabolism in primary cell walls. N. Z. J. For. Sci. 2009, 39, 125–143. [Google Scholar]
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schröder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus x domestica) fruit. BMC Plant Biol. 2012, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.R.; Ortugno, C.; Rosli, H.; Powell, A.L.T.; Greve, L.C.; Labavitch, J.M. Temporal Sequence of Cell Wall Disassembly Events in Developing Fruits. 2. Analysis of Blueberry (Vaccinium Species). J. Agric. Food Chem. 2007, 55, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, R.; Hanson, E.J.; Beggs, J.L.; Beaudry, R.M. Applying Calcium Chloride Postharvest to Improve Highbush Blueberry Firmness Applying Calcium Chloride Postharvest to Improve Highbush Blueberry Firmness. HortScience 2016, 28, 2–4. [Google Scholar]
- Angeletti, P.; Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellón, A.; Chaves, A.; Vicente, A.R. Effect of preharvest calcium applications on postharvest quality, softening and cell wall degradation of two blueberry (Vaccinium corymbosum) varieties. Postharvest Biol. Technol. 2010, 58, 98–103. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MD, USA, 2000; Volume 40. [Google Scholar]
- Lara, I.; García, P.; Vendrell, M. Modifications in cell wall composition after cold storage of calcium-treated strawberry (Fragaria× ananassa Duch.) fruit. Postharvest Biol. Technol. 2004, 34, 331–339. [Google Scholar] [CrossRef]
- Conway, W.S.; Sams, C.E. The effects of postharvest infiltration of calcium, magnesium, or strontium on decay, firmness, respiration, and ethylene production in apples. J. Am. Soc. Hortic. Sci. 1987, 112, 300–303. [Google Scholar]
- Conway, W.S. Possible Mechanisms by Which Postharvest Calcium Treatment Reduces Decay in Apples. Phytopathology 1984, 74, 208. [Google Scholar] [CrossRef]
- Sams, C.E.; Conway, W.S.; Abbott, J.A.; Lewis, R.J.; Ben-Shalom, N. Firmness and decay of apples following postharvest pressure infiltration of calcium and heat treatment. J. Am. Soc. Hortic. Sci. 1993, 118, 623–627. [Google Scholar]
- Al-Banna, M.K.S.; Jinks, J.L. Indirect selection for environmental sensitivity in Nicotiana rustica. Hered. (Edinb) 1984, 52, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Bonomelli, C.; Ruiz, R. Effects of foliar and soil calcium application on yield and quality of table grape cv.‘Thompson Seedless’. J. Plant Nutr. 2010, 33, 299–314. [Google Scholar] [CrossRef]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre-and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- García, J.M.; Herrera, S.; Morilla, A. Effects of Postharvest Dips in Calcium Chloride on Strawberry. J. Agric. Food Chem. 1996, 44, 30–33. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria x ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Estrada, A.D.; Blakley, I.; Reid, R.; Patel, K.; Meyer, M.D.; Andersen, S.U.; Brown, A.F.; Lila, M.A.; Loraine, A.E. RNA-Seq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. Gigascience 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2008, 37 (Suppl. 1), D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Mao, X.; Yang, J.; Chen, X.; Mao, F.; Xu, Y. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012, 40, W445–W451. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Gupta, P.; Bag, S.K.; Nath, P.; Trivedi, P.K. Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol. 2014, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C.; Bennett, A.B. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999, 4, 176–183. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Chandrasekar, B.; van der Hoorn, R.A.L. Beta galactosidases in Arabidopsis and tomato—A mini review. Biochem. Soc. Trans. 2016, 44, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Rana, M.M.; Kimura, Y.; Roslan, H.A. Changes in Biochemical Characteristics and Activities of Ripening Associated Enzymes in Mango Fruit during the Storage at Different Temperatures. Biomed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Gao, L.; Gao, J.; Xu, Y.; Liu, H.; Cao, X.; Zhang, B.; Chen, K. Genome-Wide Identification, Expression Patterns, and Functional Analysis of UDP Glycosyltransferase Family in Peach (Prunus persica L. Batsch). Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Preuss, A.; De Vos, R.C.H.; D’Amico, E.; Perrotta, G.; Bovy, A.G.; Martens, S.; Rosati, C. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant. Cell Environ. 2009, 32, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J.A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Moggia, C.; Beaudry, R.M.; Retamales, J.B.; Lobos, G.A. Variation in the impact of stem scar and cuticle on water loss in highbush blueberry fruit argue for the use of water permeance as a selection criterion in breeding. Postharvest Biol. Technol. 2017, 132, 88–96. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. (Amsterdam). 2016, 211, 449–457. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Chapter 2: Texture of fresh fruit. In Horticultural Reviews; Janick, J., Ed.; Wiley Online Library: Hoboken, NJ, USA, 1997; Volume 20, pp. 121–224. [Google Scholar]
- Allan-Wojtas, P.M.; Forney, C.F.; Carbyn, S.E.; Nicholas, K.U.K.G. Microstructural Indicators of Quality-related Characteristics of Blueberries—An Integrated Approach. LWT—Food Sci. Technol. 2001, 34, 23–32. [Google Scholar] [CrossRef]
- Fava, J.; Alzamora, S.M.; Castro, M.A. Structure and Nanostructure of the Outer Tangential Epidermal Cell Wall in Vaccinium corymbosum L. (Blueberry) Fruits by Blanching, Freezing-Thawing and Ultrasound. Food Sci. Technol. Int. 2006, 12, 241–251. [Google Scholar] [CrossRef]
- Blaker, K.M. Comparison of Crisp and Standard Fruit Texture in Southern Highbush Blueberry Using Instrumental and Sensory Panel Techniques; University of Florida: Gainesville, FL, USA, 2013; ISBN 1303820617. [Google Scholar]
- Abbott, J.; Watada, A.E.; Massie, D.R. Effe-gi, Magness-Taylor, and Instron fruit pressure testing devices for apples, peaches, and nectarines. J. Am. Soc. Hortic. Sci. 1976, 101, 698–700. [Google Scholar]
- Mason, H.; Mason, A. Apple Tree Named “Rosy Glow”. U.S. Patent 10/712,783, 26 August 2004. [Google Scholar]
- Braun, T. Apple tree named ‘Fuji Fubrax’. U.S. Patent 11/355,401, 29 April 2008. [Google Scholar]
- Schmider, E.; Braun, T. Apple tree named Golden Parsi. U.S. Patent 12/798,834, 13 October 2011. [Google Scholar]
- Maillard, A.; Maillard, L. Apple tree named ‘REGALSTAR’. U.S. Patent 13/999,809, 31 May 2016. [Google Scholar]
- Harker, F.R.; Maindonald, J.H.; Jackson, P.J. Penetrometer Measurement of Apple and Kiwifruit Firmness: Operator and Instrument Differences. J. Am. Soc. Hortic. Sci. 1996, 121, 927–936. [Google Scholar]
- Lehman-Salada, L. Instrument and operator effects on apple firmness readings. HortScience 1996, 31, 994–997. [Google Scholar]
- De Belie, N.; Schotte, S.; Coucke, P.; De Baerdemaeker, J. Development of an automated monitoring device to quantify changes in firmness of apples during storage. Postharvest Biol. Technol. 2000, 18, 1–8. [Google Scholar] [CrossRef]
- Plocharski, W.; Konopacka, D.; Zwierz, J. Comparison of Magness-Taylor’s pressure test with mechanical, non-destructive methods of apple and pear firmness measurements. Int. Agrophys. 2000, 14, 311–318. [Google Scholar]
- Abbott, J.A.; Liljedahl, L.A. Relationship of sonic resonant frequency to compression tests and Magness-Taylor firmness of apples during refrigerated storage. Trans. ASAE 1994, 37, 1211–1215. [Google Scholar] [CrossRef]
- Abbott, J.A.; Massie, D.R.; Upchurch, B.L.; Hruschka, W.R. Nondestructive sonic firmness measurement of apples. Trans. ASAE 1995, 38, 1461–1466. [Google Scholar] [CrossRef]
- Lu, R.; Peng, Y. Hyperspectral scattering for assessing peach fruit firmness. Biosyst. Eng. 2006, 93, 161–171. [Google Scholar] [CrossRef]
- Abbott, J.A. Firmness Measurement of Freshly Harvested ‘Delicious’ Apples by Sensory Methods, Sonic Transmission, Magness-Taylor, and Compression. J. Am. Soc. Hortic. Sci. 1994, 119, 510–515. [Google Scholar]
- Elmasry, G.; Wang, N.; Vigneault, C. Postharvest Biology and Technology Detecting chilling injury in Red Delicious apple using hyperspectral imaging and neural networks. Postharvest Biol. Technol. 2009, 52, 1–8. [Google Scholar] [CrossRef]
- Lu, R. Multispectral imaging for predicting firmness and soluble solids content of apple fruit. Postharvest Biol. Technol. 2004, 31, 147–157. [Google Scholar] [CrossRef]
- Finn, C.E.; Luby, J.J. Inheritance of fruit quality traits in blueberry. J. Am. Soc. Hortic. Sci. 1992, 117, 617–621. [Google Scholar]
- Ali, S.; Zaman, Q.U.; Schumann, A.W.; Udenigwe, C.C.; Farooque, A.A. Impact of fruit ripening parameters on harvesting efficiency of the wild blueberry harvester. In ASABE Annual International Meeting; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2016. [Google Scholar]
- Vilela, A.; Gonçalves, B.; Ribeiro, C.; Fonseca, A.T.; Correia, S.; Fernandes, H.; Ferreira, S.; Bacelar, E.; Silva, A.P. Study of Textural, Chemical, Color and Sensory Properties of Organic Blueberries Harvested in Two Distinct Years: A Chemometric Approach. J. Texture Stud. 2016, 47, 199–207. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Shelf life determination of fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. Int. J. food Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-H.; Dong, Q.-L.; Liu, B.-L.; Opara, U.L. Prediction of mechanical properties of blueberry using hyperspectral interactance imaging. Postharvest Biol. Technol. 2016, 115, 122–131. [Google Scholar] [CrossRef]
- Brazelton, D.M.; Wagner, A.L. Blueberry Plant Named ‘Last Call’. 2015. Available online: https://patents.google.com/patent/USPP25386P3 (accessed on 15 August 2018).
- NeSmith, D.S. ‘Suziblue’Southern Highbush Blueberry. HortScience 2010, 45, 142–143. [Google Scholar]
- Grajkowski, J.; Ochman, I.; Muliński, Z. Firmness and antioxidant capacity of highbush blueberry (Vaccinium corymbosum L.) grown on three types of organic bed. Veg. Crop. Res. Bull. 2007, 66, 155–159. [Google Scholar] [CrossRef]
- Blaker, K.M.; Plotto, A.; Baldwin, E.A.; Olmstead, J.W. Correlation between sensory and instrumental measurements of standard and crisp-texture southern highbush blueberries (Vaccinium corymbosum L. interspecific hybrids). J. Sci. Food Agric. 2014, 94, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- NeSmith, D.S.; Draper, A.D.; Spiers, J.M. Palmetto’Southern Highbush Blueberry. HortScience 2004, 39, 1774–1775. [Google Scholar]
- Pavlis, G.C. Blueberry fruit quality and yield as affected by fertilization. ISHS Acta Hortic. 2004, 353–356. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Skupieñ, K. Effect of substrate type on the field performance and chemical composition of highbush blueberry cv. Patriot. Agric. Food Sci. 2010, 19, 69–80. [Google Scholar] [CrossRef]
- Retamales, J.B.; Lobos, G.A.; Romero, S.; Godoy, R.; Moggia, C. Repeated applications of CPPU on highbush blueberry cv. Duke increase yield and enhance fruit quality at harvest and during postharvest. Chil. J. Agric. Res. 2014, 74, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Li, C.; Takeda, F.; Krewer, G. Visual bruise assessment and analysis of mechanical impact measurement in southern highbush blueberries. Appl. Eng. Agric. 2014, 30, 29–37. [Google Scholar] [CrossRef]
- Stringer, S.J.; Draper, A.D.; Spiers, J.M.; Marshall, D.A.; Smith, B.J. ‘Pearl’Southern Highbush Blueberry. HortScience 2013, 48, 130–131. [Google Scholar]
- Lobos, G.A.; Callow, P.; Hancock, J.F. The effect of delaying harvest date on fruit quality and storage of late highbush blueberry cultivars (Vaccinium corymbosum L.). Postharvest Biol. Technol. 2014, 87, 133–139. [Google Scholar] [CrossRef]
- Ehret, D.L.; Frey, B.; Forge, T.; Helmer, T.; Bryla, D.R. Effects of drip irrigation configuration and rate on yield and fruit quality of young highbush blueberry plants. HortScience 2012, 47, 414–421. [Google Scholar]
- Hicklenton, P.; Forney, C.; Domytrak, C. Use of row covers and post harvest storage techniques to alter maturity and marketing period for highbush blueberries. ISHS Acta Hortic. 2002, 287–295. [Google Scholar] [CrossRef]
- Sargent, S.A.; Berry, A.D.; Brecht, J.K.; Santana, M.; Zhang, S.; Ristow, N. Studies on quality of southern highbush blueberry cultivars: Effects of pulp temperature, impact and hydrocooling. ISHS Acta Hortic. 2017, 1180, 497–502. [Google Scholar] [CrossRef]
- Brazelton, D.M.; Wagner, A.L. Blueberry Plant named’CLOCKWORK’. 2013. Available online: https://patents.google.com/patent/US20130239264P1 (accessed on 15 August 2018).
- Yang, W.Q.; Andrews, H.E.; Basey, A. Blueberry rootstock: Selection, evaluation, and field performance of grafted blueberry plants. ISHS Acta Hortic. 2016, 1117, 119–124. [Google Scholar] [CrossRef]
- Brazelton, D.M.; Wagner, A.L. Blueberry Plant Named ‘Cargo’ 2014. Available online: https://patents.google.com/patent/US20130239260P1 (accessed on 15 August 2018).
- Brazelton, D.M.; Wagner, A.L. Blueberry Plant Named ‘Blue Ribbon’ 2014. Available online: https://patents.google.com/patent/US20130239265P1 (accessed on 15 August 2018).
- Brazelton, D.M.; Wagner, A.L. Blueberry Plant Named ‘Top Shelf’ 2014. Available online: https://patents.google.com/patent/US20130239261P1 (accessed on 15 August 2018).
- Moggia, C.; González, C.; Lobos, G.A.; Bravo, C.; Valdés, M.; Lara, I.; Graell, J. Changes in quality and maturity of ‘Duke’ and ‘Brigitta’ blueberries during fruit development: Postharvest implications. Acta Hortic. 2018, 1194, 1495–1501. [Google Scholar] [CrossRef]
- Patel, N. Blueberry Plant Named ‘Hortblue Poppins’ 2011. Available online: https://patents.google.com/patent/USPP21881P3 (accessed on 15 August 2018).
- Ballington, J.R.; Bland, W.T. Blueberry Plant Named ‘Heintooga’ 2017. Available online: https://patents.google.com/patent/US20170188494P1 (accessed on 15 August 2018).
- Strik, B.C.; Vance, A.J.; Finn, C.E. Northern Highbush Blueberry Cultivars Differed in Yield and Fruit Quality in Two Organic Production Systems from Planting to Maturity. HortScience 2017, 52, 844–851. [Google Scholar] [CrossRef]
- Rodríguez-Armenta, H.P.; Olmstead, J.W.; Lyrene, P.M. Characterization of backcross blueberry populations created to introgress Vaccinium arboreum traits into southern highbush blueberry. ISHS Acta Hortic. 2016, 1180, 435–444. [Google Scholar] [CrossRef]
- Ballington, J.; Rooks, S. Blueberry Named ‘Carteret’ 2007. Available online: https://patents.google.com/patent/US20070143882 (accessed on 15 August 2018).
- Ballington, J.; Rooks, S. Blueberry named ‘New Hanover’ 2007. Available online: https://patents.google.com/patent/USPP19990P3 (accessed on 15 August 2018).
- Ballington, J.R.; Rooks, S.D. Blueberry named ‘Robeson’ 2009. Available online: https://patents.google.com/patent/USPP19756P3 (accessed on 15 August 2018).
- Almutairi, K.; Bryla, D.R.; Strik, B.C. Potential of Deficit Irrigation, Irrigation Cutoffs, and Crop Thinning to Maintain Yield and Fruit Quality with Less Water in Northern Highbush Blueberry. HortScience 2017, 52, 625–633. [Google Scholar] [CrossRef]
- Strik, B.; Buller, G. Nitrogen fertilization rate, sawdust mulch, and pre-plant incorporation of sawdust-term impact on yield, fruit quality, and soil and plant nutrition in ‘elliott’. ISHS Acta Hortic. 2014, 1017, 269–275. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Prussia, S.; Tetteh, M.; Krewer, G. Firmness losses of rabbiteye blueberries (Vaccinium ashei Reade) during harvesting and handling. ISHS Acta Hortic. 2000, 287–293. [Google Scholar] [CrossRef]
- Yang, W.Q.; Harpole, J.; Finn, C.E.; Strik, B.C. Evaluating berry firmness and total soluble solids of newly released highbush blueberry cultivars. ISHS Acta Hortic. 2008, 863–868. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; MacLean, D. A novel instrument to delineate varietal and harvest effects on blueberry fruit texture during storage. J. Sci. Food Agric. 2011, 91, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- NeSmith, D.S.; Draper, A.D. ‘Camellia’southern highbush blueberry. J. Am. Pomol. Soc. 2007, 61, 34–37. [Google Scholar]
- NeSmith, D.S.; Nunez-Barrios, A.; Prussia, S.E.; Aggarwal, D. Postharvest berry quality of six rabbiteye blueberry cultivars in response to temperature. J. Am. Pomol. Soc. 2005, 59, 13–17. [Google Scholar]
- Døving, A.; Måge, F.; Vestrheim, S. Methods for Testing Strawberry Fruit Firmness Methods for Testing Strawberry Fruit Firmness: A Review. Small Fruits Rev. 2008, 8851, 37–41. [Google Scholar] [CrossRef]
- Ballington, J.R.; Ballinger, W.E.; Mainland, C.M.; Swallow, W.H.; Maness, E.P. Ripening period of Vaccinium species in southeastern North Carolina [Blueberry, breeding for both early-and late-ripening Vaccinium genotypes]. J. Am. Soc. Hortic. Sci. 1984, 109, 392–396. [Google Scholar]
- Boches, P.; Bassil, N.V.; Rowland, L. Genetic diversity in the highbush blueberry evaluated with microsatellite markers. J. Am. Soc. Hortic. Sci. 2006, 131, 674–686. [Google Scholar]
- Hancock, J.F.; Lyrene, P.; Finn, C.E.; Vorsa, N.; Lobos, G.A. Blueberries and cranberries. In Temperate Fruit Crop Breeding; James, F.H., Ed.; Springer: Berlin, Germany, 2008; pp. 115–150. [Google Scholar]
- Goldy, R.G.; Lyrene, P.M. In vitro colchicine treatment of 4× blueberries, Vaccinium sp. [Polyploid induction, genotype effect]. J. Am. Soc. Hortic. Sci. 1984, 109, 336–338. [Google Scholar]
- Chavez, D.J.; Lyrene, P.M. Effects of self-pollination and cross-pollination of Vaccinium darrowii (Ericaceae) and other low-chill blueberries. HortScience 2009, 44, 1538–1541. [Google Scholar]
- Ehlenfeldt, M.K.; Draper, A.D.; Clark, J.R. Performance of southern highbush blueberry cultivars released by the US Department of Agriculture and cooperating state agricultural experiment stations. Horttechnology 1995, 5, 127–130. [Google Scholar]
- Coville, F.V. Improving the wild blueberry. In Yearbook of Agriculture; U.S. Department of Agriculture; U.S. Government Printing Office: Washington, DC, USA, 1937; pp. 559–574. [Google Scholar]
- Sharpe, R.H.; Sherman, W.B. “Floridablue and Sharpblue”: Two new blueberries for central Florida. Circ. Fla Coop Ext. Serv. Inst. Food Agric. Sci. Univ. Fla 1976, 240, 6. [Google Scholar]
- Fillion, L.; Kilcast, D. Consumer perception of crispness and crunchiness in fruits and vegetables. Food Qual. Prefer. 2002, 13, 23–29. [Google Scholar] [CrossRef]
- Aalders, L.E.; Hall, I.V. A study of variation in fruit yield and related characters in two diallels of the lowbush blueberry, Vaccinium angustifolium Ait. Can. J. Genet. Cytol. 1975, 17, 401–404. [Google Scholar] [CrossRef]
- Erb, W.A.; Draper, A.D.; Galletta, G.J.; Swartz, H.J. Combining ability for plant and fruit traits of interspecific blueberry progenies on mineral soil. J. Am. Soc. Hortic. Sci. 1990, 115, 1025–1028. [Google Scholar]
- Scalzo, J.; Sguigna, V.; Mezzetti, B.; Stanley, J.; Alspach, P. Variation of fruit traits in highbush blueberry seedlings from a factorial cross. ISHS Acta Hortic. 2010, 79–83. [Google Scholar] [CrossRef]
- Scalzo, J.; Stanley, J.; Alspach, P.; Mezzetti, B. Preliminary evaluation of fruit traits and phytochemicals in a highbush blueberry seedling population. J. Berry Res. 2013, 3, 103–111. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Genotypic and environmental variation in antioxidant activity, total phenolic content, and anthocyanin content among blueberry cultivars. J. Am. Soc. Hortic. Sci. 2002, 127, 89–97. [Google Scholar]
- Finn, C.E.; Hancock, J.F.; Mackey, T.; Serce, S. Genotype× environment interactions in highbush blueberry (Vaccinium sp. L.) families grown in Michigan and Oregon. J. Am. Soc. Hortic. Sci. 2003, 128, 196–200. [Google Scholar]
- Isik, F.; Holland, J.; Maltecca, C. Genetic Data Analysis for Plant and Animal Breeding; Springer: New York, NY, USA, 2017; Volume 1, ISBN 3319551779. [Google Scholar]
- Hamblin, M.T.; Buckler, E.S.; Jannink, J.-L. Population genetics of genomics-based crop improvement methods. Trends Genet. 2011, 27, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Jannink, J.-L.; Lorenz, A.J.; Iwata, H. Genomic selection in plant breeding: From theory to practice. Brief. Funct. Genom. 2010, 9, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, L.F.V.; Benevenuto, J.; de Bem Oliveira, I.; Cellon, C.; Olmstead, J.; Kirst, M.; Resende, M.F.R.; Munoz, P. Insights into the genetic basis of blueberry fruit-related traits using diploid and polyploid models in a GWAS context. Front. Ecol. Evol. 2018, 6, 107. [Google Scholar] [CrossRef]
- Wang, W.; Cai, J.; Wang, P.; Tian, S.; Qin, G. Post-transcriptional regulation of fruit ripening and disease resistance in tomato by the vacuolar protease SlVPE3. Genom. Biol. 2017, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Salentijn, E.M.J.; Aharoni, A.; Schaart, J.G.; Boone, M.J.; Krens, F.A. Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol. Plant. 2003, 118, 571–578. [Google Scholar] [CrossRef]
- Roje, S. S-Adenosyl-l-methionine: Beyond the universal methyl group donor. Phytochemistry 2006, 67, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Bulens, I.; Oppermann, Y.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Sauter, M.; Geeraerd, A.H. S-adenosyl-l-methionine usage during climacteric ripening of tomato in relation to ethylene and polyamine biosynthesis and transmethylation capacity. Physiol. Plant. 2013, 148, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid Metabolism in Ripening Fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef] [Green Version]
- Moffatt, B.A.; Weretilnyk, E.A. Sustaining S-adenosyl-l-methionine-dependent methyltransferase activity in plant cells. Physiol. Plant. 2001, 113, 435–442. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q. A CRISPR–Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H. Applying CRISPR/Cas for genome engineering in plants: The best is yet to come. Curr. Opin. Plant Biol. 2017, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef] [PubMed]

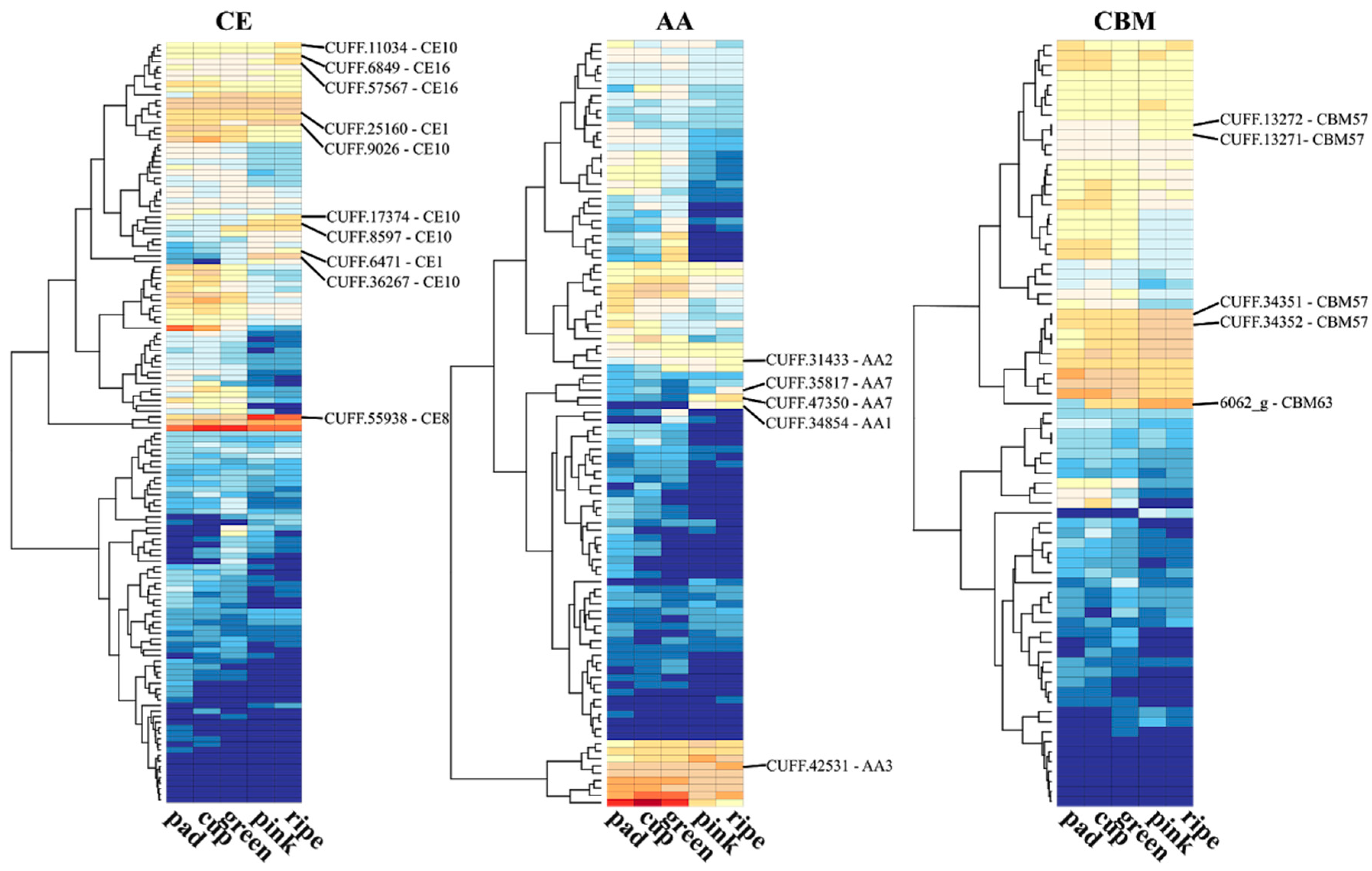
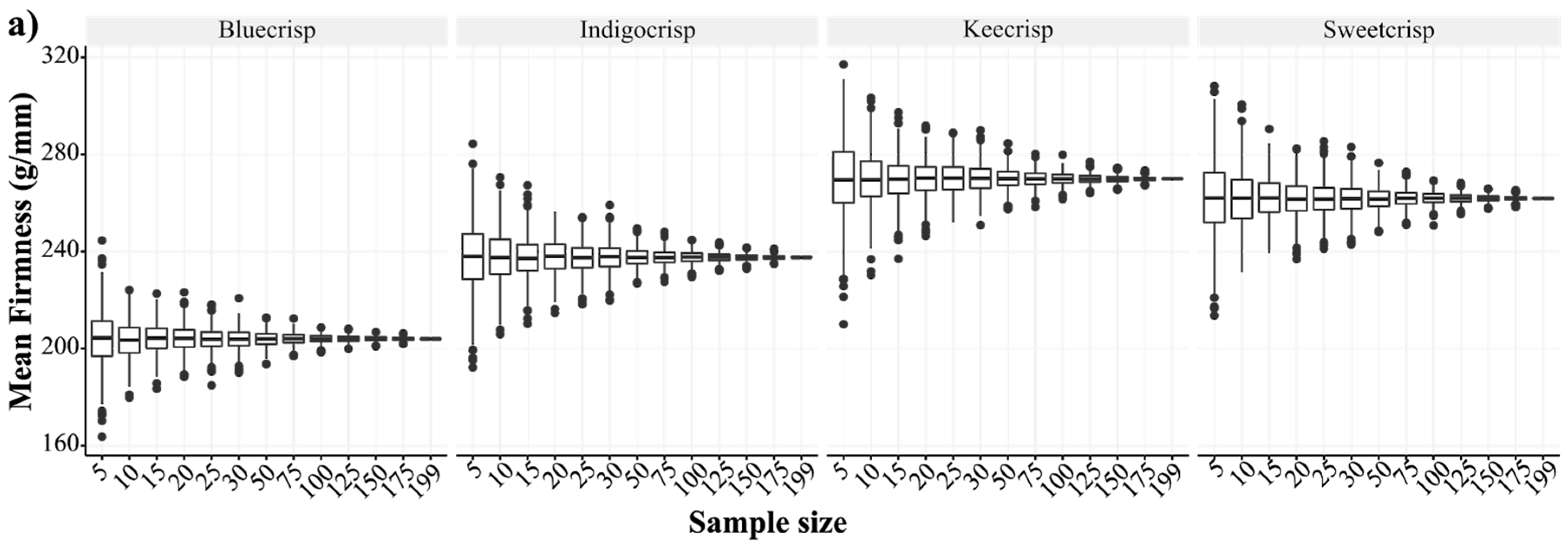
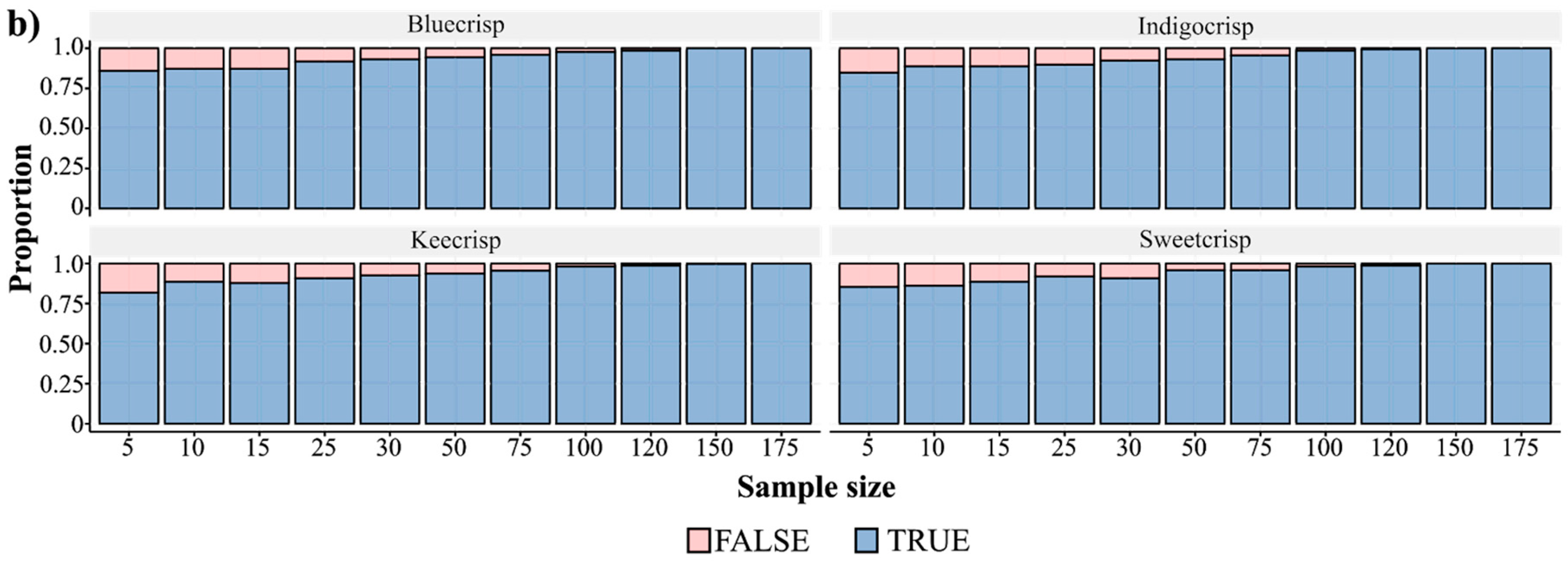
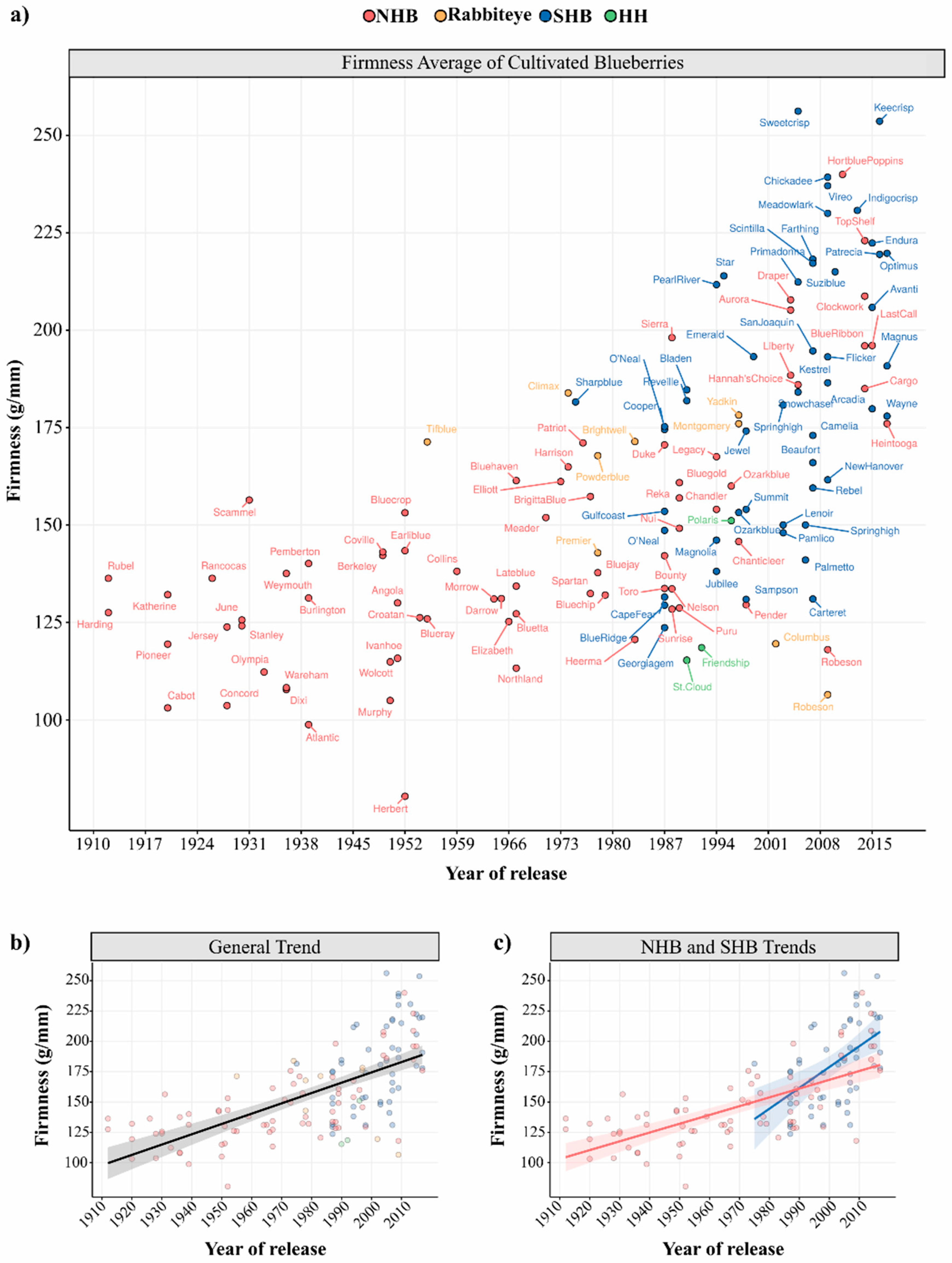

| Type * | n | Mean | St. Dev | Maximum | Minimum |
|---|---|---|---|---|---|
| SHB | 50 | 183 | 35.18 | 256 (Sweetcrisp) | 124 (Georgiagem) |
| HH | 3 | 128 | 19.79 | 151 (Polaris) | 115 (St. Cloud) |
| NHB | 74 | 145 | 31.22 | 240 (Hortblue Poppins) | 80 (Hebert) |
| Rabbiteye | 9 | 157 | 27.87 | 184 (Climax) | 106 (Robeson) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappai, F.; Benevenuto, J.; Ferrão, L.F.V.; Munoz, P. Molecular and Genetic Bases of Fruit Firmness Variation in Blueberry—A Review. Agronomy 2018, 8, 174. https://doi.org/10.3390/agronomy8090174
Cappai F, Benevenuto J, Ferrão LFV, Munoz P. Molecular and Genetic Bases of Fruit Firmness Variation in Blueberry—A Review. Agronomy. 2018; 8(9):174. https://doi.org/10.3390/agronomy8090174
Chicago/Turabian StyleCappai, Francesco, Juliana Benevenuto, Luís Felipe V. Ferrão, and Patricio Munoz. 2018. "Molecular and Genetic Bases of Fruit Firmness Variation in Blueberry—A Review" Agronomy 8, no. 9: 174. https://doi.org/10.3390/agronomy8090174





