Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Yield and Morphological Parameters of Lettuce, Escarole and Curly Endive Plants
3.2. Quality Parameters of Lettuce, Escarole and Curly Endive Plants
3.3. Principal Components Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bortels, H. Molybdän als Katalysator bei der biologischen Stickstoffbindung. Archiv. Für Mikrobiol. 1930, 1, 333–342. [Google Scholar] [CrossRef]
- Arnon, D.I.; Stout, P.R. Molybdenum as an essential element for higher plants. Plant Physiol. 1939, 14, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, B.N; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Villora, G.; Moreno, D.A.; Romero, L. Phosphorus supply influence the molybdenum, nitrate and nitrate reductase activity in eggplant. J. Hortic. Sci. Biotechnol. 2002, 77, 305–309. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Lavon, R.; Goldschmidt, E.E. Enzymatic methods for detection of mineral element deficiencies in citrus leaves. J. Plant Nutr. 1999, 22, 139–150. [Google Scholar] [CrossRef]
- Redingbaugh, M.G.; Campbell, W.H. Higher plant responses to environmental nitrate. Physiol. Plant. 1991, 82, 640–650. [Google Scholar] [CrossRef]
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.U.; Hu, C.X.; Sun, X.C.; Wang, Y.H. Influences of Mo on nitrate reductase, glutamine synthetase and nitrogen accumulation and utilization in Mo-efficient and Mo-inefficient winter wheat cultivars. Agric. Sci. China 2010, 9, 355–361. [Google Scholar]
- Agarwala, S.C.; Sharma, C.P.; Farooq, S.; Chatterjee, C. Effect of molybdenum deficiency on the growth and metabolism of corn plants raised in sand culture. Can. J. Bot. 1978, 56, 1905–1909. [Google Scholar] [CrossRef]
- Chatterjee, C.; Nautiyal, N.; Agarwala, S.C. Metabolic changes in mustard plants associated with molybdenum deficiency. New Phytol. 1985, 100, 511–518. [Google Scholar] [CrossRef]
- Spencer, D.; Wood, J.G. The role of molybdenum in nitrate reduction in higher plants. Aust. J. Biol. Sci. 1954, 7, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A survey of nitrate and oxalate content in fresh vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888. [Google Scholar] [CrossRef]
- Gupta, U.C. Molybdenum in Agriculture; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Boertje, G.A. Molybdenum and lettuce. In Annual Report—Glasshouse Crops Research and Experiment Station; Glasshouse Crops Research and Experiment Station: Naaldwijk, The Netherlands, 1969. [Google Scholar]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: New York, NY, USA, 2009. [Google Scholar]
- Goto, E.; Both, A.J.; Albright, L.D.; Langhans, R.W.; Leed, A.R. Effect of dissolved oxygen concentration on lettuce growth in floating hydroponics. Acta Hortic. 1996, 440, 205–210. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G. Reporting of objective color measurements. Hortic. Sci. 1992, 27, 1254–1255. [Google Scholar]
- Miceli, A.; Miceli, C. Effect of nitrogen fertilization on the quality of Swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production; Woodbridge Press: Santa Barbara, CA, USA, 1997. [Google Scholar]
- Islam, M.M.; Karim, M.R.; Oliver, M.M.H.; Urmi, T.A.; Hossain, M.A.; Haque, M.M. Impacts of trace element addition on lentil (Lens culinaris L.). Agronomy 2018, 8, 100. [Google Scholar] [CrossRef]
- D’Anna, F.; Miceli, A.; Vetrano, F. First results of floating system cultivation of Eruca sativa L. Acta Hortic. 2003, 609, 361–364. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F.; D’Anna, F. First results on yield and quality response of basil (Ocimum basilicum L.) grown in floating system. Acta Hortic. 2003, 609, 377–381. [Google Scholar] [CrossRef]
- Adams, P. Effect of diurnal fluctuations in humidity on the accumulation of nutrients in the leaves of tomato (Lycopersicon esculentum). J. Hortic. Sci. 1991, 66, 545–550. [Google Scholar] [CrossRef]
- Bèlanger, R. Soluble silicon: Its role in crop and disease management of greenhouse crops. Plant Dis. 1995, 79, 329–336. [Google Scholar] [CrossRef]
- Ehret, D.L.; Ho, L.C. Effects of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. J. Hortic. Sci. 1986, 61, 361–367. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon in plants: Facts vs. concepts. Stud. Plant Sci. 1999, 8, 1–15. [Google Scholar]
- Epstein, E.; Bloom, A.J. Inorganic components of plants. In Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Epstein, E., Bloom, A.J., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2005; pp. 44–45. [Google Scholar]
- Mulholland, B.J.; Taylor, I.B.; Jackson, A.C.; Thompson, A.J. Can ABA mediate responses of salinity stressed tomato. Environ. Exp. Bot. 2003, 50, 17–28. [Google Scholar] [CrossRef]
- Sonneveld, C.; van der Burg, A.M.M. Sodium chloride salinity in fruit vegetable crops in soilless culture. Neth. J. Agric. Sci. 1991, 39, 115–122. [Google Scholar]
- Zhen, Y.; Liu, S.; Liu, S. Effect of appling molybdenum fertilizer on nitrate content in lettuce and you-mai vegetable. Soil Fertil. Sci. China 2007, 1, 51–53. [Google Scholar]
- Uchida, R. Essential nutrients for plant growth: Nutrient functions and deficiency symptoms. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2000; pp. 31–55. [Google Scholar]
- Biacs, P.A.; Daood, H.G.; Kadar, I. Effect of Mo, Se, Zn, and Cr treatments on the yield, element concentration, and carotenoid content of carrot. J. Agric. Food Chem. 1995, 43, 589–591. [Google Scholar] [CrossRef]
- Breimer, T. Environmental factors and cultural measures affecting the nitrate content in spinach. Fert. Res. 1982, 3, 191–292. [Google Scholar] [CrossRef] [Green Version]
- Campo, R.J.; Araujo, R.S.; Hungria, M. Molybdenum-enriched soybean seeds enhance N accumulation, seed yield, and seed protein content in Brazil. Field Crops Res. 2009, 110, 219–224. [Google Scholar] [CrossRef]
- Vieira, R.F.; Salgado, L.T.; Ferreira, A.C.B. Performance of common bean using seeds harvested from plants fertilized with high rates of molybdenum. J. Plant Nutr. 2005, 28, 363–377. [Google Scholar] [CrossRef]
- Valenciano, J.B.; Boto, J.A.; Marcelo, V. Chickpea (Cicer arietinum L.) response to zinc, boron and molybdenum application under field conditions. N. Z. J. Crop Hortic. Sci. 2011, 39, 217–229. [Google Scholar]
- Randall, P.J. Changes in nitrate and nitrate reductase levels on restoration of molybdenum to molybdenum-deficient plants. Aust. J. Agric. Res. 1969, 20, 635–642. [Google Scholar] [CrossRef]
- Hewitt, E.J. The essential nutrient elements: requirements and interactions in plants. In Plant Physiology: A Treatise; Steward, F.C., Ed.; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Agarwala, S.C.; Hewitt, E.J. Molybdenum as a plant nutrient: IV. The interrelationships of molybdenum and nitrate supply in chlorophyll and ascorbic acid fractions in cauliflower plants grown in sand culture. J. Hortic. Sci. 1954, 29, 291–300. [Google Scholar] [CrossRef]
- Munshi, C.B.; Mondy, N.I. Effect of soil applications of sodium molybdate on the quality of potatoes: Polyphenol oxidase activity, enzymatic discoloration, phenols, and ascorbic acid. J. Agric. Food Chem. 1988, 36, 688–690. [Google Scholar] [CrossRef]
- Shinohara, Y.; Suzuki, Y. Effects of light and nutritional conditions on the ascorbic acid content of lettuce. J. Jpn. Soc. Hortic. Sci. 1981, 50, 239–246. [Google Scholar] [CrossRef]
- Biesiada, A.; Kołota, E. The effect of nitrogen fertilization on yielding and chemical composition of radicchio chicory for autumn-harvest cultivation. Acta Sci. Pol. 2010, 9, 85–91. [Google Scholar]
- Kansal, B.; Singh, B.; Bajaj, K.; Kaur, G. Effect of different levels of nitrogen and farmyard manure on yield and quality of spinach (Spinacea oleracea L.). Plant Food Hum. Nutr. 1981, 31, 163–170. [Google Scholar] [CrossRef]
- Mozafar, A. Plant Vitamins Agronomic, Physiological and Nutritional Aspects; CRC Press Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Muller, K.; Hippe, J. Influence of differences in nutrition on important quality characteristics of some agricultural crops. Plant Soil 1987, 100, 35–45. [Google Scholar] [CrossRef]
- Hulewicz, D.; Kalbarczyk, M. The influence of light on the yield of lettuce and its nutrient content. Archiv. fur Gartenbau. 1976, 24, 113–120. [Google Scholar]
- Cantliffe, D.J.; MacDonald, G.E.; Peck, N.H. Reduction of nitrate accumulation by molybdenum in spinach grown at low pH. Commun. Soil Sci. Plant Anal. 1974, 5, 273–282. [Google Scholar] [CrossRef]
- Cox, D.A. Poinsettia cultivars differ in their response to molybdenum deficiency. HortScience 1992, 27, 892–893. [Google Scholar]
- Cox, D.A. Foliar-applied molybdenum for preventing or correcting molybdenum deficiency of poinsettia. Hortscience 1992, 8, 894–895. [Google Scholar]
- Smedley, P.L.; Cooper, D.M.; Ander, E.L.; Milne, C.J.; Lapworth, D.J. Occurrence of molybdenum in British surface water and groundwater: Distributions, controls and implications for water supply. Appl. Geochem. 2014, 40, 144–154. [Google Scholar] [CrossRef]
- Martens, D.C.; Westermann, D.T. Fertilizer application for correcting micronutrient deficiencies. In Micronutrients in Agriculture, 2nd ed.; SSSA Book Series, No. 4; ACSESS DL: Madison, WI, USA, 1991; pp. 549–592. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for molybdenum. EFSA J. 2013, 11, 3333. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, K.V. Molybdenum: An essential trace element in human nutrition. Annu. Rev. Nutr. 1988, 8, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W. Nutritional Disorders of Plants—Development, Visual and Analytical Diagnosis; Spektrum Akademischer Verlag: Berlin, Germany, 1992. [Google Scholar]
- Gupta, U.C. Symptoms of molybdenum deficiency and toxicity in crops. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Hamlin, R.L. Molybdenum. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 375–394. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Molybdenum. Principles of Plant Nutrition, 5th ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 613–619. [Google Scholar]

| Leafy Vegetables (LV) | Mo (µmol L−1) | Yield (kg m−2) | Head Weigth (g) | Head Height (cm) | Stem Diameter (mm) | Number of Leaves | Head Dry Matter (%) | Root Dry Matter (%) |
|---|---|---|---|---|---|---|---|---|
| Lettuce | 0 | 1.8 ae | 147.2e | 21.3d | 14.4d | 37.6c | 10.8c | 2.0c |
| 0.5 | 3.9c | 322.3c | 34.0ab | 22.8ab | 60.7b | 13.2b | 2.4b | |
| 1.5 | 4.0c | 331.0c | 36.7a | 24.0a | 63.3b | 16.6a | 7.8a | |
| 3.0 | 3.9c | 326.2c | 32.2ab | 22.8ab | 62.3b | 17.4a | 7.0a | |
| Escarole | 0 | 2.0de | 169.2de | 17.7e | 18.7c | 42.3c | 9.2d | 2.7c |
| 0.5 | 4.5b | 374.5b | 21.8d | 23.8ab | 84.2a | 11.0c | 3.8b | |
| 1.5 | 4.6b | 379.0b | 22.8d | 21.5b | 89.8a | 10.9c | 4.5b | |
| 3.0 | 4.3b | 362.1b | 22.3d | 21.0b | 80.5a | 16.7a | 4.3b | |
| Curly Endive | 0 | 2.5d | 210.8d | 19.7de | 18.6c | 37.4c | 8.3d | 1.7d |
| 0.5 | 5.6a | 469.3a | 29.3bc | 25.0a | 81.8a | 10.3c | 2.1c | |
| 1.5 | 5.5a | 456.3a | 25.7c | 20.7b | 87.3a | 10.3c | 2.3c | |
| 3.0 | 5.6a | 464.0a | 28.0c | 21.0b | 85.8a | 9.9c | 1.8cd | |
| LV | *** b | *** | *** | ns | *** | *** | *** | |
| Mo | *** | *** | *** | *** | *** | *** | *** | |
| Interaction LV × Mo | ** | ** | *** | * | ** | *** | *** |
| Variable | Lettuce | Escarole | Curly Endive | |||
|---|---|---|---|---|---|---|
| Model a | R2 | Model a | R2 | Model a | R2 | |
| Yield | y = −0.605x2 + 2.378x + 2.146 | 0.769 **,b | y = −0.726x2 + 2.769x + 2.480 | 0.748 ** | y = −0.798x2 + 3.162x + 3.144 | 0.697 ** |
| Head weight | y = −0.605x2 + 2.378x + 2.147 | 0.769 ** | y = −0.726x2 + 2.769x + 2.481 | 0.748 ** | y = −0.798x2 + 3.162x + 3.145 | 0.697 ** |
| Head height | y = −4.092x2 + 17.596x + 23.133 | 0.824 *** | y = −1.424x2 + 5.581x + 18.285 | 0.820 *** | y = −1.466x2 + 6.176x + 22.168 | 0.352 ns |
| Stem diameter | y = −3.153x2 + 11.795x + 15.588 | 0.761 ** | y = −1.076x2 + 3.480x + 19.984 | 0.166 ns | y = −0.887x2 + 2.708x + 20.481 | 0.120 ns |
| Number of leaves | y = −6.987x2 + 27.632x + 41.531 | 0.733 *** | y = −14.428x2 + 53.331x + 49.017 | 0.798 ** | y = −13.436x2 + 53.443x + 44.925 | 0.777 ** |
| Head dry matter | y = −1.081x2 + 5.466x +10.786 | 0.981 *** | y = 0.742x2 + 0.052x + 9.759 | 0.889 *** | y = 0.327x + 9.277 | 0.101 ns |
| Root dry matter | y = −1.252x2 + 5.723x + 1.250 | 0.870 *** | y = 0.4486x + 3.2643 | 0.219 ns | y = −0.0149x + 1.9803 | 0.002 ns |
| Leafy Vegetables (LV) | Mo (µmol L−1) | TSS (°Brix) | TA (%) c | Ascorbic Acid (mg kg−1 FW) | N-NO3− (mg kg−1 FW) | Mo μg g−1 FW) |
|---|---|---|---|---|---|---|
| Lettuce | 0 | 3.1e a | 0.57c | 124.9c | 1427.3c | 0.02h |
| 0.5 | 5.4b | 0.75b | 214.3b | 1210.2d | 0.11g | |
| 1.5 | 4.7c | 0.98ab | 219.3b | 1006.9e | 0.50ef | |
| 3.0 | 4.1d | 0.93b | 320.2a | 1039.2e | 0.43f | |
| Escarole | 0 | 3.2e | 0.42d | 57.5e | 1585.2b | 0.03h |
| 0.5 | 4.3cd | 0.65c | 89.0d | 1222.3d | 0.55e | |
| 1.5 | 4.7c | 0.59c | 90.8d | 1373.3cd | 1.91c | |
| 3.0 | 5.9a | 1.23a | 139.0c | 1047.3e | 4.02a | |
| Curly Endive | 0 | 3.0e | 0.39d | 48.2f | 1927.1a | 0.05gh |
| 0.5 | 4.2d | 0.67c | 71.9e | 1606.9b | 1.31d | |
| 1.5 | 4.1d | 1.05ab | 61.3e | 1350.8c | 1.34d | |
| 3.0 | 5.1bc | 1.13ab | 102.1d | 1181.2d | 2.68b | |
| LV | *** b | ns | *** | *** | *** | |
| Mo | *** | *** | *** | *** | *** | |
| Interaction LV × Mo | *** | *** | *** | *** | *** |
| Variable | Lettuce | Escarole | Curly Endive | |||
|---|---|---|---|---|---|---|
| Model a | R2 | Model a | R2 | Model a | R2 | |
| TSS | y = 0.270x + 3.996 | 0.130 ns b | y = 0.8111x + 3.5278 | 0.891 *** | y = 0.5968x + 3.354 | 0.807 *** |
| TA | y = −0.101x2 + 0.424x + 0.568 | 0.921 *** | y = 0.0849x2 − 0.0141x + 0.4959 | 0.852 *** | y = −0.1265x2 + 0.6275x + 0.3892 | 0.963 *** |
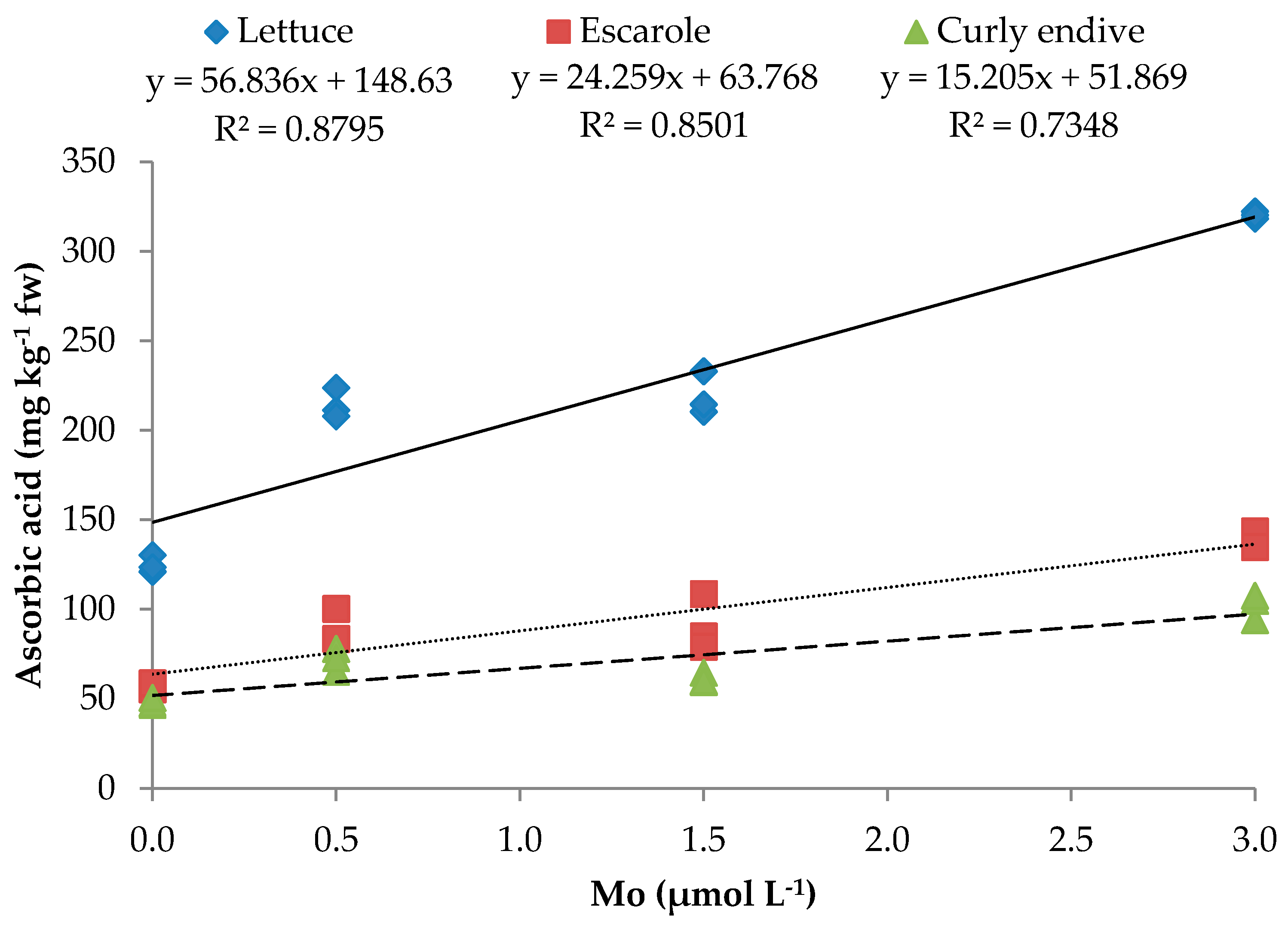
| Ascorbic acid | y = 56.836x + 148.626 | 0.880 *** | y = 24.259x + 63.768 | 0.850 *** | y = 15.205x + 51.869 | 0.735 *** |
| N-NO3− | y = 83.52x2 − 384.17x + 1411 | 0.917 *** | y = −137.56x + 1479 | 0.618 ** | y = 97.928x2 − 531.12x + 1898.9 | 0.966 *** |
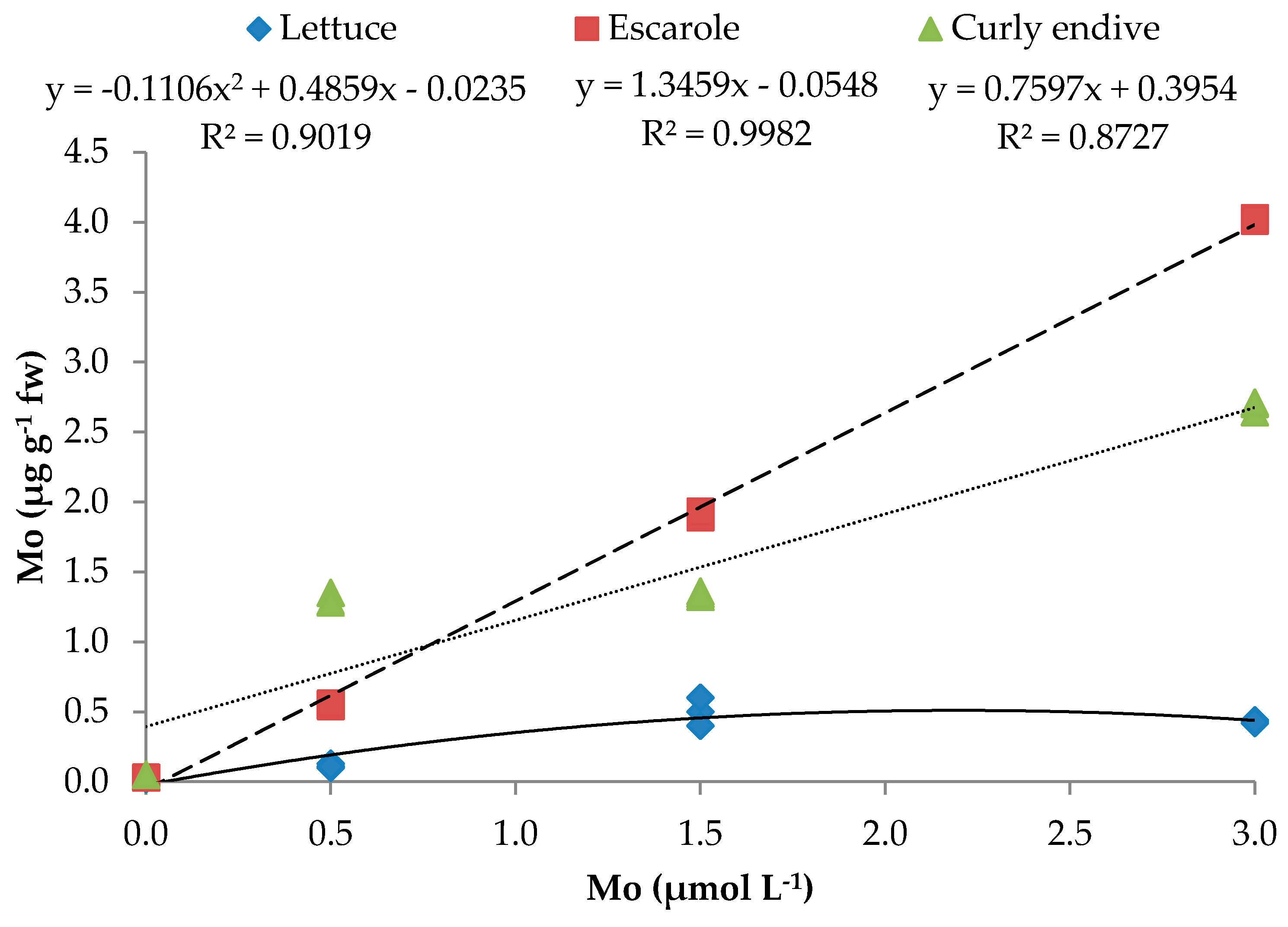
| Mo | y = −0.111x2 + 0.486x − 0.024 | 0.902 ** | y = 1.3459x − 0.0548 | 0.998 *** | y = 0.7597x + 0.3954 | 0.873 *** |
| L * | y = −1.116x + 46.648 | 0.194 ns | y = −1.0054x + 52.669 | 0.315 ns | y = 1.734x2 − 6.562x + 55.851 | 0.661 ** |
| Chroma | y = −1.145x2 + 4.506x + 28.991 | 0.615 * | y = −1.862x2 + 6.320x + 28.851 | 0.781 ** | y = −1.142x2 + 3.759x + 28.743 | 0.392 ns |
| Hue angle | y = −2.178x2 + 8.324x + 116.31 | 0.526 * | y = −2.420x2 + 9.736x + 113.03 | 0.659 ** | y = −2.545x2 + 9.950x + 113.81 | 0.656 ** |
| L * | Chroma | Hue Angle | |
|---|---|---|---|
| Leafy Vegetable (LV) | |||
| Lettuce | 45.3b a | 31.4a | 120.5a |
| Escarole | 51.4a | 31.4a | 118.3b |
| Curly Endive | 52.7a | 30.4b | 118.9ab |
| Mo (µmol L−1) | |||
| 0 | 53.4a | 28.2b | 112.9b |
| 0.5 | 48.2b | 32.1a | 121.2a |
| 1.5 | 48.9b | 32.4a | 121.5a |
| 3.0 | 48.5b | 31.4a | 121.3a |
| LV | *** b | * | * |
| Mo | *** | *** | *** |
| Interaction LV × Mo | ns | ns | ns |
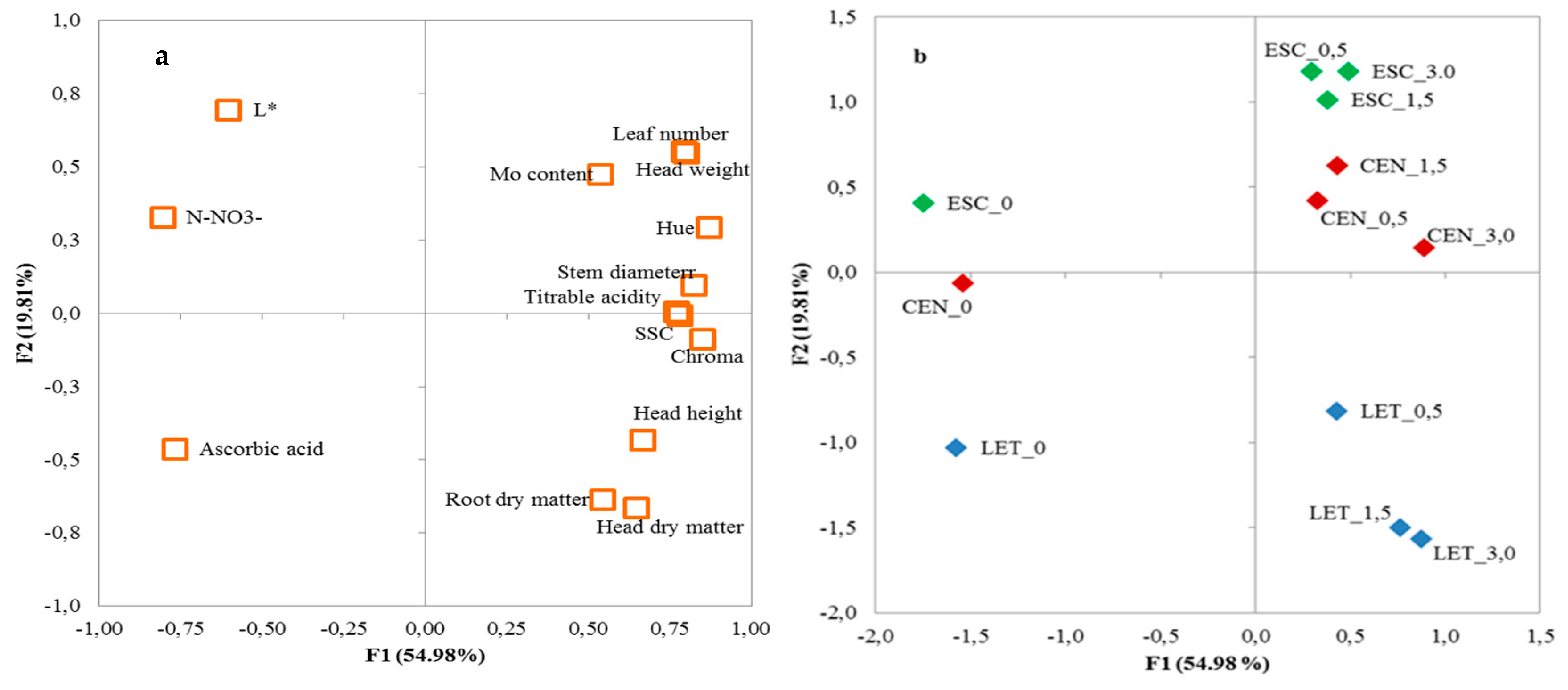
| Variable | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Head weight | 0.795 | 0.552 | −0.138 |
| Head height | 0.666 | −0.433 | −0.310 |
| Stem diameter | 0.826 | 0.096 | −0.478 |
| Leaf number | 0.800 | 0.548 | 0.006 |
| Head dry matter | 0.649 | −0.664 | 0.234 |
| Root dry matter | 0.543 | −0.635 | −0.109 |
| TSS | 0.782 | −0.006 | 0.471 |
| TA | 0.771 | 0.005 | 0.458 |
| Ascorbic acid | −0.768 | −0.463 | 0.256 |
| N-NO3− | −0.806 | 0.328 | −0.369 |
| Mo | 0.538 | 0.476 | 0.652 |
| L* | −0.603 | 0.695 | 0.087 |
| Chroma | 0.851 | −0.088 | −0.295 |
| Hue angle | 0.871 | 0.293 | −0.293 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System. Agronomy 2018, 8, 171. https://doi.org/10.3390/agronomy8090171
Moncada A, Miceli A, Sabatino L, Iapichino G, D’Anna F, Vetrano F. Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System. Agronomy. 2018; 8(9):171. https://doi.org/10.3390/agronomy8090171
Chicago/Turabian StyleMoncada, Alessandra, Alessandro Miceli, Leo Sabatino, Giovanni Iapichino, Fabio D’Anna, and Filippo Vetrano. 2018. "Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System" Agronomy 8, no. 9: 171. https://doi.org/10.3390/agronomy8090171





