Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review
Abstract
:1. Introduction
2. The Quantitative Use of 13C NMR Spectroscopy in Soil Organic Matter Research
3. The Concept of Humic Substances in Soil Organic Matter Research
- The dead organic matter in soil arising mainly from plants and soil microorganisms is partially degraded and split into small units.
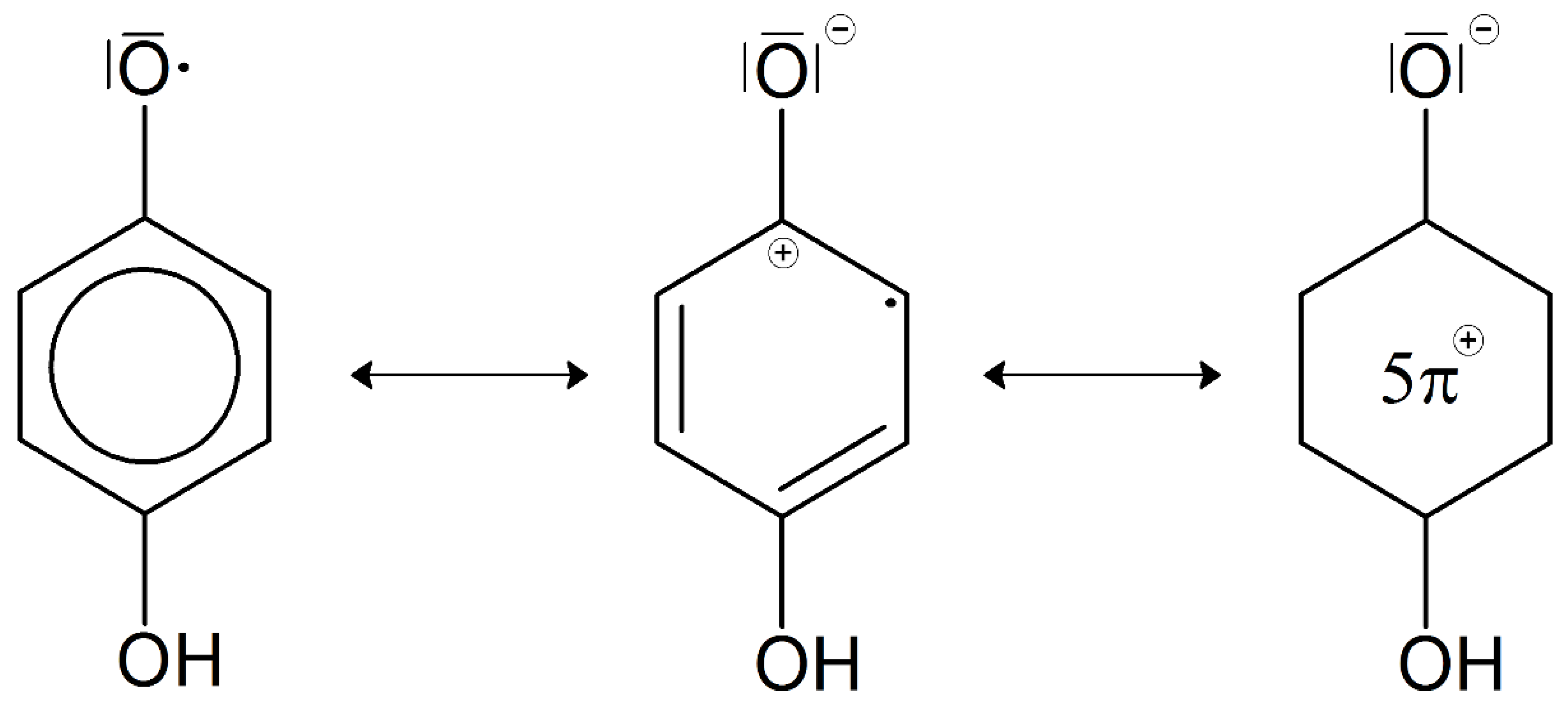
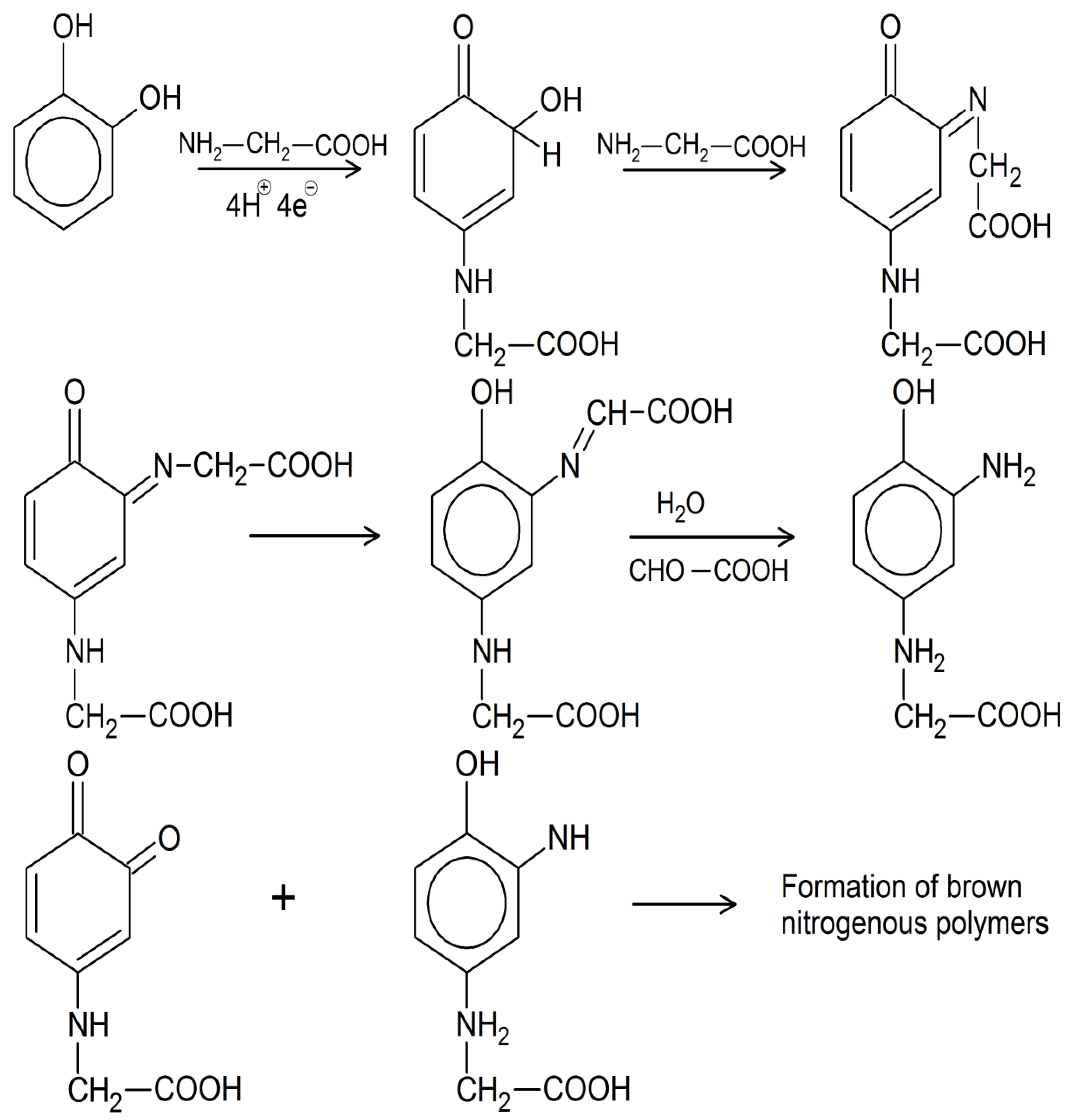
- The remaining molecules such as phenols, phenylpropene units, amino acids, peptides, amino sugars, and sugars react, polymerize, and polycondensate as described, partly catalyzed by soil oxidoreductases or mineral surfaces, and partly mediated by soil microorganisms to form medium to higher molecular weight organic substances, specifically humic substances. Humic molecules of different molecular masses form supramolecular bonds resulting in a humic network.
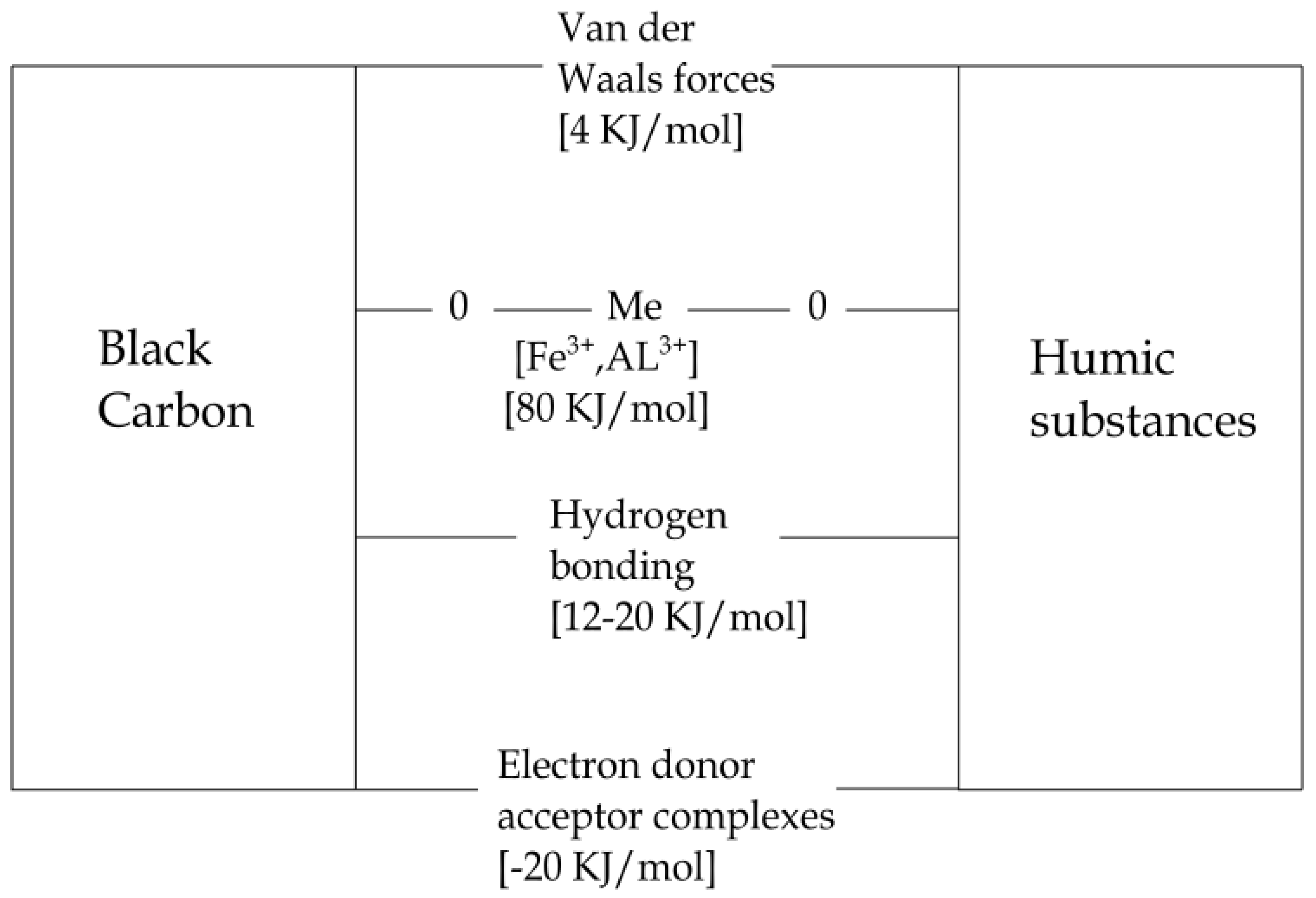
- The humic substances show high resistance and stability against microbial degradation partly because of the biochemical stability of the molecules against microbial attack (Haider [31,32]), and partly by the reaction of humic substances with inorganic soil components during and after the formation of the humic substances. As a result, clay-humic substances complexes are formed or humic substances form complexes with di- or trivalent cations, such as Ca2+, Cu(II), Fe(III), or Al(III). Both sorption to mineral surfaces and complexation of cations have been shown to reduce the rate of degradation of organic molecules in soil [33,34,35,36].
- The chemical results allow the formulation of structural models for humic substances. Stevenson ([6], chapter 12, pp. 285–302) gave an overview on various models of humic substances. Most of them show an aromatic core; however, the models vary in the degree of aromaticity, molecular weight, and intramolecular bonding (Schnitzer [1]; Schulten and Schnitzer [37]).
4. Criticism of the Concept of Humic Substances
5. Black Carbon in Soil
6. Humic Substances in Soils: A Reformulated View
6.1. Reactions of Humic Substances with Organic Xenobiotics
6.1.1. Reaction with Triazines
6.1.2. Interaction of Humic Substances and Surfactants
6.2. Reactions of Humic Substances with Inorganic Ions in Soil
6.2.1. Reactions with Micronutrient Metals
6.2.2. Humic Al(Fe)-Phosphate Complexes in Soil
6.3. Effects of Humic Substances on Plant Growth in Higher Plants
7. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Schnitzer, M. Humic substances: Chemistry and reactions. In Soil Organic Matter; Schnitzer, M., Kahn, S.U., Eds.; Elsevier: New York, NY, USA, 1978; pp. 1–64. [Google Scholar]
- Schnitzer, M. A lifetime perspective on the chemistry of soil organic matter. Adv. Agron. 2000, 68, 1–58. [Google Scholar]
- Flaig, W.; Beutelspacher, H.; Rietz, E. Chemical composition and physical properties of humic substances. In Soil Components; Gieseking, J.E., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1975; Volume 1, pp. 1–211. [Google Scholar]
- Ziechmann, W. Huminstoffe; Verlag Chemie: Weinheim, Germany, 1980. [Google Scholar]
- Aiken, G.R.; Mc Knight, D.M.; Wershaw, R.; MacCarthy, P. Humic Substances in Soil, Sediment and Water; John Wiley: New York, NY, USA, 1985. [Google Scholar]
- Stevenson, F.J. Humus Chemistry. Genesis, Composition, Reactions; John Wiley: New York, NY, USA, 1994. [Google Scholar]
- Hatcher, P.G.; Breger, J.A.; Dennis, L.W.; Maciel, G.E. Solid-state 13C NMR of sedimentary humic substances: New revelations on their chemical composition. In Aquatic and Terrestrial Humic Materials; Christman, R.F., Gjessing, E.T., Eds.; Ann Arbor Science: Ann Arbor, MI, USA, 1983; pp. 37–82. [Google Scholar]
- Hasselmann, N. Untersuchungenzum Einbau von Kohlenhydraten in die Huminstoff-Matrix. Ph.D. Thesis, Georg-August Universität, Göttingen, Germany, 1987. [Google Scholar]
- Smernik, R.J.; Oades, J.M. The use of spin counting for determining quantitation in solid state 13C NMR spectra of natural organic matter. 1. Model systems and the effect of paramagnetic impurities. Geoderma 2000, 96, 101–129. [Google Scholar] [CrossRef]
- Mao, J.-D.; Hu, W.-G.; Schmidt-Rohr, K.; Davies, G.; Ghabbour, E.A.; Xing, B. Quantitative characterization of humic substances by solid-state Carbon-13 nuclear magnetic resonance. Soil Sci. Soc. Am. J. 2000, 64, 873–884. [Google Scholar] [CrossRef]
- Keeler, C.; Maciel, G.E. Quantitation in the solid-state 13C NMR analysis of soil and organic soil fractions. Anal. Chem. 2003, 75, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-D.; Schmidt-Rohr, K. Accurate quantification of aromaticity and nonprotonated aromatic carbon fraction in natural organic matter by 13C solid-state nuclear magnetic resonance. Environ. Sci. Technol. 2004, 38, 2680–2684. [Google Scholar] [CrossRef] [PubMed]
- Kögl-Knabner, I.; Zech, W.; Hatcher, P.G.; de Leeuw, J.W. Fate of plant components during biodegradation and humification. In Forest Soils: Evidence from Structural Characterization of Individual Biomolecules; Wilson, W.S., Ed.; Royal Society of Chemistry: Cambridge, UK, 1991; pp. 61–70. [Google Scholar]
- Urbanski, L.; Kölbl, A.; Lehndorff, E.; Houtermans, M.; Schad, P.; Zhang, G.-L.; Utami, S.R.; Kögl-Knabner, I. Paddy management on different soil types does not promote lignin accumulation. J. Plant Nutr. Soil Sci. 2017, 180, 366–380. [Google Scholar] [CrossRef]
- Peerson, O.B.; Wu, X.; Kustanovick, I.; Smith, S.O. Variable-amplitude cross-polarization MAS NMR. J. Magn. Res. 1993, 104, 334–339. [Google Scholar] [CrossRef]
- Cook, R.L.; Langford, C.H.; Yamdagni, R.; Preston, C.M. A modified cross-polarization magic angle spinning 13C NMR procedure for the study of humic materials. Anal. Chem. 1996, 68, 3979–3986. [Google Scholar] [CrossRef]
- Mao, J.; Cao, X.; Olk, D.L.; Chu, W.; Schmidt-Rohr, K. Advanced solid-state NMR spectroscopy of natural organic matter. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100, 17–51. [Google Scholar] [CrossRef] [PubMed]
- Schöning, I.; Knicker, H.; Kögl-Knabner, I. Intimate association between O/N-alkyl carbon and iron oxides in clay fractions of forest soils. Org. Geochem. 2005, 36, 1378–1390. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Kögl-Knabner, I. Soil organic matter stabilization in acidic forest soils is preferential and soil type specific. Eur. J. Soil Sci. 2008, 674–692. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J.P. Synthesis and transformation of phenolic compounds by Epicoccum nigrum in relation to humic acid formation. Proc. Soil Sci. Soc. Am. 1967, 31, 766–772. [Google Scholar] [CrossRef]
- Weichelt, T. Chemical alteration of natural lignin by interactions with humic like autoxidation products of pyrogallol (1,2,3-trihydroxybenzene). In Soil Organic Matter Studies; IAEA, FAO, Eds.; IAEA: Wien, Austria, 1977; Volume II, pp. 67–82. [Google Scholar]
- Haider, K.; Nagar, B.R.; Saiz, C.; Meuzelaar, H.L.C.; Martin, J.P. Studies on soil humic compounds, fungal melanins, and model polymers by pyrolysis. In Soil Organic Matter Studies; IAEA, FAO, Eds.; IAEA: Wien, Austria, 1977; Volume II, pp. 213–220. [Google Scholar]
- Martin, J.P.; Haider, K.; Bondietti, E. Properties of model humic acids synthesized by phenoloxidase and autoxidation of phenols and other components formed by soil fungi. In Proceedings Intern Meeting Humic Substances; Pudoc: Wageningen, The Netherlands, 1975; pp. 171–186. [Google Scholar]
- Piccolo, A.; Cozzolino, A.; Conte, P.; Spaccini, R. Polymerization of humic substances by an enzyme catalyzed oxidative coupling. Naturwissenschaften 2000, 87, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Cozzolini, A.; Piccolo, A. Polymerization of dissolved humic substances catalyzed by peroxidase. Effects of pH and humic composition. Org. Geochem. 2002, 33, 281–294. [Google Scholar] [CrossRef]
- Wang, T.S.C.; Li, S.W.; Huang, P.M. Catalytic polymerization of phenolic compounds by a latosol. Soil Sci. 1978, 126, 81–86. [Google Scholar] [CrossRef]
- Wang, T.S.C.; Wang, M.C.; Yue, L.; Huang, P.M. Catalytic synthesis of humic substances by natural clays, silts and soils. Soil Sci. 1983, 135, 350–360. [Google Scholar] [CrossRef]
- Wang, M.C.; Huang, P.M. Catalytic polymerization of hydroquinone by nontronite. Can. J. Soil Sci. 1987, 67, 867–875. [Google Scholar] [CrossRef]
- Shino, H. Catalytic synthesis of humic acids from phenolic compounds by Mn(IV) oxide (Birnessite). Soil Sci. Plant Nutr. 1990, 36, 679–682. [Google Scholar] [CrossRef]
- Ziechmann, W. Über modellreaktionenzur bildungsynthetischer huminsäuren. 2. Die synthese von huminsäuren im neutralen millieu. Brennstoff-Chemie 1960, 41, 334–340. [Google Scholar]
- Haider, K. Biochemie der umsetzung von pflanzenrückständenzu huminstoffen. In Refraktäre Organische Säuren in Gewässern; Frimmel, F.H., Abbt-Braun, G., Eds.; Verlag Chemie: Bonn, Germany, 1993; pp. 215–232. [Google Scholar]
- Haider, K. Problems related to the humification processes in soils of temperate climates. In Soil Biochemistry; Bollag, J.M., Stotzky, G., Eds.; Marcel Dekker: New York, NY, USA, 1992; Volume 7, pp. 55–94. [Google Scholar]
- Boudot, J.P. Relative efficiency of complexed aluminum, noncrystalline Al hydroxide, allophone and imogolite in retarding the biodegradation of citric acid. Geoderma 1992, 52, 29–39. [Google Scholar] [CrossRef]
- Jones, D.L.; Edwards, A.C. Influence of sorption on the biological utilization of two simple carbon substrates. Soil Biol. Biochem. 1998, 30, 1895–1902. [Google Scholar] [CrossRef]
- Gerke, J. Humic (organic matter)-Al(Fe)-phosphate complexes: An underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. A state of the art structural concept for humic substances. Naturwissenschaften 1993, 80, 29–30. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Skjemstad, J.O.; Gehrt, E.; Kögl-Knabner, I. Charred organic carbon in German chernozemic soils. Eur. J. Soil Sci. 1999, 50, 351–365. [Google Scholar] [CrossRef]
- Glaser, B.; Haumeier, L.; Guggenberger, G.; Zech, W. The Terra preta phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Von Lützow, M.; Kögl-Knabner, I.; Ekschmidt, R.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions: A review. Eur. J Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Kögl-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral-organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.H.B. Solvent systems for the isolation of organic components from soils. Soil Sci. Soc. Am. J. 2006, 70, 986–994. [Google Scholar] [CrossRef]
- Perdue, E.M. Acidic functional groups of humic substances. In Humic Substances in Soil, Sediment and Water; Aiken, G.R., McKnight, D., Wershaw, R., MacCarthy, P., Eds.; John Wiley: New York, NY, USA, 1985; pp. 493–526. [Google Scholar]
- Goldberg, E.D. Black Carbon in the Environment; John Wiley: New York, NY, USA, 1985. [Google Scholar]
- Masiello, C.A. New directions in black carbon organic geochemistry. Mar. Chem. 2004, 92, 201–213. [Google Scholar] [CrossRef]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermal altered Pinusresinosa (Red pine) wood. Org. Geochem. 2002, 34, 1093–1109. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Skjemstad, J.-O.; Czimczik, C.I.; Glaser, B.; Prentice, K.M.; Gelinas, Y.; Kuhlbusch, T.A. Comparative analysis of black carbon in soils. Glob. Biochem. Cycles 2001, 15, 163–167. [Google Scholar] [CrossRef]
- Hammes, K.; Schmidt, M.W.I.; Smernik, R.J.; Currie, L.A.; Ball, W.P.; Nguyen, T.H.; Louchouran, P.; Houel, S.; Gustafsson, O.; Elmquist, M.; et al. Comparison of quantification methods to measure fire-derived (black/elemental) carbon in soils and sediments using reference materials from soil, water, sediment and the atmosphere. Glob. Biochem. Cycles 2007, 21, 1–18. [Google Scholar] [CrossRef]
- Glaser, B.; Haumeier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Clarke, J.A.; Taylor, J.A.; Oades, J.M.; McClure, S.G. The chemistry and nature of protected carbon in soil. Aust. J. Soil Res. 1996, 34, 251–271. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Taylor, J.A.; Smernik, R.J. Estimation of charcoal (char) in soils. Commun. Soil Sci. Plant Anal. 1999, 30, 2283–2298. [Google Scholar] [CrossRef]
- Simpson, M.J.; Hatcher, P.G. Overestimates of black carbon in soils and sediments. Naturwissenschaften 2004, 91, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, S.; Rodionow, A.; Haumeier, L.; Glaser, B.; Amelung, W. Revised black carbon assessment using benzene polycarboxylic acids. Org. Geochem. 2005, 36, 1299–1310. [Google Scholar] [CrossRef]
- Francko, D.A.; Heath, R.T. Functionally distinct classes of complex phosphorus compounds in lake waters. Limnol. Oceanogr. 1979, 24, 463–473. [Google Scholar] [CrossRef]
- Zika, R.G.; Cooper, W.J. Photochemistry of Environmental Aquatic Systems; American Chemical Society (ACS): Washington, DC, USA, 1987; Volume 327. [Google Scholar]
- Hermann, R.; Gerke, J.; Ziechmann, W. Photodegradation of the surfactants Na-dodecylbenzenesulfate and dodecylpyridinium-chloride as affected by humic substances. Water Air Soil Pollut. 1997, 98, 43–55. [Google Scholar] [CrossRef]
- Hens, M. Aqueous Phase Speciation of Phosphorus in Sandy Soils. Ph.D. Thesis, University of Leuven, Leuven, Belgium, 1999. [Google Scholar]
- Kappenberg, A.; Bläsing, M.; Lehndorff, E.; Amelung, W. Black carbon assessment using benzene polycarboxylic acids: Limitations for organic-rich matrices. Org. Geochem. 2016, 94, 47–51. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Skjemstad, J.O.; Jäger, C. Carbon isotope geochemistry and nanomorphology of soil black carbon: Black chernozemic soils in central Europe originate from ancient biomass burning. Glob. Biogeochem. Cycles 2002, 16, 1123–1131. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Reicosky, D.C.; Wilts, A.R.; Mcgowan, J.A. Charcoal carbon in U.S. agricultural soils. Soil Sci. Soc. Am. J. 2002, 66, 1249–1255. [Google Scholar] [CrossRef]
- Erro, J.; Urrutia, O.; Baigorri, R.; Fuentes, M.; Zamerreno, G.; Garcia-Mina, J.M. Incorporation of humic-derived active molecules into compound NPK granulated fertilizers: Main technical difficulties and potential solutions. Chem. Biol. Technol. Agric. 2016, 3, 18–33. [Google Scholar] [CrossRef]
- Glaser, B.; Balashov, E.; Haumeier, L.; Guggenberger, G.; Zech, W. Black carbon in density fractions of anthropogenic soils of the Brazilian amazon region. Org. Geochem. 2000, 31, 669–678. [Google Scholar] [CrossRef]
- Senesi, N.; Testini, C.; Miano, T.M. Interaction mechanisms between humic acids of different origin and nature and electron donor herbicides: A comparative IR and ESR study. Org. Geochem. 1987, 11, 25–30. [Google Scholar] [CrossRef]
- Müller-Wegener, U. Einfluss von huminstoffen auf den eintrag von pflanzenschutzmitteln in das grundwasser. In Refraktäre Organische Säuren in Gewässern; Frimmel, F.H., Abbt-Braun, G., Eds.; Verlag-Chemie: Weinheim, Germany, 1993; pp. 79–87. [Google Scholar]
- Müller-Wegener, U. Electron donor acceptor complexes between organic nitrogen heterocycles and humic acid. Sci. Total Environ. 1987, 62, 297–304. [Google Scholar] [CrossRef]
- Gerke, J. Untersuchungenzur Bindung des Kationischen Tensids Laurylpyridiniumchlorid und des Anionischen Tensids Dodecylbenzolsulfonat an Huminstoffe-ein Modell der Reaktion in Böden und Gewässern. Ph.D. Thesis, Georg-August Universität, Göttingen, Germany, 1988. [Google Scholar]
- Wershaw, R.L. Molecular aggregation of humic substances. Soil Sci. 1999, 164, 803–813. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil sciences. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Sutton, R.; Sposito, G. Molecular structure in humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Von Wandruszka, R. The micellar model of humic acid: Evidences from pyrene fluorescence measurements. Soil Sci. 1998, 163, 921–930. [Google Scholar] [CrossRef]
- Engebretson, R.R.; von Wandruszka, R. Kinetic aspects of cation enhanced aggregation in aqueous humic acids. Environ. Sci. Technol. 1998, 32, 488–493. [Google Scholar] [CrossRef]
- Nuzzo, A.; Sanchez, A.; Fontaine, B.; Piccolo, A. Conformational changes of dissolved humic and fulvic superstructures with progressive iron complexation. J. Geochem. Explor. 2013, 129, 1–5. [Google Scholar] [CrossRef]
- Helal, H.-M.; Dressler, A. Mobilization and turnover of soil phosphorus in the rhizosphere. J. Plant Nutr. Soil Sci. 1989, 152, 175–180. [Google Scholar] [CrossRef]
- Müller-Wegener, U. Neuere Erkenntnissezur Wechselwirkung Zwischen s-Triazinen und Organischen Stoffen. Ph.D. Thesis, Georg-August Universität, Göttingen, Germany, 1984. [Google Scholar]
- Gerke, J.; Ziechmann, W. Adsorption des kationischen tensids laurylpyridiniumchloridan huminstoffe, böden und komposte. Chem. Erde 1990, 51, 247–253. [Google Scholar]
- Hermann, R. Modifiziertes Reaktionsverhalten von Huminstoffen Infolge Photochemisch Induzierter Prozesse Gegenüber Ausgewählten Organischen Substanzen und Anorganischen Ionen. Ph.D. Thesis, Georg-August Universität, Göttingen, Germany, 1993. [Google Scholar]
- Gerke, J. Chemische Prozesse der Nährstoffmobilisierung in der Rhizosphäre und ihre Bedeutungfür den Übergangvom Boden in die Pflanze. Ph.D. Thesis, Georg-August Universität, Göttingen, Germany, 1995. [Google Scholar]
- Gerke, J. Aluminum and iron(III) species in the soil solution including organic complexes with citrate and humic substances. J. Plant Nutr. Soil Sci. 1997, 160, 427–432. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V. Strategies of plants for acquisition of iron. Plant Soil 1994, 165, 261–274. [Google Scholar] [CrossRef]
- Cesco, S.; Nikolic, M.; Römheld, V.; Varanini, Z.; Pinton, R. Uptake of 59Fe from soluble 59Fe-humate by cucumber and barley plants. Plant Soil 2002, 241, 121–128. [Google Scholar] [CrossRef]
- Varanini, Z.; Pinton, R. Plant-soil relationship: Role of humic substances in iron nutrition. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadia, J., Eds.; Springer: Heidelberg, Germany, 2006; pp. 153–168. [Google Scholar]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Spectroscopic characterization of metal-humic acid like complexes of earthworm-composted organic wastes. Sci. Total Environ. 1992, 117–118, 111–120. [Google Scholar] [CrossRef]
- Levesque, M.; Schnitzer, M. Organic metallic interactions in soil: 6. Preparation and properties of fulvic acid-metal phosphates. Soil Sci. 1967, 103, 183–190. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Raben-Lange, B.; Gimsing, A.L.; Strobel, B.W. Influence of humic substances on phosphate adsorption by aluminum and iron oxides. Geoderma 2005, 127, 270–279. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Song, K.; Lin, Y.; Bai, Y.; Zhu, Y.; Giesy, J.P. Competitive interaction between soil-derived humic acid and phosphate. Appl. Geochem. 2013, 36, 125–131. [Google Scholar] [CrossRef]
- Urrutria, O.; Erro, J.; Guardado, I.; San Francisco, S.; Mandado, M.; Baigorri, R.; Yvin, J.C.; Garcia-Mina, J.M. Physico-chemical characterization of humic-metal-phosphate complexes and their potential application to the manufacture of new types of phosphate-based fertilizers. J. Plant Nutr. Soil Sci. 2014, 177, 128–136. [Google Scholar] [CrossRef]
- De Haan, H.; Jones, R.I.; Salonen, K. Abiotic transformations of iron and phosphate in humic lake water revealed by double-isotopic labeling and gel filtration. Limnol. Oceanogr. 1990, 35, 491–497. [Google Scholar] [CrossRef]
- Jones, R.I.; Salonen, K.; De Haan, H. Effect of dissolved humic substances on the speciation of iron and phosphate at different pH and ionic strength. Environ. Sci. Technol. 1993, 27, 1052–1059. [Google Scholar] [CrossRef]
- Flaig, W. Mögliche beeinflussung von stoffwechsel und ertrag der pflanzendurch huminstoffe. In Refraktäre Organische Säuren in Gewässern; Frimmel, F.H., Abbt-Braun, G., Eds.; Verlag Chemie: Bonn, Germany, 1993; pp. 233–252. [Google Scholar]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.; Nebioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015. [Google Scholar] [CrossRef]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerke, J. Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review. Agronomy 2018, 8, 76. https://doi.org/10.3390/agronomy8050076
Gerke J. Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review. Agronomy. 2018; 8(5):76. https://doi.org/10.3390/agronomy8050076
Chicago/Turabian StyleGerke, Jörg. 2018. "Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review" Agronomy 8, no. 5: 76. https://doi.org/10.3390/agronomy8050076



