Short-Term Effects of Biochar Amendment on Greenhouse Gas Emissions from Rainfed Agricultural Soils of the Semi–Arid Loess Plateau Region
Abstract
:1. Introduction
2. Materials and Methods
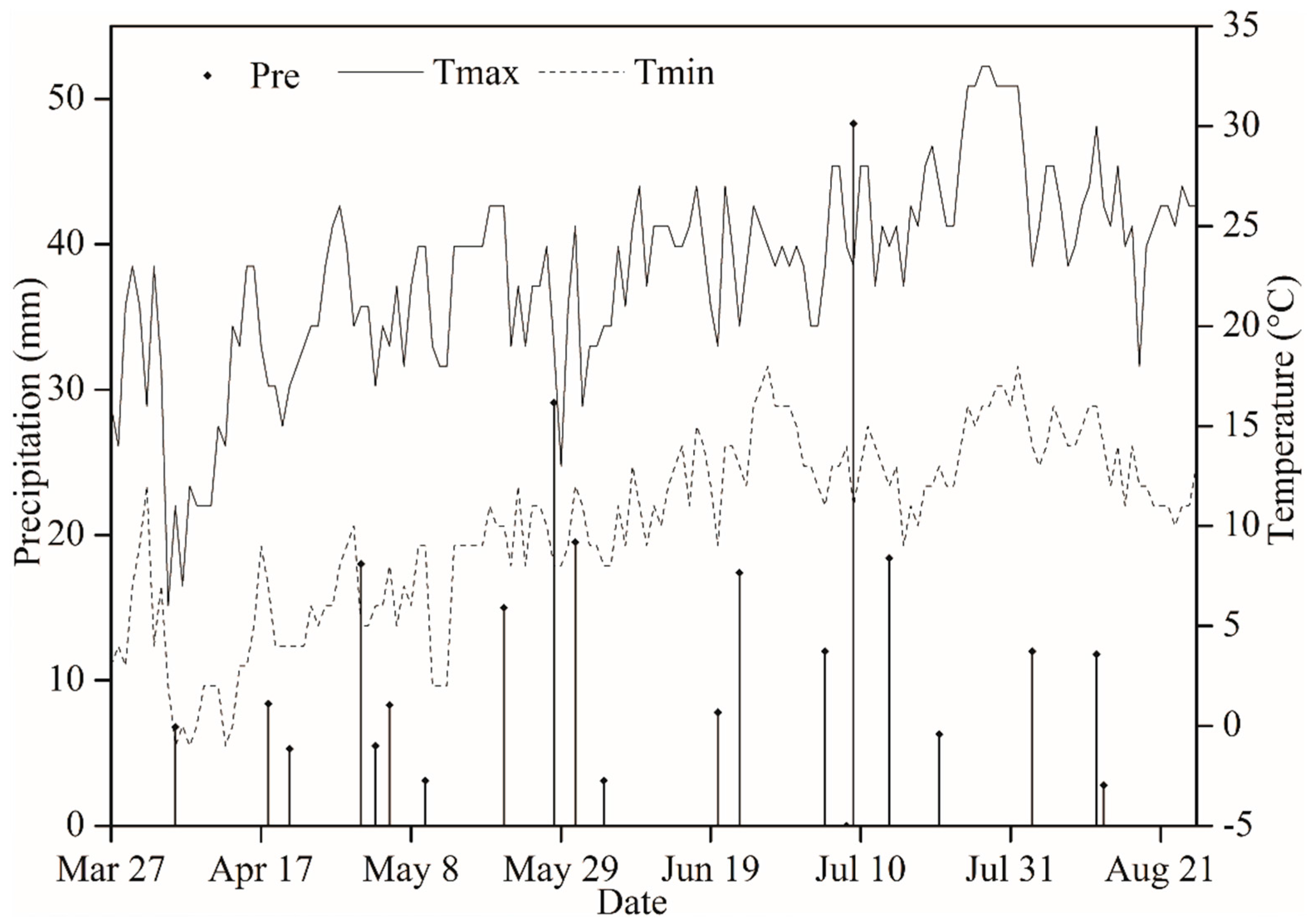
2.1. Site Description
2.2. Experimental Design
2.3. Production and Basic Properties of Biochar
2.4. Soil Sampling and Analysis
2.5. CH4, N2O, and CO2 Emission Monitoring
2.6. Calculation and Statistics
3. Results
3.1. Soil Properties
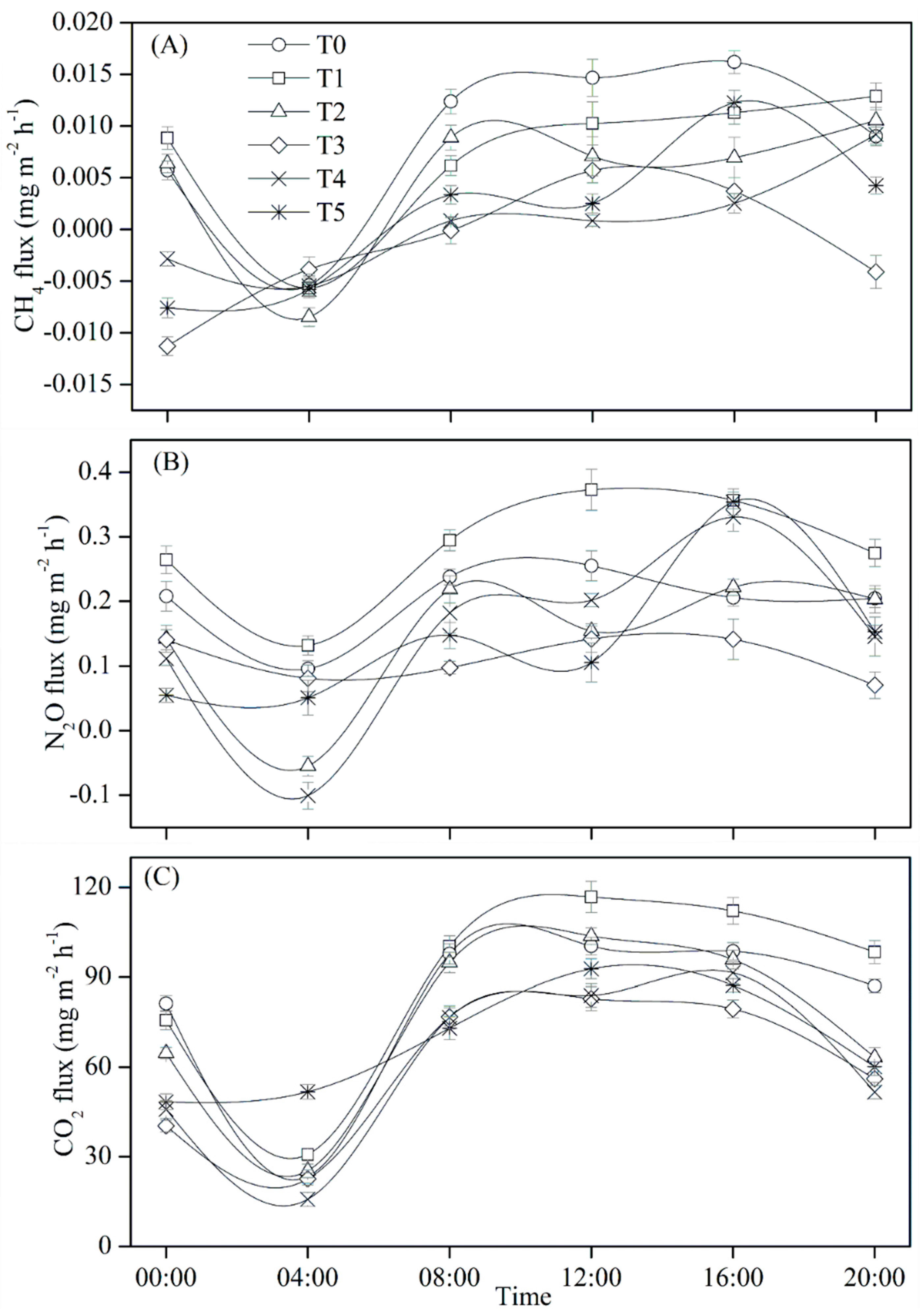
3.2. Diurnal Dynamics of GHG Flux
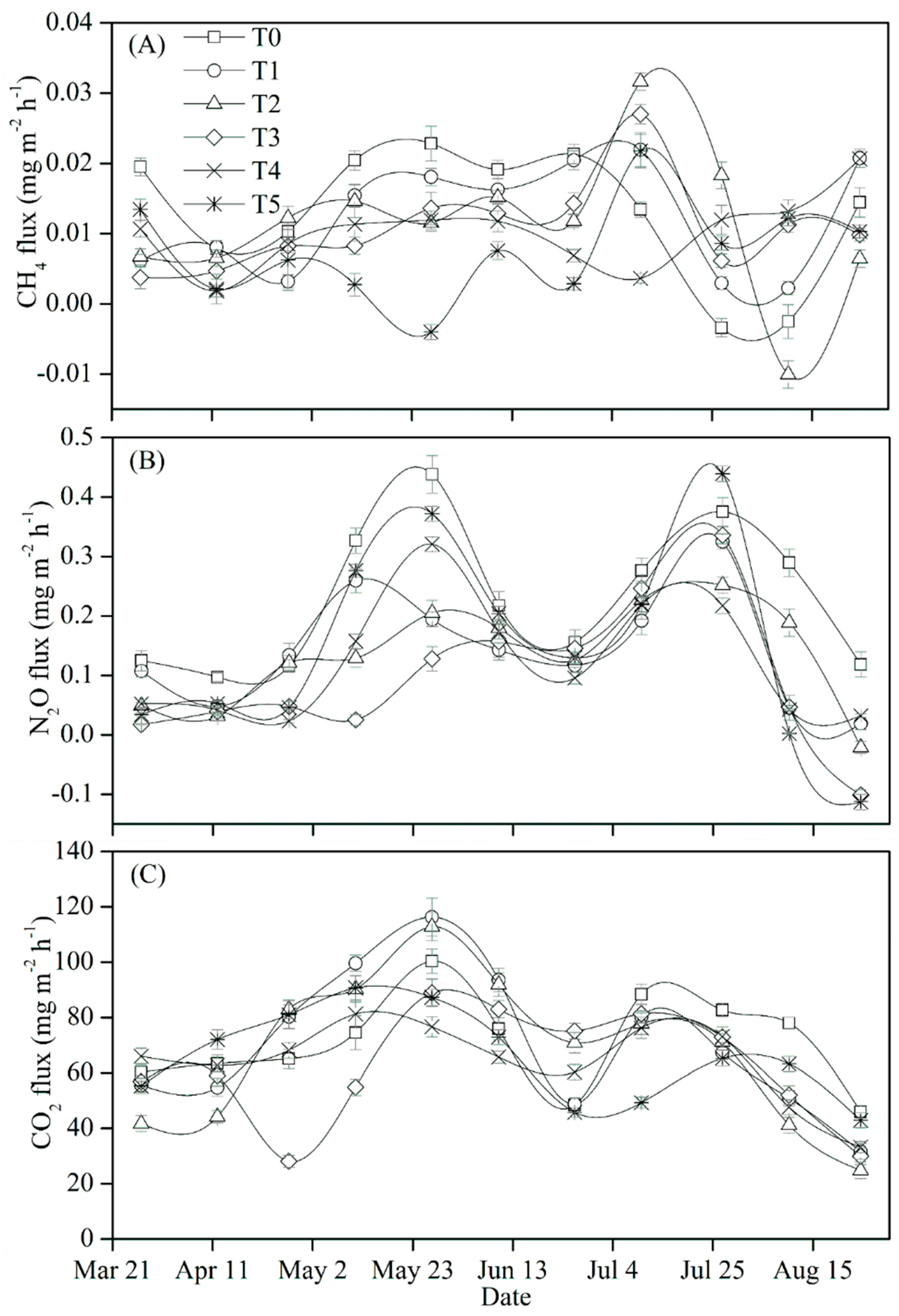
3.3. Seasonal Dynamics of GHG Flux
3.4. GHG Flux Correlated with Soil Temperature at Topsoil
3.5. Grain Yield, Total GHG Emissions and GWP
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Change, I.C. The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2013; Volume 1, pp. 531–535. [Google Scholar]
- Intergovernmental Panel on Climate Change Working Group 3; Metz, B.; Davidson, O.; Bosch, P.; Dave, R.; Meyer, L. Climate Change 2007: Mitigation: Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change: Summary for Policymakers and Technical Summary; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- IPoCCWG; Metz, B.; Davidson, O.; Bosch, P.; Dave, R.; Meyer, L. Climate Change 2007: Mitigation: Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change: Summary for Policymakers and Technical Summary; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Solomon, S. Climate Change 2007−The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Xiong, Z.; Liu, P.; Pan, G. Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol. Fertil. Soils 2011, 47, 887–896. [Google Scholar] [CrossRef]
- Ventura, M.; Alberti, G.; Viger, M.; Jenkins, J.; Girardin, C.; Baronti, S.; Zaldei, A.; Taylor, G.; Rumpel, C.; Miglietta, F.; et al. Biochar mineralization and priming effect on SOM decomposition in two European short rotation coppices. Glob. Chang. Biol. Bioeng. 2015, 7, 1150–1160. [Google Scholar] [CrossRef]
- Castaldi, S.; Riondino, M.; Baronti, S.; Esposito, F.; Marzaioli, R.; Rutigliano, F.; Vaccari, F.; Miglietta, F. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 2011, 85, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- FAO. Soil Map of the World: Revised Legend; World Soil Resources Report 60; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- Nelson, D.W.; Sommers, L.W. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, Agronomy Monograph, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 301–312. [Google Scholar]
- Ladd, J.N.; Amato, M. Relationship between microbial biomass carbon in soils and absorbance of extracts of fumigated soils. Soil Biol. Biochem. 1989, 21, 457–459. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Jiang, J.; Zheng, X.; Sass, R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Yeboah, S.; Zhang, R.; Cai, L.; Song, M.; Li, L.L.; Xie, J.; Luo, Z.; Wu, J.; Zhang, J. Greenhouse gas emissions in a spring wheat–field pea sequence under different tillage practices in semi-arid Northwest China. Nutr. Cycl. Agroecosyst. 2016, 106, 77–91. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, C.S.; Zhang, J.B.; Dong, W.X.; Wang, Y.Y.; Song, L.N. Research advances on source/sink intensities and greenhouse effects of CO2, CH4 and N2O in agricultural soils. Chin. J. Eco-Agric. 2011, 19, 966–975. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Yang, X.; Xu, H.; Jia, Y. Key microbial processes in nitrous oxide emissions of agricultural soil and mitigation strategies. Huan Jing Ke Xue 2014, 35, 792–800. [Google Scholar] [PubMed]
- XinBxin, W.; Ping, Z.; JianBrong, F. Effects of bamboo biochar amendments on methane and nitrous oxide emission from paddy soil. J. Agric. Environ. Sci. 2014, 33, 198–204. [Google Scholar]
- De-Cai, G.; Zhang, L.; Qiang, L. Effects of biochar on CO2, CH4, N2O emission and its environmental benefits in dryland soil. Acta Ecol. Sin. 2015, 35, 3615–3624. [Google Scholar]
- Baritz, R.; Seufert, G.; Montanarella, L.; Van Ranst, E. Carbon concentrations and stocks in forest soils of Europe. For. Ecol. Manag. 2010, 260, 262–277. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Zheng, J.; Zhao, S.; Zhang, X. Effect of Biochar on Soil Greenhouse Gas Emissions in Semi-arid Region. Huan Jing Ke Xue 2015, 36, 3393–3400. [Google Scholar] [PubMed]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.D.; Rutherford, D.W.; Sparrevik, M.; Hale, S.E.; Obia, A.; Mulder, J. Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Xu, Y.; Liu, G.; Liu, Q.; Zhu, J.; Tu, C.; Amonette, J.E.; Cadisch, G.; Yong, J.W.; Hu, S. Impact of biochar application on nitrogen nutrition of rice, greenhouse−gas emissions and soil organic carbon dynamics in two paddy soils of China. Plant Soil 2013, 370, 527–540. [Google Scholar] [CrossRef]
- Wu, J.; Guo, W.; Feng, J.; Li, L.; Yang, H.; Wang, X.; Bian, X. Greenhouse gas emissions from cotton field under different irrigation methods and fertilization regimes in arid Northwestern China. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
| Treatments | Microbial Biomass Carbon | Soil Organic Carbon | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–5 | 5–10 | 10–30 | 0–5 | 5–10 | 10–30 | ||||||
| g kg−1 | |||||||||||
| T0 | 205 b | 185 a,b | 160 a | 7.44 c | 7.56 b | 6.57 b | |||||
| T1 | 262 a | 149 b | 216 a | 10.76 b,c | 8.13 a,b | 6.33 a,b | |||||
| T2 | 219 b | 154 b | 187 a | 13.07 b | 9.09 a,b | 7.12 a,b | |||||
| T3 | 277 a | 258 a | 239 a | 16.97 a | 11.56 a,b | 7.17 a,b | |||||
| T4 | 160 c | 179 a,b | 194 a | 18.86 a | 10.34 a,b | 6.89 a,b | |||||
| T5 | 119 c | 159 b | 161 a | 17.16 a | 12.17 a | 7.84 a | |||||
| GHG Flux | Soil Layer (cm) | Regression Equation | Coefficient of Determination (R2) |
|---|---|---|---|
| CH4 flux | 5 | F = 0.006 × Ts − 0.095 | 0.887 * a |
| 10 | F = 0.016 × Ts − 0.243 | 0.684 * | |
| 15 | F = 0.021× Ts − 0.325 | 0.714 * | |
| N2O flux | 5 | F = 0.081× Ts − 1.128 | 0.723 * |
| 10 | F = 0.210 × Ts − 3.119 | 0.545 | |
| 15 | F = 0.246 × Ts − 3.764 | 0.575 | |
| CO2 flux | 5 | F = 11.89 × Ts − 118.1 | 0.801 * |
| 10 | F = 29.34 × Ts − 387.7 | 0.590 | |
| 15 | F = 39.55 × Ts − 558.5 | 0.497 |
| Treatments | CH4–C | N2O–N | CO2–C | Grain Yield | GWP | GHGI |
|---|---|---|---|---|---|---|
| kg ha−1 | kg CO2-e ha−1 | kg CO2-e t−1 | ||||
| T0 | 0.190 ab | 8.69 a | 2627 a | 2476 a | 5222 a | 2.11 a |
| T1 | 0.165 ab | 5.48 c | 2647 a | 2495 a | 4284 b | 1.72 b |
| T2 | 0.145 b | 5.31 cd | 2580 a | 2577 a | 4164 b | 1.62 b |
| T3 | 0.133 b | 4.07 e | 2300 c | 2557 a | 3515 d | 1.37 d |
| T4 | 0.076 c | 4.80 d | 2381 b | 2553 a | 3813 c | 1.49 c |
| T5 | 0.002 d | 6.13 b | 2436 b | 2551 a | 4263 b | 1.67 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeboah, S.; Lamptey, S.; Cai, L.; Song, M. Short-Term Effects of Biochar Amendment on Greenhouse Gas Emissions from Rainfed Agricultural Soils of the Semi–Arid Loess Plateau Region. Agronomy 2018, 8, 74. https://doi.org/10.3390/agronomy8050074
Yeboah S, Lamptey S, Cai L, Song M. Short-Term Effects of Biochar Amendment on Greenhouse Gas Emissions from Rainfed Agricultural Soils of the Semi–Arid Loess Plateau Region. Agronomy. 2018; 8(5):74. https://doi.org/10.3390/agronomy8050074
Chicago/Turabian StyleYeboah, Stephen, Shirley Lamptey, Liqun Cai, and Min Song. 2018. "Short-Term Effects of Biochar Amendment on Greenhouse Gas Emissions from Rainfed Agricultural Soils of the Semi–Arid Loess Plateau Region" Agronomy 8, no. 5: 74. https://doi.org/10.3390/agronomy8050074




