First Case of Glufosinate-Resistant Rigid Ryegrass (Lolium rigidum Gaud.) in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Screening
2.2. Dose–Response Experiments
2.3. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Screening
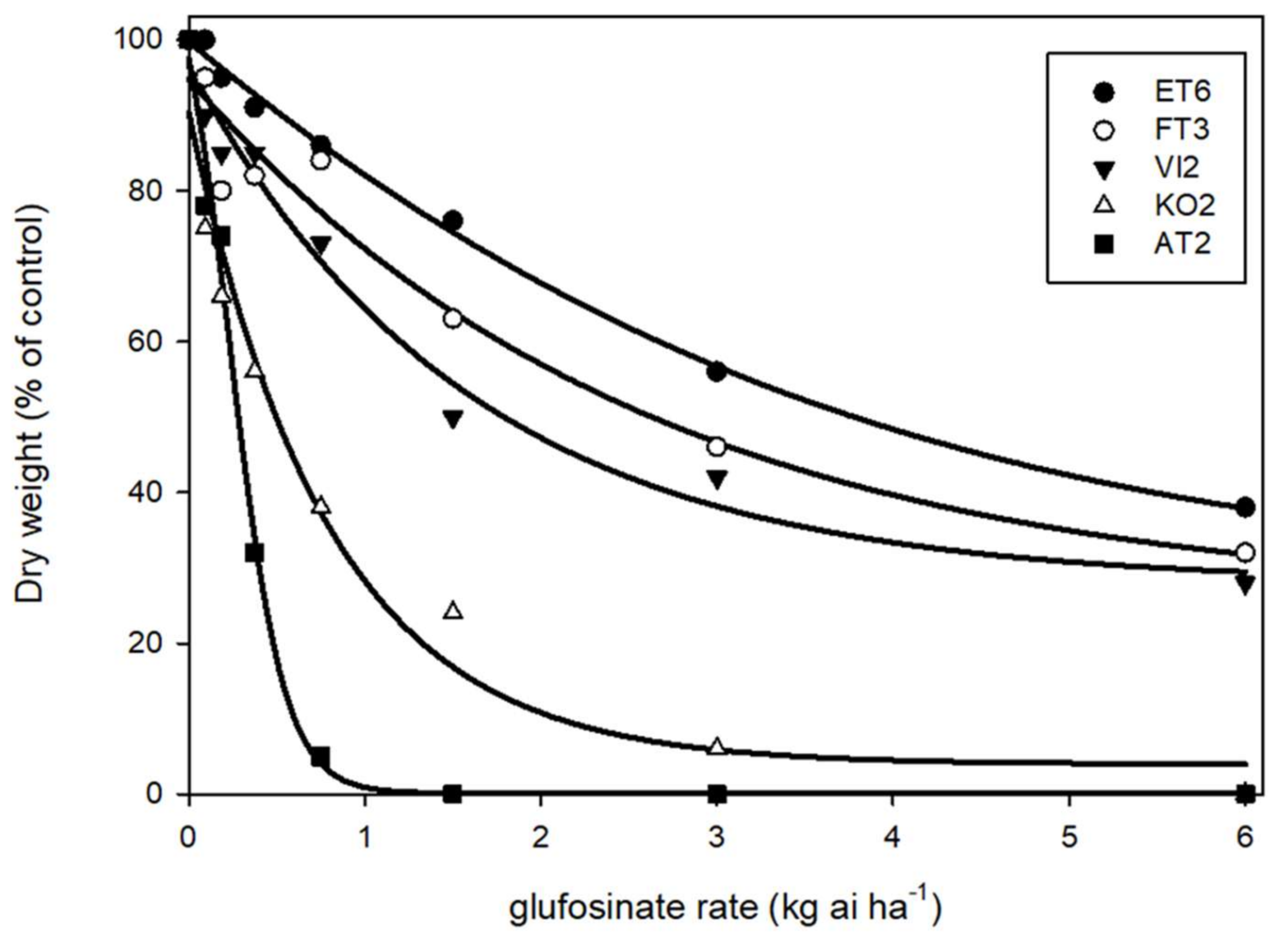
3.2. Dose–Response Experiments
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Senseman, S.A. Herbicide Handbook; Weed Science Society of America: Lawrence, KS, USA, 2007. [Google Scholar]
- Devine, M.; Duke, S.O.; Fedtke, C. Physiology of Herbicide Action; PTR Prentice Hall: Englewood Cliffs, NJ, USA, 1992; pp. xii–441. [Google Scholar]
- Duke, S.O. Overview of herbicide mechanisms of action. Environ. Health Perspect. 1990, 87, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wendler, C.; Putzer, A.; Wild, A. Effect of glufosinate (phosphinothricin) and inhibitors of photorespiration on photosynthesis and ribulose-1,5-bisphosphate carboxylase activity. J. Plant Physiol. 1992, 139, 666–671. [Google Scholar] [CrossRef]
- The Organisation for Economic Co-operation and Development(OECD). Module II: Herbicide Biochemistry, Herbicide-Metabolism and the Residues in Glufosinateammonium (Phosphinothricin)-Tolerant Transgenic Crops; ENV/JM/MONO: Paris, France, 2002. [Google Scholar]
- Chahal, G.S.; Johnson, W.G. Influence of glyphosate or glufosinate combinations with growth regulator herbicides and other agrochemicals in controlling glyphosate-resistant weeds. Weed Technol. 2017, 26, 638–643. [Google Scholar] [CrossRef]
- Kaur, S.; Sandell, L.D.; Lindquist, J.L.; Jhala, A.J. Glyphosate-resistant giant ragweed (Ambrosia trifida) control in glufosinate-resistant soybean. Weed Technol. 2014, 28, 569–577. [Google Scholar] [CrossRef]
- Seng, C.T.; Van Lun, L.O.W.; San, C.T.; Sahid, I.B. Initial report of glufosinate and paraquat multiple resistance that evolved in a biotype of goosegrass (Eleusine indica) in Malaysia. Weed Biol. Manag. 2010, 10, 229–233. [Google Scholar] [CrossRef]
- Jalaludin, A.; Ngim, J.; Bakar, B.H.J.; Alias, Z. Preliminary findings of potentially resistant goosegrass (Eleusine indica) to glufosinate-ammonium in Malaysia. Weed Biol. Manag. 2010, 10, 256–260. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide-Resistant Weeds. Available online: http://www.weedscience.org/ (accessed on 29 October 2017).
- Olofsdotter, M.; Valverde, B.E.; Madsen, K.H. Herbicide resistant rice (Oryza sativa L.): Global implications for weedy rice and weed management. Ann. Appl. Biol. 2000, 137, 279–295. [Google Scholar] [CrossRef]
- Kumar, V.; Bellinder, R.R.; Brainard, D.C.; Malik, R.K.; Gupta, R.K. Risks of herbicide-resistant rice in India: A review. Crop Protect. 2008, 27, 320–329. [Google Scholar] [CrossRef]
- Madsen, K.H.; Valverde, B.E.; Jensen, J.E. Risk assessment of herbicide-resistant crops: A latin american perspective using rice (Oryza sativa) as a model. Weed Technol. 2002, 16, 215–223. [Google Scholar] [CrossRef]
- Monaghan, N.M. The biology and control of Lolium rigidum as a weed of wheat. Weed Res. 1980, 20, 117–121. [Google Scholar] [CrossRef]
- Recasens, J.; Riba, F.; Izquierdo, J.; Forn, R.; Taberner, A. Gramíneas infestantes de los cereales de invierno de Cataluña. ITEA 1996, 92, 116–130. [Google Scholar]
- Gill, G.S. Ecology of annual ryegrass. Plant Protect. Q. 1996, 11, 195–198. [Google Scholar]
- Travlos, I.; Tabaxi, I.; Papadimitriou, D.; Bilalis, D.; Chachalis, D. Lolium rigidum Gaud. biotypes from Greece with resistance to glyphosate and other herbicides. Bull. UASVM Hortic. 2016, 73, 1–3. [Google Scholar] [CrossRef]
- Yu, Q.; Cairns, A.; Powles, S. Glyphosate, paraquat and ACCase multiple herbicide resistance evolved in a Lolium rigidum biotype. Planta 2007, 225, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.; Alcántara, R.; Osuna, M.D.; Vila-Aiub, M.M.; Prado, R.D. Forward selection for multiple resistance across the non-selective glyphosate, glufosinate and oxyfluorfen herbicides in Lolium weed species. Pest Manag. Sci. 2016, 73, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Nandula, V.K.; Reddy, K.N.; Duke, S.O.; Poston, D.H. Glyphosate-resistant weeds: Current status and future outlook. Outlooks Pest Manag. 2005, 16, 183. [Google Scholar] [CrossRef]
- Travlos, I.S.; Chachalis, D. Glyphosate-resistant hairy fleabane (Conyza bonariensis) is reported in Greece. Weed Technol. 2010, 24, 569–573. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- Gawronski, S.; Gadamski, G. Negative Cross Resistance in the Weeds with Different Resistance Mechanisms. In Weed and Crop Resistance to Herbicides; University of Cordoba: Cordoba, Spain, 1995; pp. 80–81. [Google Scholar]
- VanGessel, M.J.; Scott, B.A.; Johnson, Q.R.; White-Hansen, S.E. Influence of glyphosate-resistant horseweed (Conyza canadensis ) growth stage on response to glyphosate applications. Weed Technol. 2009, 23, 49–53. [Google Scholar] [CrossRef]
- Travlos, I.S. Competition between ACCase-Inhibitor resistant and susceptible sterile wild oat (Avena sterilis) biotypes. Weed Sci. 2013, 61, 26–31. [Google Scholar] [CrossRef]
- Kumaratilake, A.R.; Lorraine-Colwill, D.F.; Preston, C. A comparative study of glufosinate efficacy in rigid ryegrass (Lolium rigidum) and sterile oat (Avena sterilis). Weed Sci. 2002, 50, 560–566. [Google Scholar] [CrossRef]
- Avila-Garcia, W.V.; Mallory-Smith, C. Glyphosate-resistant Italian ryegrass (Lolium perenne) populations also exhibit resistance to glufosinate. Weed Sci. 2011, 59, 305–309. [Google Scholar] [CrossRef]
- Carlson, K.L.; Burnside, O.C. Comparative phytotoxicity of glyphosate, SC-0224, SC-0545, and HOE-00661. Weed Sci. 1984, 32, 841–844. [Google Scholar]
- Kumaratilake, A.R.; Preston, C. Low temperature reduces glufosinate activity and translocation in wild radish (Raphanus raphanistrum). Weed Sci. 2005, 53, 10–16. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the Risks of Herbicide Resistance: Best Management Practices and Recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Broster, J.; Pratley, J. A decade of monitoring herbicide resistance in Lolium rigidum in Australia. Aust. J. Exp. Agric. 2006, 46, 1151–1160. [Google Scholar] [CrossRef]
- Mueller, T.C.; Mitchell, P.D.; Young, B.G.; Culpepper, A.S. Proactive Versus Reactive Management of Glyphosate-Resistant or -Tolerant Weeds. Weed Technol. 2017, 19, 924–933. [Google Scholar] [CrossRef]
| Prefecture | Code | Positions | Crops | No. of Accessions |
|---|---|---|---|---|
| Attiki | AT | 37°56′–37°59′ N, 23°53′–23°57′ E | Vineyards, Olive orchards | 2 |
| Etoloakarnania | ET | 38°23′–38°32′ N, 21°15′–21°28′ E | Olive orchards, Orchards | 9 |
| Fthiotida | FT | 39°08′–39°09′ N, 22°16′–22°27′ E | Vineyards, Orchards | 5 |
| Korinthia | KO | 37°54′–37°57′ N, 22°41′–22°51′ E | Orchards, Vineyards | 2 |
| Viotia | VI | 38°19′–38°20′ N, 23°05′–23°15′ E | Vineyards, Olive orchards | 2 |
| Category | Accessions (no.) | Accessions (%) |
|---|---|---|
| Potentially susceptible | 6 | 30 |
| Intermediate | 9 | 45 |
| Potentially resistant | 5 | 25 |
| Total | 20 | 100 |
| AT2 | ET6 | ΚΟ2 | FT3 | VI2 | |
|---|---|---|---|---|---|
| GR50 a | 0.52 (0.22) c | 3.84 (0.61) a | 0.61 (0.13) c | 2.8 (0.48) ab | 1.6 (0.36) b |
| RI | 1.00 c | 7.38 a | 1.17 c | 5.38 ab | 3.08 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travlos, I.S.; Cheimona, N.; De Prado, R.; Jhala, A.J.; Chachalis, D.; Tani, E. First Case of Glufosinate-Resistant Rigid Ryegrass (Lolium rigidum Gaud.) in Greece. Agronomy 2018, 8, 35. https://doi.org/10.3390/agronomy8040035
Travlos IS, Cheimona N, De Prado R, Jhala AJ, Chachalis D, Tani E. First Case of Glufosinate-Resistant Rigid Ryegrass (Lolium rigidum Gaud.) in Greece. Agronomy. 2018; 8(4):35. https://doi.org/10.3390/agronomy8040035
Chicago/Turabian StyleTravlos, Ilias S., Nikolina Cheimona, Rafael De Prado, Amit J. Jhala, Demosthenis Chachalis, and Eleni Tani. 2018. "First Case of Glufosinate-Resistant Rigid Ryegrass (Lolium rigidum Gaud.) in Greece" Agronomy 8, no. 4: 35. https://doi.org/10.3390/agronomy8040035





