Phosphate Sources and Their Placement Affecting Soil Phosphorus Pools in Sugarcane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Treatments
2.2. Soil Sampling and Phosphorus Analyses
2.3. Statistical Analysis
3. Results
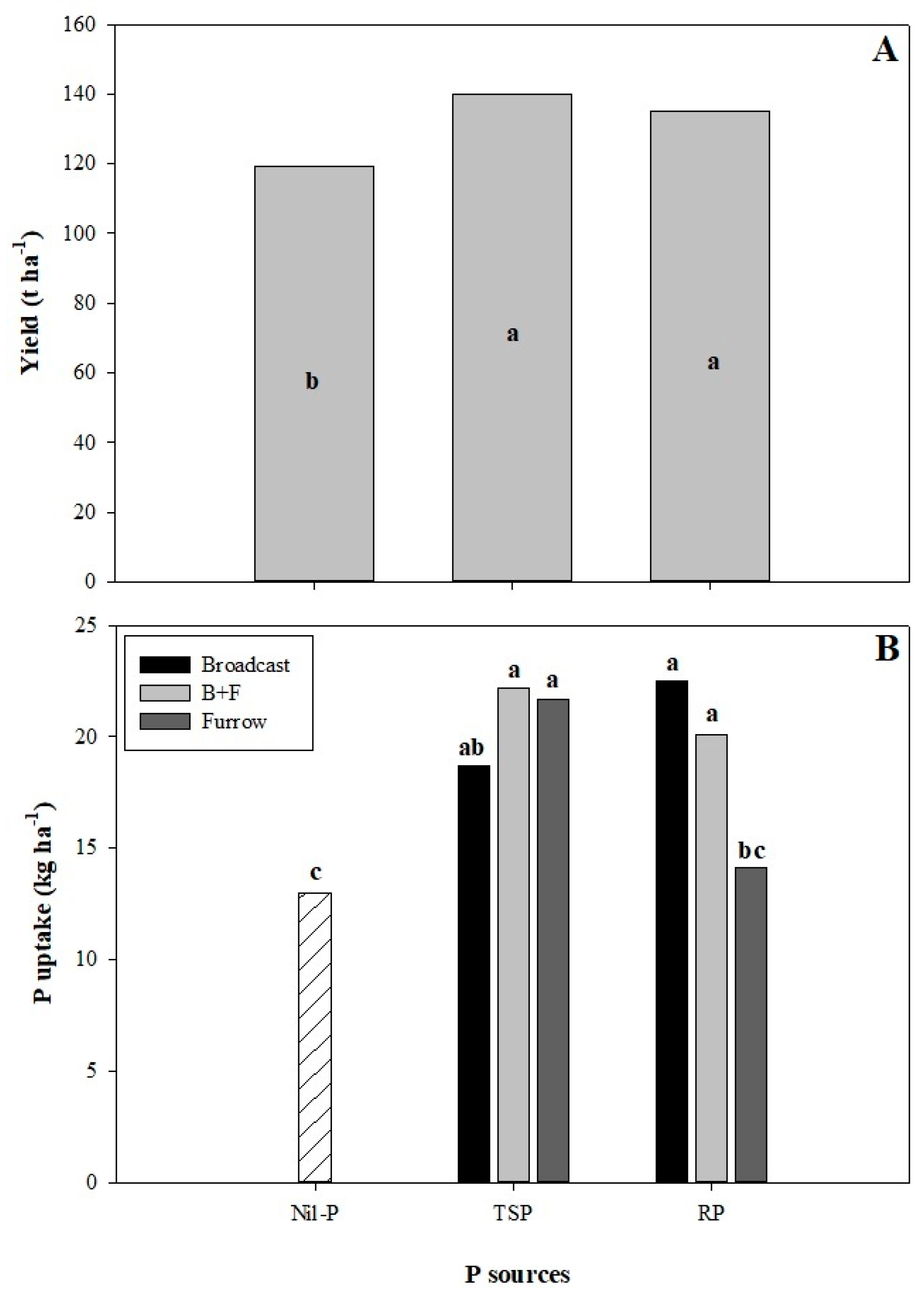
3.1. Cane Yield and P Uptake
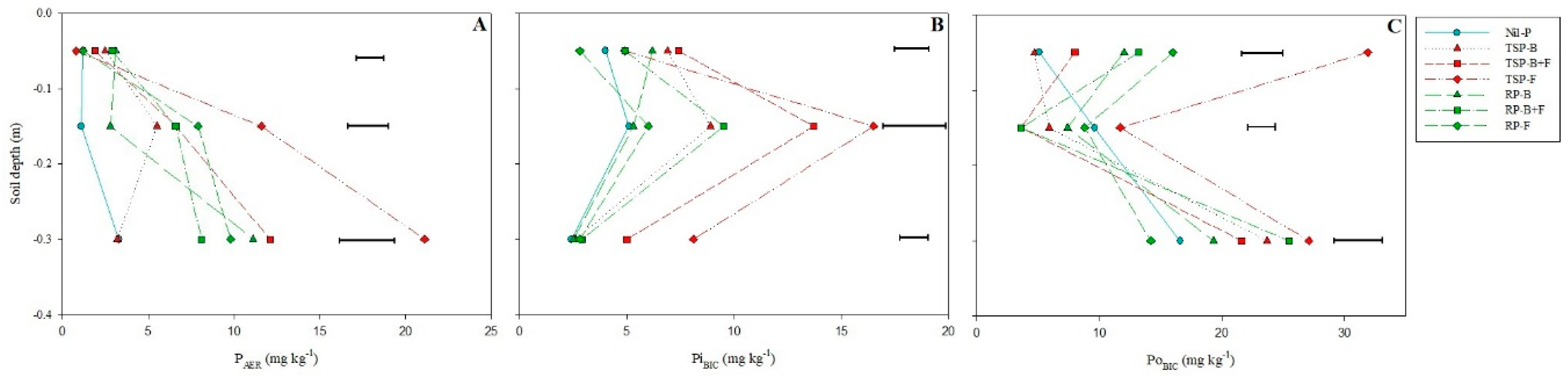
3.2. Labile P Fractions (PAER, PiBIC and PoBIC)
3.3. Moderately Labile P Fractions (PiHID-0.1, PoHID-0.1 and PHCl)
3.4. Non-Labile P Fractions (PiHID-0.5, PoHID-0.5 and PResidual)
3.5. Soil P Balance over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldemberg, J.; Mello, F.F.; Cerri, C.E.; Davies, C.A.; Cerri, C.C. Meeting the global demand for biofuels in 2021 through sustainable land use change policy. Energy Policy 2014, 69, 14–18. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento—CONAB. Acompanhamento da Safra Brasileira de Cana-de-açúcar, Segundo Levantamento; CONAB: Brasília, Brazil, 2016; pp. 1–33. [Google Scholar]
- Walter, A.; Galdos, M.V.; Scarpare, F.V.; Leal, M.R.L.V.; Seabra, J.E.A.; da Cunha, M.P.; de Oliveira, C.O.F. Brazilian sugarcane ethanol: Developments so far and challenges for the future. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 70–92. [Google Scholar] [CrossRef]
- Rossetto, R.; Cantarella, H.; Dias, F.L.F.; Landell, M.G.A.; Vitti, A.C. Conservation management and nutrient recycling in sugarcane with a view to mechanical harvesting. Informações Agronômicas 2008, 124, 8–13. [Google Scholar]
- Devi, T.C.; Bharathalakshmi, M.; Kumari, M.B.G.S.; Naidu, N.V. Effect of sources and levels of phosphorus with zinc on yield and quality of sugarcane. Sugar Tech. 2012, 14, 195–198. [Google Scholar] [CrossRef]
- Savini, I.; Kihara, J.; Koala, S.; Mukalama, J.; Waswa, B.; Bationo, A. Long-term effects of TSP and Minjingu phosphate rock applications on yield response of maize and soybean in a humid tropical maize–legume cropping system. Nutr. Cycl. Agroecosyst. 2016, 104, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Novais, R.D.; Braga, J.M.; Martins Filho, C.A.S. Efeito do tempo de incubação do fosfato-de-araxá em solos sobre o fósforo disponível. Rev. Bras. Cien. Solo 1980, 4, 153–155. [Google Scholar]
- Raij, B.V.; Cantarella, H.; Quaggio, J.; Furlani, A. Outras culturas industriais. In Recomendações de Adubação e Calagem Para o Estado de São Paulo; Instituto Agronômico de Campinas—IAC: Campinas, Brazil, 1997; Volume 100, pp. 233–243. [Google Scholar]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Della Coletta, L.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Zapata, F.; Roy, R.N. Use of phosphate rocks for sustainable agriculture. FAO Fertil. Plant Nutr. Bull. 2004, 13, 1–148. [Google Scholar]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Crews, T.E.; Brookes, P.C. Changes in soil phosphorus forms through time in perennial versus annual agroecosystems. Agric. Ecosyst. Environ. 2014, 184, 168–181. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Turner, B.L.; Cade-Menun, B.J.; Condron, L.M.; Newman, S. Extraction of soil organic phosphorus. Talanta 2005, 66, 294–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Empresa Brasileira de Pesquisa Agropecuaria—EMBRAPA. Sistema Brasileiro de Classificação do solo, 3rd ed.; EMBRAPA: Brasília, Brazil, 2013; p. 353. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture—Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 360. [Google Scholar]
- Universidade Federal de São Carlos—UFSCar. Programa de Melhoramento Genético da Cana-de-açúcar—PMGCA, Censo Varietal 2014; UFSCar: São Carlos, Brazil, 2016. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do estado nutricional das plantas: Princípios e aplicações, 2nd ed.; POTAFOS: Piracicaba, Brazil, 1997; p. 319. [Google Scholar]
- Condron, L.M.; Goh, K.M.; Newman, R.H. Nature and distribution of soil phosphorus as revealed by a sequential extraction method followed by 31P nuclear magnetic resonance analysis. J. Soil Sci. 1985, 36, 199–207. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S. Preventing phosphorus losses during perchloric acid digestion of sodium bicarbonate soil extracts. J. Sci. Food Agri. 1981, 32, 671–674. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M.A. Determination of orthophosphate in aqueous solutions containing labile organic and inorganic phosphorus compounds. J. Environ. Qual. 1977, 6, 82–85. [Google Scholar] [CrossRef]
- USEPA—United States Environmental Protection Agency. Methods of Chemical Analysis for Water and Wastes; Environmental Protection Agency: Cincinnati, OH, USA, 1971; p. 312. [Google Scholar]
- Sui, Y.; Thompson, M.L. Phosphorus sorption, desorption, and buffering capacity in a biosolids-amended mollisol. Soil Sci. Soc. Am. J. 2000, 64, 164–169. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis—Advanced Course, 2nd ed.; University of Wisconsin, College of Agric., Department of Soils: Madison, WI, USA, 1979. [Google Scholar]
- Box, G.E.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar]
- Caione, G.; de Mello Prado, R.; Campos, C.N.S.; Rodrigues, M.; Pavinato, P.S.; Agostinho, F.B. Phosphorus fractionation in soil cultivated with sugarcane fertilized by filter cake and phosphate sources. Commun. Soil Sci. Plant Anal. 2015, 46, 2449–2459. [Google Scholar] [CrossRef]
- Dos Santos, V.R.; Filho, G.M.; de Albuquerque, A.W.; da Costa, J.P.; dos Santos, C.G.; dos Santos, A.C. Crescimento e produtividade agrícola de cana-de-açúcar em diferentes fontes de fósforo. Rev. Bras. Eng. Agríc. Ambient. 2009, 13, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Tsado, P.A.; Lawal, B.A.; Igwe, C.A.; Adeboye, M.K.A.; Odofin, A.J.; Adekambi, A.A. Effects of sources and levels of phosphorus on yield and quality of sugarcane in Southern Guinea Savanna Zone of Nigeria. Agric. Sci. Dev. 2013, 2, 25–27. [Google Scholar]
- De Sousa, R.T.X.; Korndörfer, G.H.; Brem Soares, R.A.; Fontoura, P.R. Phosphate fertilizers for sugarcane used at pre-planting (phosphorus fertilizer application). J. Plant Nutr. 2015, 38, 1444–1455. [Google Scholar] [CrossRef]
- Hammond, L.L.; Chien, S.H.; Easterwood, G.W. Agronomic effectiveness of Bayovar phosphate rock in soil with induced phosphorus retention. Soil Sci. Soc. Am. J. 1986, 50, 1601–1606. [Google Scholar] [CrossRef]
- Ruaysoongnern, S.; Keerati-Kasikorn, P. Role of phosphorus fertilization in improving the soil fertility of acid tropical and subtropical soils in Asia. In Nutrient Management for Sustainable Crop Production in Asia; Johnston, A.E., Syers, J.K., Eds.; CAB International: Oxon, UK; New York, NY, USA, 1998; p. 394. [Google Scholar]
- Negassa, W.; Leinweber, P. How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: A review. J. Plant Nutr. Soil Sci. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Conte, E.; Anghinoni, I.; Rheinheimer, D.D.S. Frações de fósforo acumuladas em Latossolo argiloso pela aplicação de fosfato no sistema plantio direto. Bras. Cien. Solo 2003, 27, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Tiessen, H.J.W.B.; Stewart, J.W.B.; Cole, C.V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Merlin, A.; Rosolem, C.A. Phosphorus fractions in Brazilian Cerrado soils as affected by tillage. Soil Till. Res. 2009, 105, 149–155. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Zoysa, A.K.N.; Loganathan, P.; Hedley, M.J. Comparison of the agronomic effectiveness of a phosphate rock and triple superphosphate as phosphate fertilisers for tea (Camellia sinensis L.) on a strongly acidic Ultisol. Nutr. Cycl. Agroecosyst. 2001, 59, 95–105. [Google Scholar] [CrossRef]
- Hocking, P.J. Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. Adv. Agron. 2001, 74, 63–97. [Google Scholar]
- Tambunan, D.; Hedley, M.J.; Bolan, N.S.; Turner, M.A. A comparison of sequential extraction procedures for measuring phosphate rock residues in soils. Fert. Res. 1993, 35, 183–191. [Google Scholar] [CrossRef]
- Chien, S.H.; Sale, P.W.G.; Hammond, L.L. Comparison of the Effectiveness of Phosphorus Fertilizer Products; International Rice Research Institute: Manila, Philippines, 1990; pp. 143–156. [Google Scholar]
- Shigaki, F.; Sharpley, A. Phosphorus source and soil properties effects on phosphorus availability. Soil Sci. 2011, 176, 502–507. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Relationship between soil test phosphorus and phosphorus release to solution in three soils after long-term mineral and manure application. Agric Ecosyst. Environ. 2016, 233, 214–223. [Google Scholar] [CrossRef]
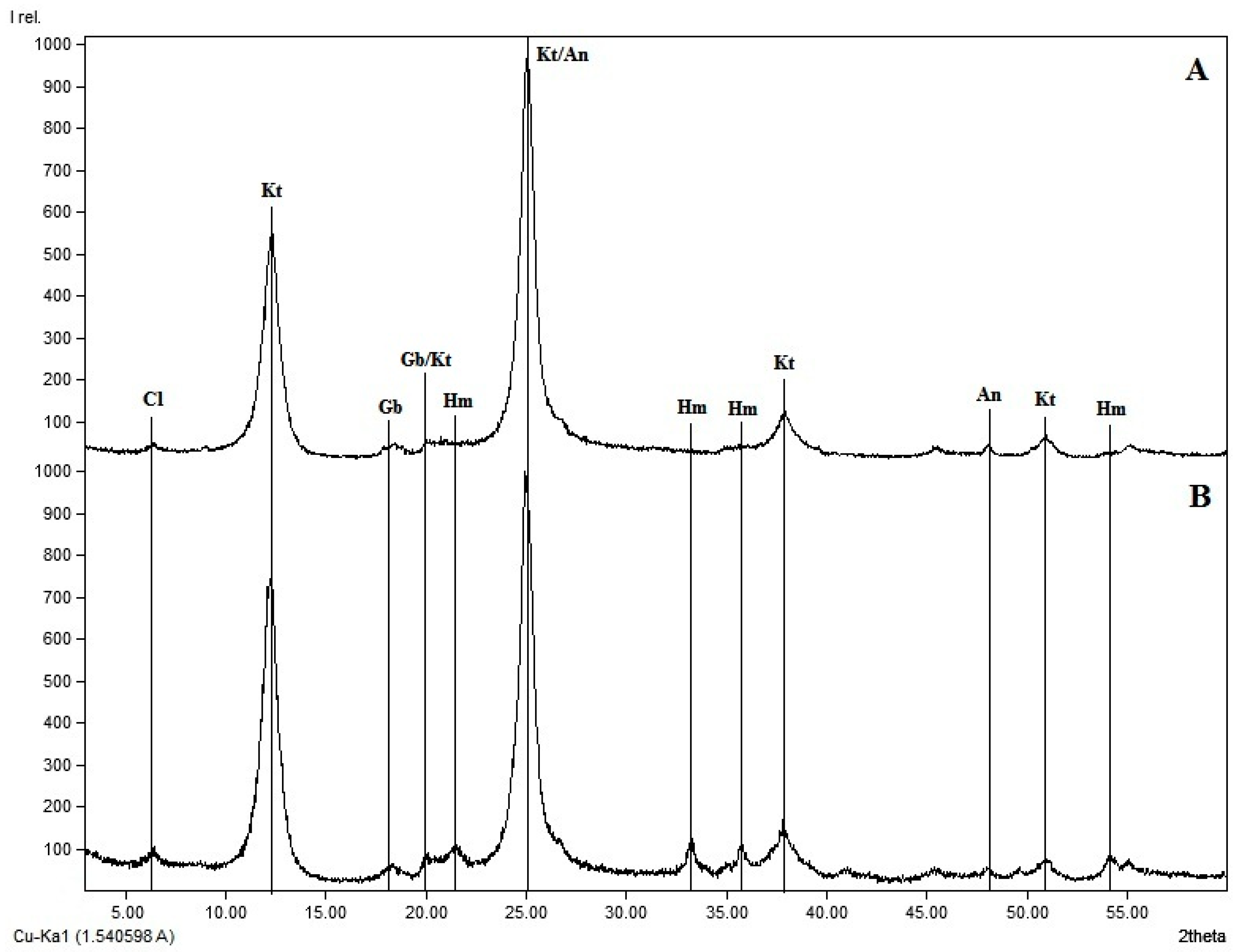


| Depth | pH CaCl2 | OM a | P-resin | K | Ca | Mg | Al | H + Al | SB b | CEC | BS c | m d | Texture | DCB | Oxalate | PMAC g | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Fed e | Ald e | Feo f | Alo f | ||||||||||||||
| cm | g kg−1 | mg kg−1 | mmolc kg−1 | % | g kg−1 | mg kg−1 | ||||||||||||||
| 0−20 | 4.3 | 12.5 | 4 | 1.6 | 6.0 | 4 | 3 | 27 | 11.8 | 38.1 | 31 | 16 | 884 | 4 | 112 | 15.3 | 42.3 | 0.6 | 1.4 | 342 |
| 20−40 | 3.9 | 3 | 1.9 | 3.0 | 2 | 6 | 38 | 6.8 | 44.8 | 15 | 48 | 835 | 27 | 138 | ||||||
| 40−60 | 4.0 | 2 | 0.8 | 2.8 | 0.8 | 7 | 34 | 4.3 | 38.5 | 11 | 63 | 832 | 17 | 151 | ||||||
| 60−80 | 3.9 | 1 | 0.7 | 2.8 | 0.9 | 9 | 31 | 3.8 | 34.6 | 11 | 70 | 825 | 24 | 151 | ||||||
| Management | PAER | PiBIC | PoBIC | PiHID-0.1 | PoHID-0.1 | PHCl | PiHID-0.5 | PoHID-0.5 | PResidual | PTotal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSP | RP | TSP | RP | TSP | RP | TSP | RP | TSP | RP | TSP | RP | TSP | RP | TSP | RP | TSP | RP | TSP | RP | |
| mg kg−1 | ||||||||||||||||||||
| 0–40 cm | ||||||||||||||||||||
| Broadcast | 1.4 | 4.8 | 1.8 | 0.7 | 2.5 | 2.5 | 5.4 | 1.5 | −11.9 | −2.2 | −1.9 | 49.5 | 0.0 | 6.1 | −1.2 | 12.3 | 3.3 | −0.9 | −0.6 | 74.2 |
| B + F | 6.0 | 4.2 | 4.3 | 1.6 | 1.7 | 5.0 | 14.9 | 4.6 | 7.2 | 33.9 | 2.5 | 55.3 | 3.7 | 9.5 | −4.3 | −1.4 | 6.6 | −2.1 | 42.6 | 110.5 |
| Furrow | 11.4 | 5.0 | 5.9 | 0.1 | 12.5 | 1.3 | 17.2 | 1.1 | 16.8 | 5.2 | 3.2 | 42.0 | 6.1 | 3.6 | −1.9 | 9.3 | 0.5 | −0.3 | 71.7 | 67.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosendo dos Santos, V.; Soltangheisi, A.; Junqueira Franco, H.C.; Kolln, O.; Vitti, A.C.; Santos Dias, C.T.d.; Pavinato, P.S. Phosphate Sources and Their Placement Affecting Soil Phosphorus Pools in Sugarcane. Agronomy 2018, 8, 283. https://doi.org/10.3390/agronomy8120283
Rosendo dos Santos V, Soltangheisi A, Junqueira Franco HC, Kolln O, Vitti AC, Santos Dias CTd, Pavinato PS. Phosphate Sources and Their Placement Affecting Soil Phosphorus Pools in Sugarcane. Agronomy. 2018; 8(12):283. https://doi.org/10.3390/agronomy8120283
Chicago/Turabian StyleRosendo dos Santos, Valdevan, Amin Soltangheisi, Henrique Coutinho Junqueira Franco, Oriel Kolln, André Cesar Vitti, Carlos Tadeu dos Santos Dias, and Paulo Sergio Pavinato. 2018. "Phosphate Sources and Their Placement Affecting Soil Phosphorus Pools in Sugarcane" Agronomy 8, no. 12: 283. https://doi.org/10.3390/agronomy8120283





