Impact of Pre-Anthesis Water Deficit on Yield and Yield Components in Barley (Hordeum vulgare L.) Plants Grown under Controlled Conditions
Abstract
:1. Introduction
2. Materials and methods
2.1. Plant Material
2.2. Genotyping of Major Flowering Time Genes
2.3. Greenhouse Experiment and Stress Treatments
2.4. Statistical Analysis
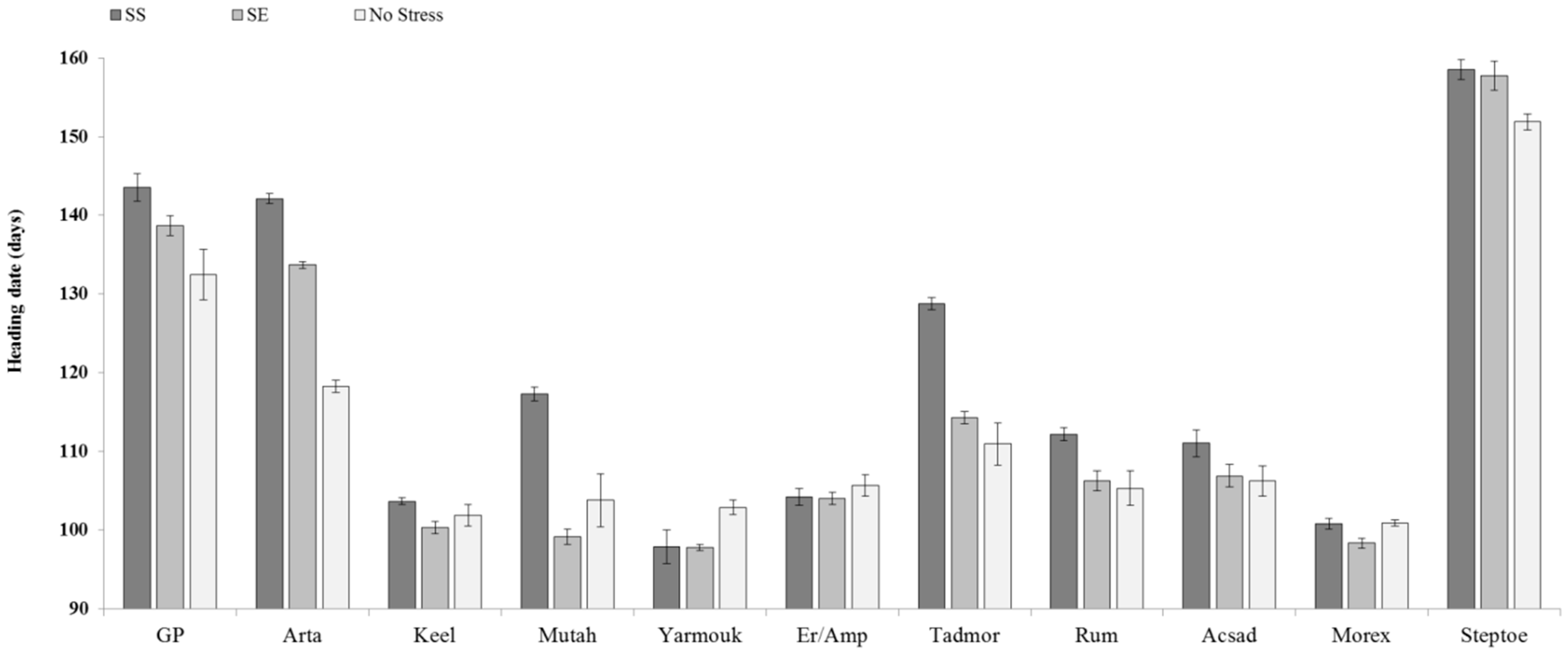
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ceccarelli, S.; Grando, S. Drought as a challenge for the plant breeder. Plant Growth Regul. 1996, 20, 149–155. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Nogues, S.; Ferrio, P.; Voltas, J.; Araus, J.L. Does higher yield potential improve barley performance in Mediterranean conditions? A case study. Field Crop. Res. 2005, 91, 149–160. [Google Scholar] [CrossRef]
- Rizza, F.; Badeck, F.W.; Cattivelli, L.; Lidestri, O.; Di Fonzo, N.; Stanca, A.M. Use of a water stress index to identify barley genotypes adapted to rainfed and irrigated conditions. Crop Sci. 2004, 44, 2127–2137. [Google Scholar] [CrossRef]
- Samarah, N. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; et al. Plant breeding and climate changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Blum, A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Dodig, D.; Zoric, M.; Jovic, M.; Kandic, V.; Stanisavljevic, R.; Šurlan-Momirovic, G. Wheat seedlings growth response to water deficiency and how it correlates with adult plant tolerance to drought. J. Agric. Sci. 2014, 153, 466–480. [Google Scholar] [CrossRef]
- Fischer, R.A.; Turner, N.C. Plant productivity in the arid and semiarid zones. Annu. Rev. Plant Physiol. 1978, 29, 277–317. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; MomčIlović, I.; Ristić, Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Miralles, D.J.; Slafer, G.A. Wheat development. In Wheat: Ecology and Physiology of Yield Determination; Satorre, E.H., Slafer, G.A., Eds.; Food Product Press: New York, NY, USA, 1999; pp. 13–43. [Google Scholar]
- Gonzalez, A.; Bermejo, V.; Gimeno, B.S. Effect of different physiological traits on grain yield in bar-ley grown under irrigated and terminal water deficit conditions. J. Agric. Sci. 2010, 148, 319–328. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crop. Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- Maccaferri, M.; Sangunneti, M.C.; Demonts, A.; El-Ahmed, A.; Del moral, L.G.; Maalouf, F.; Nachit, M.; Nserallah, N.; Ouabbou, H.; Rhoua, S.; et al. Association mapping in durum wheat grown across a broad range of water regimes. J. Exp. Bot. 2011, 62, 409–438. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castaneda, C.; Richards, R.A.; Farquhar, G.D. Variation in early vigour between wheat and barley. Crop Sci. 1995, 35, 472–479. [Google Scholar] [CrossRef]
- Baurle, I.; Dean, C. The timing of developmental transitions in plants. Cell 2006, 125, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Bernier, G.; Havelange, A.; Houssa, C.; Petitjean, A.; Lejeune, P. Physiological signals thatinduce flowering. Plant Cell 1993, 5, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 2009, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Winnepenninckx, B.; Backeljau, T.; De Wachter, R. Extraction of high molecular weight DNA from mollusks. Trends Genet. 1993, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Drosse, B.; Campoli, C.; Mulki, A.M.; von Korff, M. Genetic control of reproductive development. In Biotechnological Approaches to Barley Improvement; Kumlehn, J., Stein, N., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2014; Volume 6, pp. 81–98. [Google Scholar]
- Hemming, M.N.; Fieg, S.; Peacock, W.J.; Dennis, E.S.; Trevaskis, B. Regions associated with repression of the barley Hordeumvulgare. VERNALIZATION1 gene are not required for cold induction. Mol. Genet. Genom. 2009, 282, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Amasino, R.M. Remembering winter: Toward a molecular understanding of vernalization. Annu. Rev. Cell Dev. Biol. 2005, 56, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Szucs, P.; Yan, L.; Helguera, M.; Skinner, J.S.; von Zitzewitz, J.; Hayes, P.M.; Dubcovsky, J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 273, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Casao, M.C.; Karsai, I.; Igartua, E.; Gracia, M.P.; Veisz, O.; Casas, A.M. Adaptation of barley to mild winters: A role for PPDH2. BMC Plant Biol. 2011, 11, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakhrabekova, S.; Gough, S.P.; Braumann, I.; Muller, A.H.; Lundqvist, J.; Ahmann, K.; Dockter, C.; Matyszczak, I.; Kurowska, M.; Druka, A.; et al. Induced mutations in circadian clock regulator Mat-a facilitated short-season adaptation and range extension in cultivated barley. Proc. Natl. Acad. Sci. USA 2012, 109, 4326–4331. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Kawahigashi, H.; Ando, T.; Tonooka, T.; Handa, H. Molecular and functional characterization of PEBP genes in barley reveals the diversification of their roles in flowering. Plant Physiol. 2009, 149, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Borras-Gelonch, G.; Slafer, G.; Casas, A.; van Eeuwijk, F.; Romagosa, I. Genetic control of pre-heading phases and other traits related to development in a double-haploid barley (Hordeumvulgare L.) population. Field Crop. Res. 2009, 119, 36–47. [Google Scholar] [CrossRef]
- Francia, E.; Tondelli, A.; Rizza, F.; Badeck, F.; Li Destri Nicosia, O.; Akar, T.; Grando, S.; Al-Yassin, A.; Benbelkacem, A.; Thomas, W.; et al. Determinants of barley grain yield in a wide range of Mediterranean environments. Field Crop. Res. 2011, 120, 169–178. [Google Scholar] [CrossRef]
- Rajala, A.; Hakala, K.; Mäkelä, P.; Peltonen-Sainio, P. Drought effect on grain number and grain weight at spike and spikelet level in six-row spring barley. J. Agron. Crop Sci. 2011, 197, 103–112. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Day, W.; Johnston, A.E.; Legg, B.J.; Parkinson, K.J. Growth of spring barley under drought: Crop development, dry matter accumulation and nutrient content. J. Agric. Sci. 1981, 96, 167–186. [Google Scholar] [CrossRef]
- Arisnabarreta, S.; Miralles, D.J. Critical period for grain number establishment of near isogenic lines of two- and six-rowed barley. Field Crop. Res. 2008, 107, 196–202. [Google Scholar] [CrossRef]
- Lizana, X.; Calderini, D. Yield and grain quality of wheat in response to increased temperatures at key periods for grain number and grain weight determination: Considerations for the climatic change scenarios of Chile. J. Agric. Sci. 2013, 151, 209–221. [Google Scholar] [CrossRef]
- Slafer, G.A.; Araus, J.L.; Richards, R.A. Physiological traits that increase the yield potential of wheat. In Wheat: Ecology and Physiology of Yield Determination; Satorre, E.H., Slafer, G.A., Eds.; Food Products Press: New York, NY, USA, 1999; pp. 379–415. [Google Scholar]
- Bingham, I.J.; Blake, J.; Foulkes, M.J.; Spink, J. Is barley yield in the UK sink limited? I. Post-anthesis radiation interception, radiation use efficiency and source-sink balance. Field Crop. Res. 2007, 101, 198–211. [Google Scholar] [CrossRef]
- Voltas, J.; Romagosa, I.; Lafarga, A.; Armesto, A.P.; Sombrero, A.; Araus, J.L. Genotype by environment interaction for grain yield and carbon isotope discrimination of barley in Mediterranean Spain. Aust. J. Agric. Res. 1999, 50, 263–1271. [Google Scholar] [CrossRef]
- Scott, R.W.; Appleyard, M.; Fellowes, G.; Kirby, E.J.M. Effect of genotype and position in the ear on carpel and grain growth and mature grain weight of spring barley. J. Agric. Sci. 1983, 100, 383–391. [Google Scholar] [CrossRef]
- Calderini, D.F.; Abeledo, L.G.; Savin, R.; Slafer, G.A. Effect of temperature and carpel size during pre-anthesis on potential grain weight in wheat. J. Agric. Sci. 1999, 132, 453–460. [Google Scholar] [CrossRef]
- Calderini, D.F.; Reynolds, M.P. Changes in grain weight as a consequence of de-graining treatment satpre- and post-anthesis in synthetic hexaploid lines of wheat (Triticum durum×T. tauschii). Aust. J. Plant Physiol. 2000, 27, 183–191. [Google Scholar]
- Comstock, J.P. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. J. Exp. Bot. 2002, 53, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Westgate, M.E.; Passioura, J.B.; Munns, R. Water status and ABA content of floral organs in drought-stressed wheat. Aust. J. Plant Physiol. 1996, 23, 763–772. [Google Scholar] [CrossRef]
- Gallagher, J.N.; Biscope, P.V. Radiation absorption, growth and yield of cereals. J. Agric. Sci. Camb. 1978, 91, 47–60. [Google Scholar] [CrossRef]
- Samarah, N.; Alqudah, A.; Amayreh, J.; McAndrews, G. The effect of late-terminal drought stress on yield components of four barley cultivars. J. Agron. Crop Sci. 2009, 195, 427–441. [Google Scholar] [CrossRef]
- Mickelson, S.; Deven, S.; Fletcher, DM.; John, PG.; Curt, RF.; Tom, KB.; Andreas, MF. Mapping of QTL associated with nitrogen storage and remobilization in barley (Hordeum vulgare L.) leaves. J. Exp. Botany. 2003, 54, 801–812. [Google Scholar] [CrossRef]
| Genotype | Row Type | Pedigree/Source |
|---|---|---|
| Acsad176 | 6 | (CM872-189-3Y-1B-2Y-1BX1Y-OB) X (Cr.366/16/2) |
| Arta | 2 | A single spike collected from a field of Arabi Abiad in the Hourani plateau. Released as Arabi Abiad Mohassan. |
| ER/Amp | 2 | A selected ICARDA line released in Tunisia |
| GP | 2 | Gamma-Ray mutant created from Maythorpe cultivar |
| Keel | 2 | Clipper/CPI18197 and WI 2645 |
| Morex | 6 | Derived from Manchurian barley (Cree/Bonanza). |
| Mutah | 2 | Roho/Arabi Abiad/6250 developed in ICARDA and certified in NCARE, Jordan |
| Rum | 6 | Originated from Harbinger-ArivatX Attiki in CIMMYT (Mexico) and certified in the National Center for Agriculture Research and Extension (NCARE), Jordan. |
| Steptoe | 6 | Derived from Coast-type barley originating in North Africa. Washington Selection 3564/Unitan |
| Tadmor | 2 | A single spike collected from a field of Arabi Aswad (ICARDA). |
| Yarmouk | 2 | Esp/1808-4L//Harmal developed in ICARDA and certified in NCARE, Jordan |
| Genotype | PPDH1 | PPDH2 | HvVrn1 | HvVrn2 | HvVrn3 | Expected Response to Photoperiod and Vernalization |
|---|---|---|---|---|---|---|
| Rum | Responsive | Responsive | HvVrn1-7 | Winter | Winter | Long and short day with no vernalization |
| Acsad | Responsive | Responsive | HvVrn1-7 | Winter | Winter | Long and short day with no vernalization |
| Morex | Non-Responsive | Responsive | HvVrn1-1 | Spring | Spring | Short day with no vernalization |
| Steptoe | Responsive | Non-responsive | HvVrn1-4 | Spring | Winter | Long day with no vernalization |
| Mutah | Responsive | Responsive | HvVrn1-7 | Winter | Winter | Long and short day with no vernalization |
| Yarmouk | Responsive | Responsive | HvVrn1-4 | Winter | Winter | Long and short day with no vernalization |
| Keel | Responsive | Responsive | HvVrn1-7 | Spring | Winter | Long and short day with no vernalization |
| Arta | Responsive | Responsive | HvVrn1-6 | Winter | Spring | Long and short day with vernalization |
| GP | Non-Responsive | Responsive | HvVrn1-1 | Spring | Winter | Short day with no vernalization |
| Tadmor | Responsive | Responsive | HvVrn1-6 | Winter | Winter | Long and short day with vernalization |
| Er/Amp | Responsive | Responsive | Spring | Winter | Winter | Long and short day with short vernalization |
| Source of Variation | DF | Total Weight | Grain Weight | Grain Number | Thousand Kernel Weight | Spikes Number | Tiller Number | Grain/Spike | Harvest Index |
|---|---|---|---|---|---|---|---|---|---|
| Replication | 5 | 16.4 ns | 9.2 ns | 998 ns | 340.9 ns | 37.83 * | 8.9 ns | 40.4 ns | 0.0035 ns |
| Genotype | 10 | 214 ** | 144.4 ** | 483004 ** | 1050 ** | 801.7 ** | 810.6 ** | 92.3 ** | 0.0668 ** |
| Stress Treatment | 2 | 6462 ** | 2398.6 ** | 583325 ** | 2806 ** | 108.6 ** | 185.9 ** | 454.1 ** | 0.371 ** |
| Stress Treatment X Genotype | 20 | 34.7 * | 15.1 ** | 106064 ** | 183.5 * | 43.12 ** | 8.9* | 34.4 ** | 0.0045 ** |
| Error | 160 | 19.8 | 4.6 | 997 | 107.3 | 16.35 | 25.6 | 10.8 | 0.0019 |
| Total | 197 |
| Total Weight (g) | Grain Weight (g) | Grain Number | 1000 Grain Weight | |||||||||||||
| Genotype | NS | SE | SS | Mean | NS | SE | SS | Mean | NS | SE | SS | Mean | NS | SE | SS | Mean |
| Acsad | 62.1 b–d | 46.3 ab | 45.9 b–d | 51.4 b–d | 22.8 a | 8.4 ab | 9.2 bc | 13.45 a | 441.7 a | 191.2 a | 199.0 ab | 277.3 a | 51.6 cd | 44.4 b–e | 46.2 d | 47.4 cd |
| Arta | 61.5 b–e | 41.8 d | 45.0 b–e | 49.4 c–e | 19.6 bc | 4.4 c | 9.1 bc | 11.05 cd | 298.0 c–e | 91.33 d | 163.7 cd | 184.3 f | 66.2 a | 47.9 a–d | 55.8 a | 56.6 ab |
| ER/Amp | 65.0 b | 45.9 a–c | 46.4 a–c | 52.4 b | 18.2 b–d | 8.6 ab | 10.2 ab | 12.35 a–c | 343.8 bc | 181.7 a | 203.5 ab | 240.8 b | 53.0 cd | 47.7 a–d | 50.3 a–d | 50.4 b–d |
| GP | 63.3 bc | 42.3 b–d | 43.3 c–e | 49.6 b–e | 16.8 de | 5.0 c | 5.0 e | 9.43 e | 357.5 b | 144.7 bc | 148.8 de | 226.1 b–d | 47.3 d | 37.9 c–f | 33.6 e | 39.6 ef |
| Keel | 56.1 d–e | 44.3 b–d | 45.2 b–e | 48.5 de | 19.4 b–d | 8.9 a | 11.5 a | 13.28 ab | 338.5 b–d | 147.8 bc | 214.3 a | 233.6 bc | 57.4 bc | 60.7 ab | 53.8 ab | 57.3 a |
| Morex | 54.7 e | 37.1 e | 40.9 de | 44.2 f | 14.6 e | 5.7 bc | 9.5 bc | 9.91 de | 267.5 e | 137.7 c | 204.3 ab | 203.2 ef | 54.9 bc | 34.6 d–f | 46.3 cd | 45.3 de |
| Mutah | 58.3 b–e | 41.9 cd | 41.9 de | 47.3 e | 20.0 b | 8.8 a | 8.4 cd | 12.41 a–c | 313.7 b–e | 142.3 bc | 177.3 bc | 211.1 de | 64.3 a | 62.2 a | 48.1 b–d | 58.2 a |
| Rum | 63.5 bc | 45.1 b–d | 46.5 a–c | 51.6 bc | 20.5 ab | 6.5 a–c | 7.3 bd | 12.01 bc | 347.5 bc | 137.2 c | 128.2 e | 213.3 c–e | 59.9 ab | 46.5 a–e | 48.1 b–d | 51.6 a–d |
| Steptoe | 74.6 a | 49.2 a | 50.2 a | 58.0 a | 8.8 f | 0.3 d | 0.3 f | 3.29 f | 168.5 f | 6.33 e | 15.6 f | 66.3 g | 52.3 cd | 36.2 d–f | 22.4 f | 34.8 f |
| Tadmor | 63.7 bc | 44.3 b–d | 47.9 ab | 51.9 bc | 18.8 b–d | 8.2 ab | 9.8 bc | 11.85 c | 292.6 c–e | 155.6 bc | 191.5 ab | 208.5 de | 64.2 a | 52.5 a–c | 50.8 a–d | 55.8 ab |
| Yarmouk | 57.8 ce | 45.1 b–d | 49.0 ab | 50.6 b–d | 17.2 cd | 7.2 a–c | 10.3 ab | 11.53 cd | 287.0 fe | 159.5 b | 192.5 ab | 213.0 c–e | 59.9 ab | 44.4 b–e | 53.2 a–c | 52.5 a–c |
| Mean | 61.9 a | 43.9 c | 45.7 b | 16.9 a | 5.8 c | 7.7 b | 314.5 a | 136.4 c | 170 b | 54.0 a | 43.0 b | 44.6 b | ||||
| Spike Number | Tiller Number | Grains/Spike | Harvest Index | |||||||||||||
| Genotype | NS | SE | SS | Mean | NS | SE | SS | Mean | NS | SE | SS | Mean | NS | SE | SS | Mean |
| Acsad | 18.0 cd | 15.0 c–d | 17.7 de | 16.6 cd | 18.0 cd | 20.2 c–e | 20.8 de | 19.7 cd | 26.9 b | 11.4 b–d | 12.9 b | 17.1 a | 0.37 a | 0.18 ab | 0.22 b | 0.25 ab |
| Arta | 23.7 ab | 22.3 b | 25.3 ab | 23.8 ab | 23.7 ab | 34.5 a | 30.7 ab | 29.6 a | 12.7 b | 6.7 e | 5.0 de | 8.1 gf | 0.32 b–d | 0.11 c | 0.20 b | 0.22 c |
| ER/Amp | 23.6 ab | 28.5 a | 25.3 ab | 25.8 a | 24.3 ab | 33.3 ab | 27.2 b–d | 28.3 ab | 15.4 c | 7.5 de | 6.5 c–e | 9.5 ef | 0.28 de | 0.19 a | 0.22 ab | 0.23 bc |
| GP | 26.0 a | 16.5 cd | 22.7 bc | 22.4 b | 26.8 a | 22.0 c–e | 27.5 a–d | 25.9 b | 13.9 c | 6.8 e | 10.4 bc | 10.4 ef | 0.26 e | 0.12 bc | 0.12 d | 0.17 d |
| Keel | 20.7 bc | 15.3 cd | 16.3 de | 17.4 c | 21.3 bc | 20.2 c–e | 19.8 de | 20.4 dc | 16.1 c | 13.5 b | 9.9 bc | 13.4 cd | 0.35 ab | 0.20 a | 0.25 a | 0.27 a |
| Morex | 7.7 e | 5.67 e | 6.8 f | 6.7 e | 7.7 f | 7.3 f | 6.8 f | 7.3 f | 36.3 a | 32.3 a | 27.4 a | 32.0 a | 0.27 e | 0.15 a–c | 0.23 ab | 0.22 c |
| Mutah | 17.3 bc | 17.5 b–d | 19.7 cd | 19.2 c | 21.0 bc | 18.8 c–e | 23.2 c–e | 21.0 c | 15.3 c | 9.5 c–e | 8.2 b–d | 11.0 de | 0.34 ab | 0.21 a | 0.20 b | 0.25 ab |
| Rum | 13.5 d | 14.8 d | 14.2 e | 14.1 d | 15.2 de | 18.6 de | 17.2 e | 16.9 de | 23.3 b | 11.8 bc | 9.2 b–d | 14.8 bc | 0.32 bc | 0.14 a–c | 0.16 c | 0.22 c |
| Steptoe | 13.0 d | 3.0 e | 2.0 g | 6.2 e | 12.3 e | 15.2 e | 17.6 e | 14.9 e | 13.1 c | 1.5 f | 2.8 e | 5.8 g | 0.12 f | 0.01 d | 0.01 e | 0.04 e |
| Tadmor | 20.6 bc | 20.5 bc | 27.7 a | 23.1 b | 21.2 bc | 26.5 bc | 34.5 a | 27.8 ab | 14.4 c | 7.0 e | 7.8 cd | 9.7 ef | 0.29 c–e | 0.18 ab | 0.20 b | 0.23 bc |
| Yarmouk | 24.5 a | 23.2 ab | 25.3 ab | 24.3 ab | 26.2 a | 25.8 b–d | 28.8 a–c | 26.9ab | 11.9 c | 7.7 de | 6.9 c–e | 8.8 ef | 0.30 c–e | 0.16 a–c | 0.21 b | 0.22 c |
| Mean | 18.9 a | 16.6 b | 18.78 a | 19.8 b | 22.1 a | 23.1 a | 18.2 a | 10.5 b | 9.7 b | 0.29 a | 0.151 c | 0.18 b | ||||
| NS | TW | GW | GN | TKW | SN | TN | GS | HI |
| GW | −0.50 ns | |||||||
| GN | −0.37 ns | 0.87 ** | ||||||
| TKW | −0.30 ns | 0.34 ns | −0.18 ns | |||||
| SN | −0.02 ns | 0.34 ns | 0.34 ns | 0.04 ns | ||||
| TN | −0.02 ns | 0.40 ns | 0.37 ns | 0.12 ns | 0.98 ** | |||
| GS | −0.40 | 0.13 ns | 0.22 ns | −0.15 ns | −0.78 ** | −0.75 ** | ||
| HI | −0.72 * | 0.95 ** | 0.80 ** | 0.37 ns | 0.25 ns | 0.33 ns | 0.23 ns | |
| HD | 0.82 ** | −0.70 * | −0.52 ns | −0.38 ns | 0.02 ns | −0.07 ns | −0.43 ns | −0.82 ** |
| SE | TW | GW | GN | TKW | SN | TN | GS | HI |
| GW | −0.17 ns | |||||||
| GN | −0.23 ns | 0.90 ** | ||||||
| TKW | 0.03 ns | 0.68 * | 0.32 ns | |||||
| SN | 0.07 ns | 0.59 * | 0.58 * | 0.45 ns | ||||
| TN | 0.26 ns | 0.23 ns | 0.22 ns | 0.32 ns | 0.88 ** | |||
| GS | −0.70 * | 0.25 ns | 0.40 ns | −0.26 ns | −0.37 ns | −0.64 * | ||
| HI | −0.31 ns | 0.99 ** | 0.88 ** | 0.67 * | 0.55 ns | 0.18 ns | 0.33 ns | |
| HD | 0.34 ns | −0.86 ** | −0.78 ** | −0.46 ns | −0.34 ns | 0.07 ns | −0.52 ns | −0.88 ** |
| SS | TW | GW | GN | TKW | SN | TN | GS | HI |
| GW | −0.30 ns | |||||||
| GN | −0.46 ns | 0.95 ** | ||||||
| TKW | −0.20 ns | 0.94 ** | 0.81 ** | |||||
| SN | 0.03 ns | 0.57 ns | 0.56 ns | 0.62 * | ||||
| TN | 0.36 ns | 0.19 ns | 0.16 ns | 0.29 ns | 0.87 ** | |||
| GS | −0.57 ns | 0.23 ns | 0.30 ns | 0.07 ns | −0.54 | −0.80 ** | ||
| HI | −0.43 ns | 0.98 ** | 0.97 ** | 0.90 ** | 0.48 | 0.07 ns | 0.34 ns | |
| HD | 0.24 ns | −0.79 ** | −0.76 ** | −0.66 * | −0.16 ns | 0.26 ns | −0.45 ns | −0.80 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ajlouni, Z.I.; Al-Abdallat, A.M.; Al-Ghzawi, A.L.A.; Ayad, J.Y.; Abu Elenein, J.M.; Al-Quraan, N.A.; Baenziger, P.S. Impact of Pre-Anthesis Water Deficit on Yield and Yield Components in Barley (Hordeum vulgare L.) Plants Grown under Controlled Conditions. Agronomy 2016, 6, 33. https://doi.org/10.3390/agronomy6020033
Al-Ajlouni ZI, Al-Abdallat AM, Al-Ghzawi ALA, Ayad JY, Abu Elenein JM, Al-Quraan NA, Baenziger PS. Impact of Pre-Anthesis Water Deficit on Yield and Yield Components in Barley (Hordeum vulgare L.) Plants Grown under Controlled Conditions. Agronomy. 2016; 6(2):33. https://doi.org/10.3390/agronomy6020033
Chicago/Turabian StyleAl-Ajlouni, Zakaria I., Ayed M. Al-Abdallat, Abdul Latief A. Al-Ghzawi, Jamal Y. Ayad, Jamal M. Abu Elenein, Nisreen A. Al-Quraan, and P. Stephen Baenziger. 2016. "Impact of Pre-Anthesis Water Deficit on Yield and Yield Components in Barley (Hordeum vulgare L.) Plants Grown under Controlled Conditions" Agronomy 6, no. 2: 33. https://doi.org/10.3390/agronomy6020033






