1. Introduction
Acid soils represent a significant percentage of the world’s arable lands [
1]. Aluminium toxicity is one of the major constrains for crop production on acid soils [
2]. Great inter- and intra-species differences exist in Al resistance, which have successfully been exploited for the breeding of better yielding Al-resistant cultivars of crop species on acid, Al-toxic soils [
3,
4,
5]. The understanding of the physiological and molecular mechanisms underlying Al resistance could contribute to further intensify the breeding of Al-resistant crop cultivars [
6,
7]. Generally, plant Al resistance may be achieved either through Al exclusion from uptake and binding in the apoplast of the most Al-sensitive root apex or Al uptake and detoxification within the root symplast, thus Al tolerance [
2,
8]. For most crop species, Al exclusion through sequestration of Al in the root apoplast with organic acid anions released from the root symplast is the most common mechanism of Al resistance [
9]. Less binding of Al in the cell walls owing to a lower content of unmethylated pectins [
10,
11] or hemicellulose [
12] of the cell wall may also contribute to Al exclusion. There is recent evidence that both Al exclusion and Al tolerance contribute to Al resistance in a coordinated way in Arabidopsis [
13] and particularly in the Al-resistant cereal crop rice [
14]. In some plant species Al tolerance is combined with the capacity to translocate Al to the shoots where Al is accumulated in large concentrations in the leaves [
15,
16]. Among these plant species are tea (
Camellia sinensis var.
sinensis), buckwheat (
Fagopyrum esculentum) and hortensia (
Hydrangea macrophylla).
Whereas the physiological and molecular understanding of resistance mechanisms in Al-excluding plant species has made considerable progress in recent years [
7,
14,
17], the understanding of Al accumulation in relation to Al tolerance still widely lags behind. The Al accumulator buckwheat is characterized by both, Al exclusion from uptake through Al-induced release by root tips of oxalate [
18] and symplastic sequestration of Al by oxalate [
19]. Although the role of oxalate release from the roots in Al exclusion is well established, references [
20,
21] could not relate differences in oxalate exudation to genotypic differences in Al resistance between buckwheat genotypes. Comparing genotypes of
Fagopyrum tataricum Yang
et al. [
21] also concluded that root exudation of oxalate could not explain genotypic differences in Al resistance. They suggested that the differences in Al accumulation of root tips were related to differences in cell-wall negativity conferred by lower pectin contents and a higher degree of pectin methylation. Al accumulation in shoots requires radial transfer of Al from the root surface to the xylem where Al is transported primarily as Al-citrate to the shoot [
22,
23]. Based on sophisticated physiological approaches references [
24,
25,
26] proposed a hypothesis reconciling Al exclusion and Al accumulation in which an Al oxalate (Ox)
+ plasma-membrane transporter in the root cortex and a xylem-loading Al citrate (Cit)
n− transporter in the xylem parenchyma cells are key elements of Al tolerance and accumulation in buckwheat.
The aim of the present study was to enhance the physiological understanding of Al exclusion on the one hand and Al accumulation on the other hand by exploiting differences in Al resistance and Al accumulation within a large set of genotypes of the species F. esculentum (buckwheat). To even widen the genetic background we included into the genotype selection also genotypes from further species of the Fagopyrum genus namely F. tataricum and F. acutatum.
2. Results
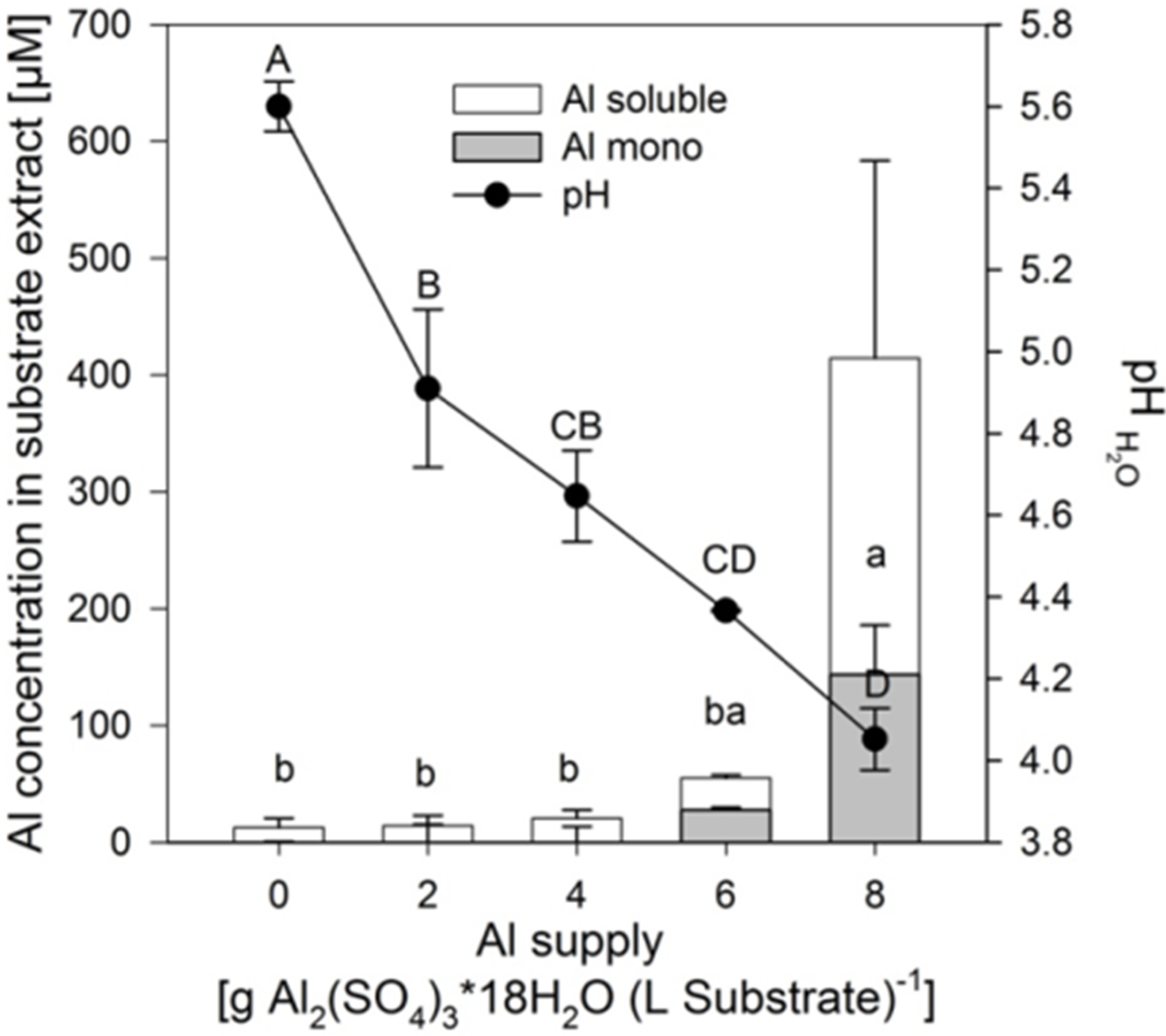
Addition of 2–8 g Al
2(SO
4)
3·18H
2O decreased the pH of the substrate from 5.6 to 4.0 approximately linearly (
Figure 1). In contrast, the soluble and mononuclear Al concentrations increased exponentially from an Al application of 6 g on. Neither the pH reduction, nor the related increase of the soluble Al in the substrate lead to reduced growth of the plants of the buckwheat cultivar “Lifago” (
Figure 2).
Figure 1.
Total soluble, mononuclear Al (Al mono) concentrations and substrate pH in the aqueous 1:3 (v/v) substrate extract as affected by increasing supply of Al2(SO4)3·18H2O. Bars represent means ± SE, n = 3. Different capital and lower case letters denote differences between treatment durations for pH values and for Al mono concentrations, respectively, at p < 0.05 (Tukey test).
Figure 1.
Total soluble, mononuclear Al (Al mono) concentrations and substrate pH in the aqueous 1:3 (v/v) substrate extract as affected by increasing supply of Al2(SO4)3·18H2O. Bars represent means ± SE, n = 3. Different capital and lower case letters denote differences between treatment durations for pH values and for Al mono concentrations, respectively, at p < 0.05 (Tukey test).
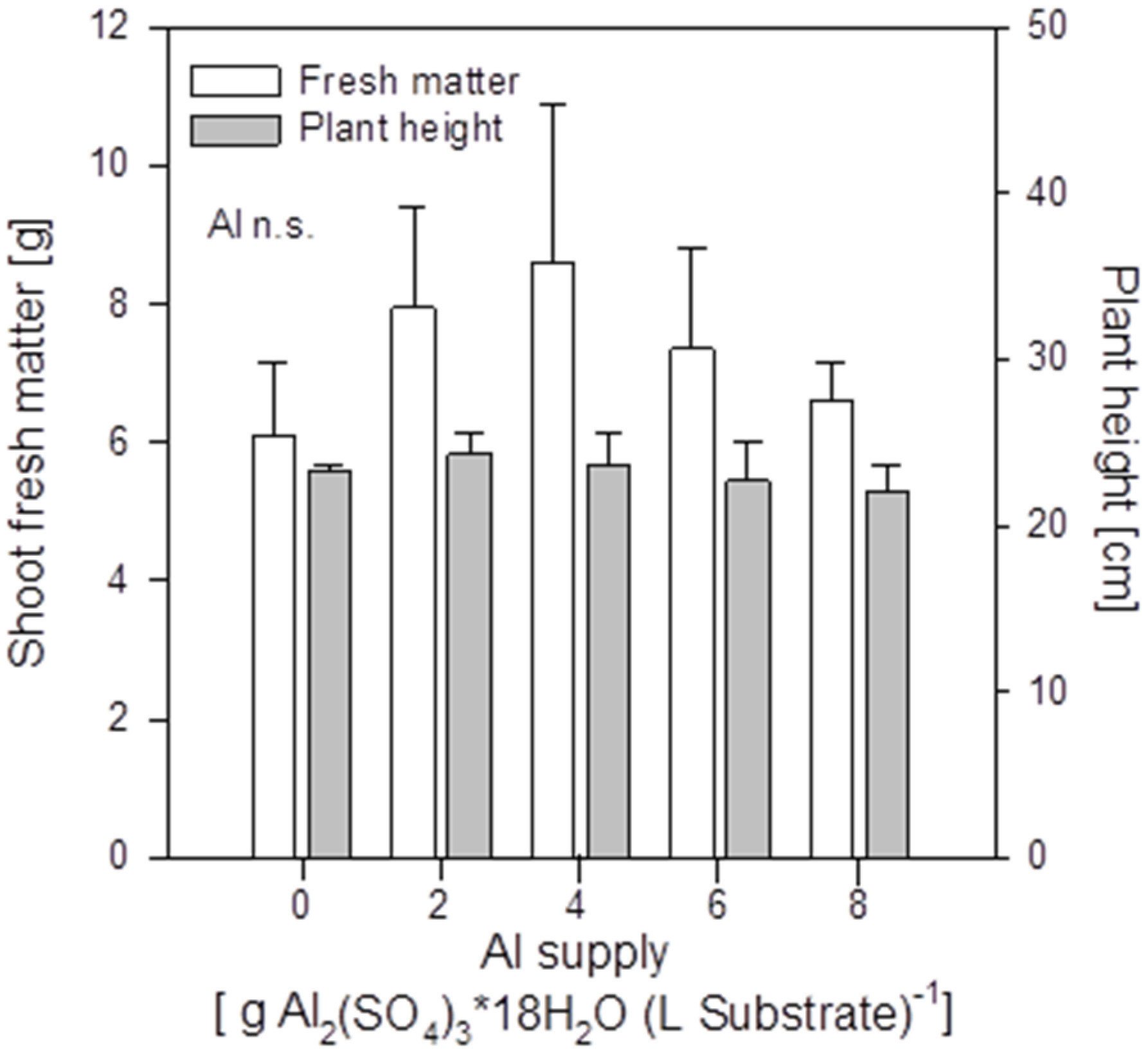
Increasing Al supply did not induce mineral element deficiency-symptoms in shoots of the buckwheat cultivar “Lifago”. Also growth, height, and fresh matter of shoots were not significantly affected (
Figure 2). There was a tendency of optimum shoot growth at 4 g Al sulfate supply which was also especially true for root growth (
Figure S1). This might indicate a growth-promoting effect of small amounts of Al in buckwheat.
Although the soluble Al concentration in the substrate only significantly increased at 8 g Al sulfate supply (see above,
Figure 1), the Al concentrations in the primary leaves steadily increased from the lowest Al application rate to reach about 500 μg (g dry weight)
−1 at the highest Al supply (
Figure 3A).
Aluminium is readily transported from the roots to the shoots via the xylem typical for Al accumulators. The Al concentration in the xylem sap increased with increasing Al supply to a level of about 400 μM at an Al sulfate application of 6 g (
Figure 3A). The xylem-sap citrate-concentration which was more than 10 times higher than the Al concentration increased with the Al supply (
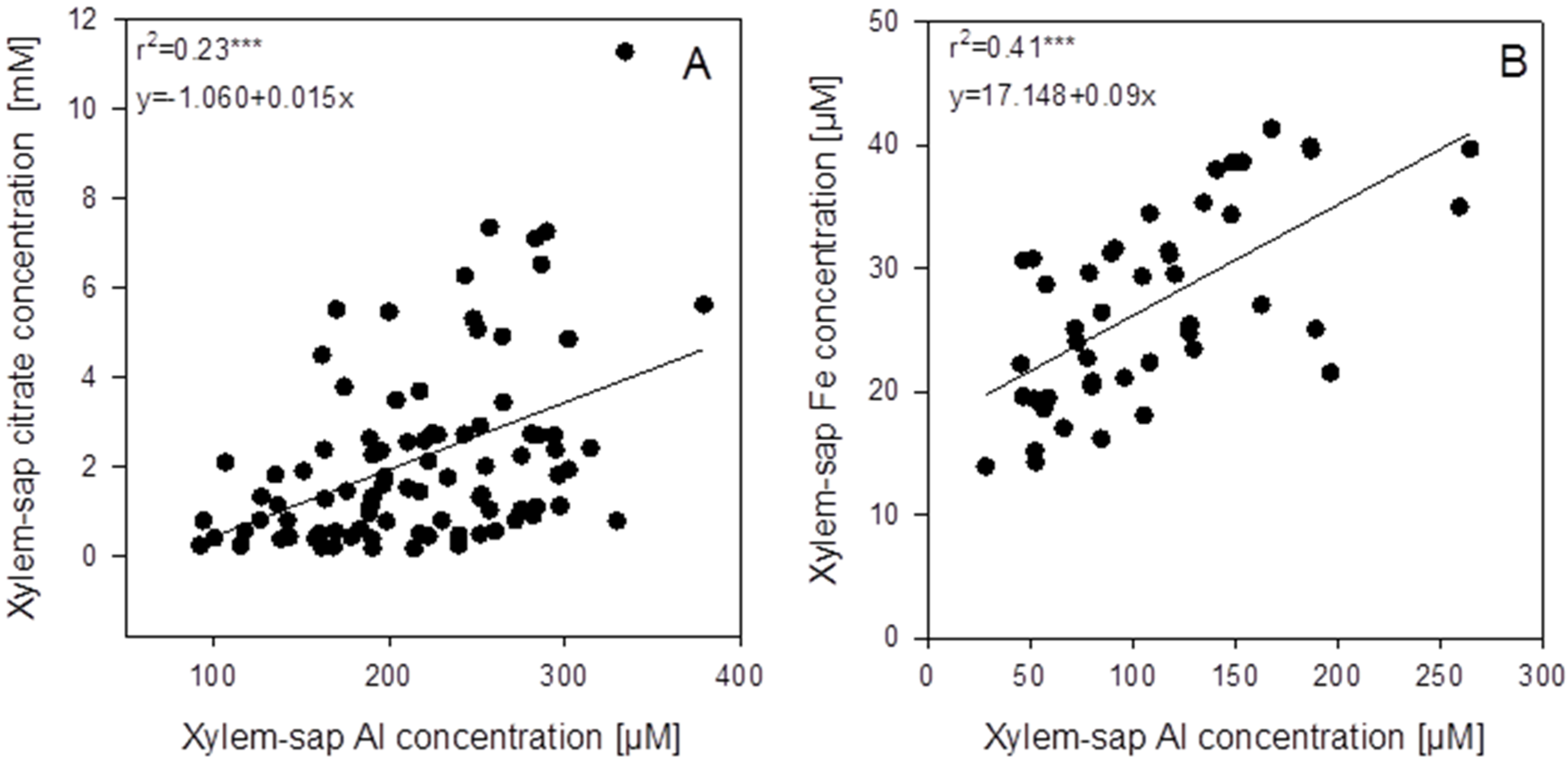
Figure 3B). In spite of great variability between individual plants, there was a significant positive correlation between Al and citrate xylem-sap concentrations.
Figure 2.
Shoot fresh matter production and height of buckwheat cv. “Lifago” plants as affected by increasing Al2(SO4)3·18H2O supply to the substrate, three weeks after germination. Bars represent means ± SE, n = 3.
Figure 2.
Shoot fresh matter production and height of buckwheat cv. “Lifago” plants as affected by increasing Al2(SO4)3·18H2O supply to the substrate, three weeks after germination. Bars represent means ± SE, n = 3.
Figure 3.
Primary leaf and xylem-sap Al concentrations of buckwheat cv. “Lifago” plants as affected by increasing Al2(SO4)3·18H2O supply to the substrate, three weeks after germination (A), and relationship between xylem-sap Al and citrate concentrations (B). Bars represent means ± SE, n = 3. In (A), different capital and lower-case letters denote significant (p < 0.05) differences between leaf and xylem-sap Al concentrations, respectively; In (B), *** denote significance of the regression coefficient at p < 0.001.
Figure 3.
Primary leaf and xylem-sap Al concentrations of buckwheat cv. “Lifago” plants as affected by increasing Al2(SO4)3·18H2O supply to the substrate, three weeks after germination (A), and relationship between xylem-sap Al and citrate concentrations (B). Bars represent means ± SE, n = 3. In (A), different capital and lower-case letters denote significant (p < 0.05) differences between leaf and xylem-sap Al concentrations, respectively; In (B), *** denote significance of the regression coefficient at p < 0.001.
Based on this experiment the whole set of genotypes of the genus
Fagopyrum was cultivated at an Al supply of 8 g Al
2(SO
4)
3·18H
2O. The fresh matter production of the 94 genotypes varied between 5 g and 10 g per plant after three weeks of cultivation. The number of leaves per plant was particularly variable. Some genotypes showed about five times more leaves than others. Since this biased the comparison of the Al concentrations of the leaves owing to dilution or concentration effects in the analyzed leaf tissue, the xylem-sap Al concentration appeared to be the more suitable parameter for the characterization of the Al accumulation capacity. The comparison of the xylem-sap Al concentrations reveals a broad genotypic variation (
Figure 4). The highest Al concentrations reached about 350 μM. None of the genotypes showed Al concentrations lower than 100 μM which could have been indicative of Al excluders. Thus all genotypes can be classified as Al accumulators. However the Al accumulation capacity differed greatly between the genotypes across and within the
Fagopyrum species.
Figure 4.
Xylem-sap Al concentration of 94 genotypes of the genus Fagopyrum after three weeks of substrate culture with an Al supply of 8 g Al2(SO4)3·18H2O. Xylem exudates of three plants per 1 L pot were combined to one composite sample. Bars represent means ± SE, n = 3. Different letters denote significant differences between Al xylem concentrations at p < 0.05 (Tukey test).
Figure 4.
Xylem-sap Al concentration of 94 genotypes of the genus Fagopyrum after three weeks of substrate culture with an Al supply of 8 g Al2(SO4)3·18H2O. Xylem exudates of three plants per 1 L pot were combined to one composite sample. Bars represent means ± SE, n = 3. Different letters denote significant differences between Al xylem concentrations at p < 0.05 (Tukey test).
In the xylem sap not only Al but also citrate and other mineral elements were determined. In spite of high variability of the xylem-sap Al and citrate concentrations, a highly significant positive correlation between the Al and citrate concentrations across the genotypes existed (
Figure 5A). The mean xylem-sap citrate-concentration was 10 times higher than the Al concentration. Among all mineral elements determined in the xylem sap, only Fe was highly significantly positively correlated with the Al concentration (
Figure 5B and
Figure S2 ). The mean Fe xylem-sap concentration was 10 times lower than the Al concentration. Also citrate and Fe xylem-sap concentrations were highly significantly positively correlated (
p < 0.001,
Figure S3). However, the slope of the regression suggests that on average across genotypes, 93 times more citrate than Fe was loaded into the xylem.
For a further in-depth study in hydroponics, six
F. esculentum genotypes (grey columns in
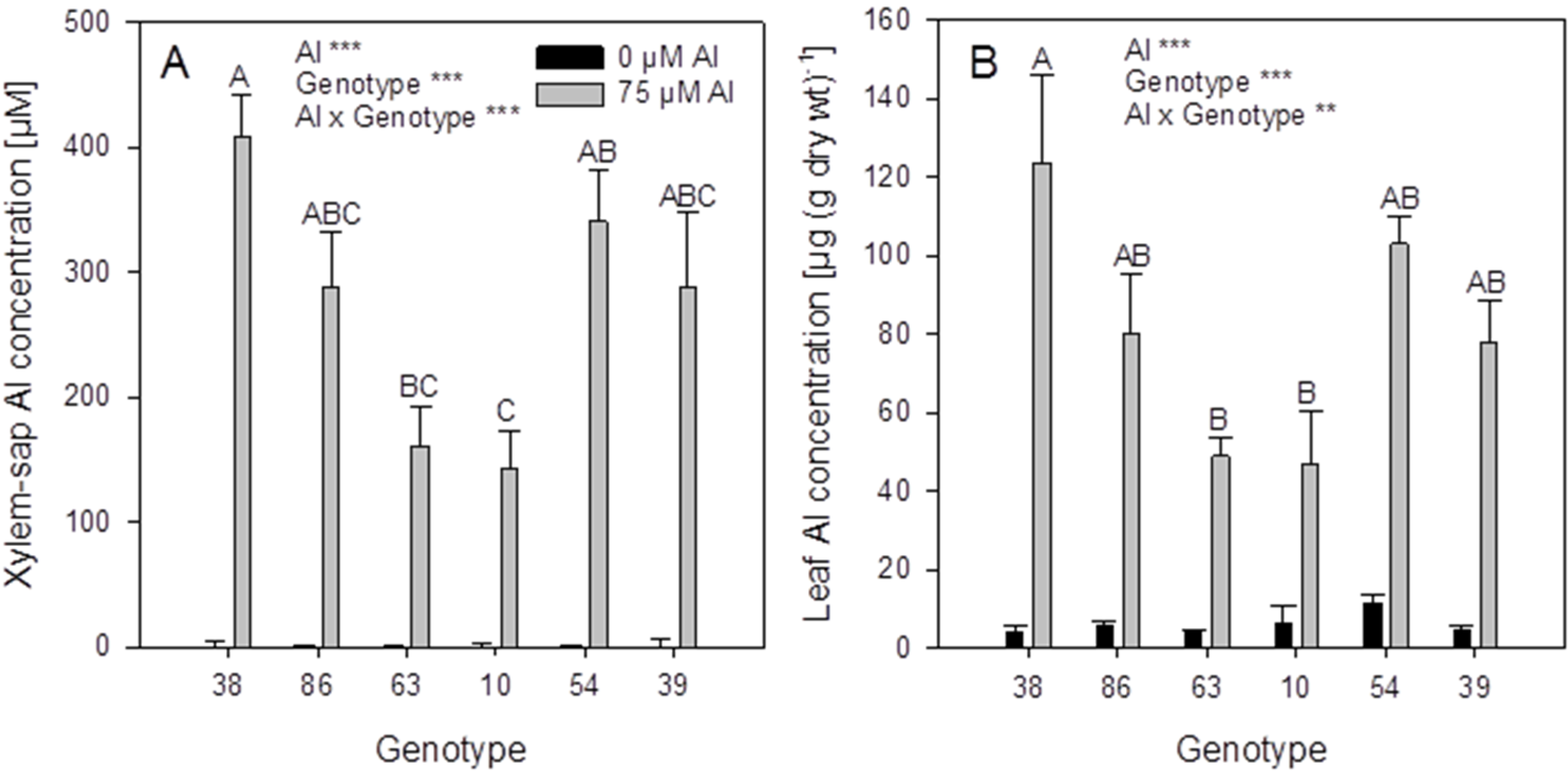
Figure 4) were selected on the basis of significantly different xylem-sap Al concentrations. In the hydroponic system the ranking of the genotypes for Al accumulation based on the screening in substrate could only partially be confirmed: the genotypes 38 and 86 proved to be more efficient Al accumulators than the genotypes 63 and 10 based on high xylem (
Figure 6A) and leaf (
Figure 6B) Al concentrations. However, the genotypes 54 and 39 with the lowest xylem Al concentrations in the substrate experiment had equally high Al accumulation capacity compared to the genotypes 38 and 86. This indicates that the conditions in the substrate and the hydroponic experiment differed greatly with regard to form and concentration of plant-available Al.
Figure 5.
Correlations between xylem-sap Al concentrations and the xylem-sap citrate (A) and iron (B) concentrations of 94 (A) or 46 (B) genotypes of the genus Fagopyrum after 3 weeks of substrate culture with an Al supply of 8 g Al2(SO4)3·18H2O. Xylem sap was collected for 30 min after cutting off the shoots. Points represent means ± SE, n = 3. *** denote significance of the regression coefficient at p < 0.001.
Figure 5.
Correlations between xylem-sap Al concentrations and the xylem-sap citrate (A) and iron (B) concentrations of 94 (A) or 46 (B) genotypes of the genus Fagopyrum after 3 weeks of substrate culture with an Al supply of 8 g Al2(SO4)3·18H2O. Xylem sap was collected for 30 min after cutting off the shoots. Points represent means ± SE, n = 3. *** denote significance of the regression coefficient at p < 0.001.
Figure 6.
Xylem-sap (A) and leaf (B) Al concentrations of six selected Fagopyrum esculentum genotypes. Plants were pre-cultured in complete nutrient solution and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. Bars represent means ± SE, n = 3. For the ANOVA, ** and *** denote significant effects at p < 0.01 and p < 0.001, respectively.
Figure 6.
Xylem-sap (A) and leaf (B) Al concentrations of six selected Fagopyrum esculentum genotypes. Plants were pre-cultured in complete nutrient solution and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. Bars represent means ± SE, n = 3. For the ANOVA, ** and *** denote significant effects at p < 0.01 and p < 0.001, respectively.
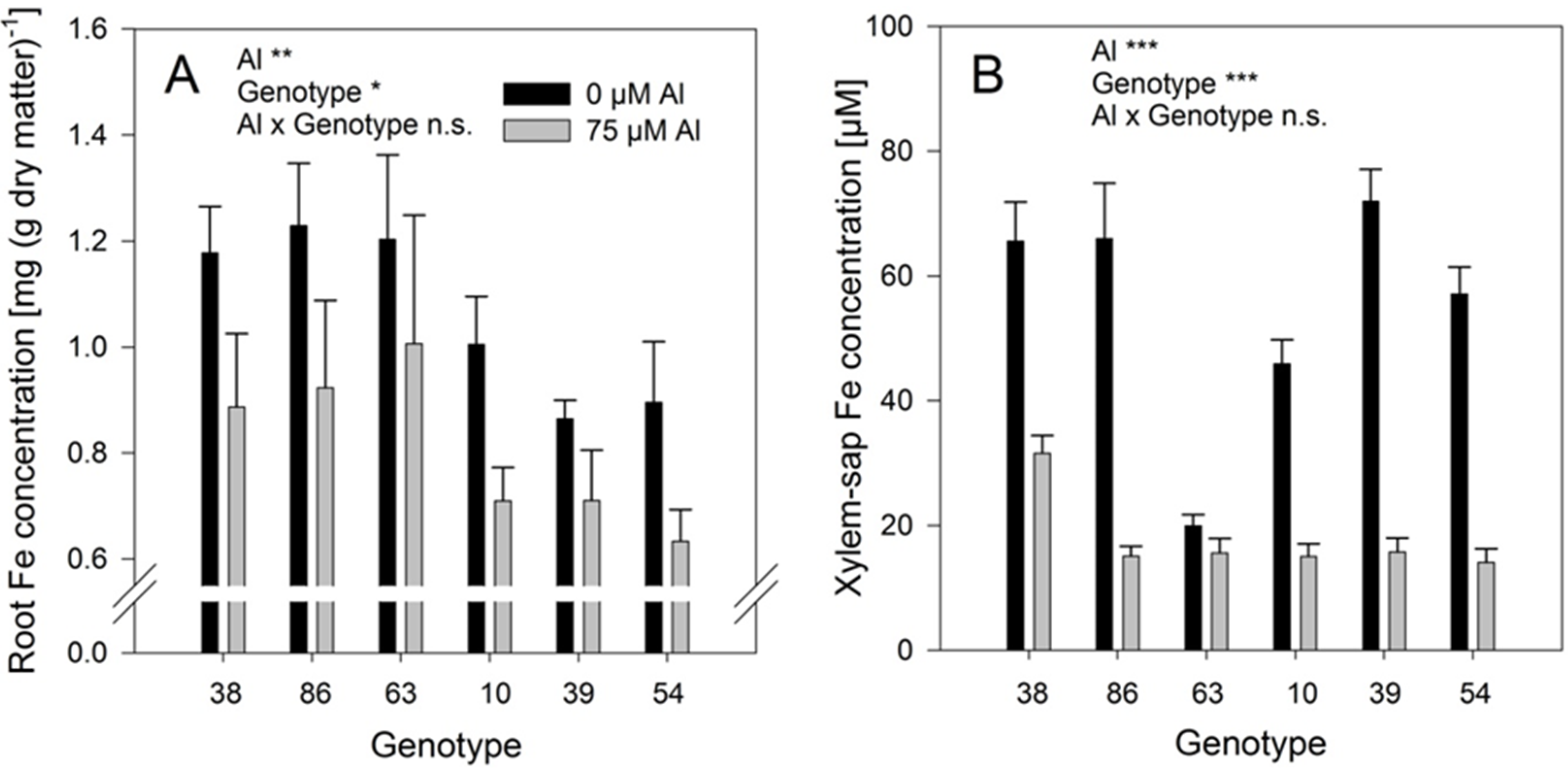
Al treatment significantly reduced the Fe concentrations in the roots (
Figure 7A) and even more in the xylem-sap (
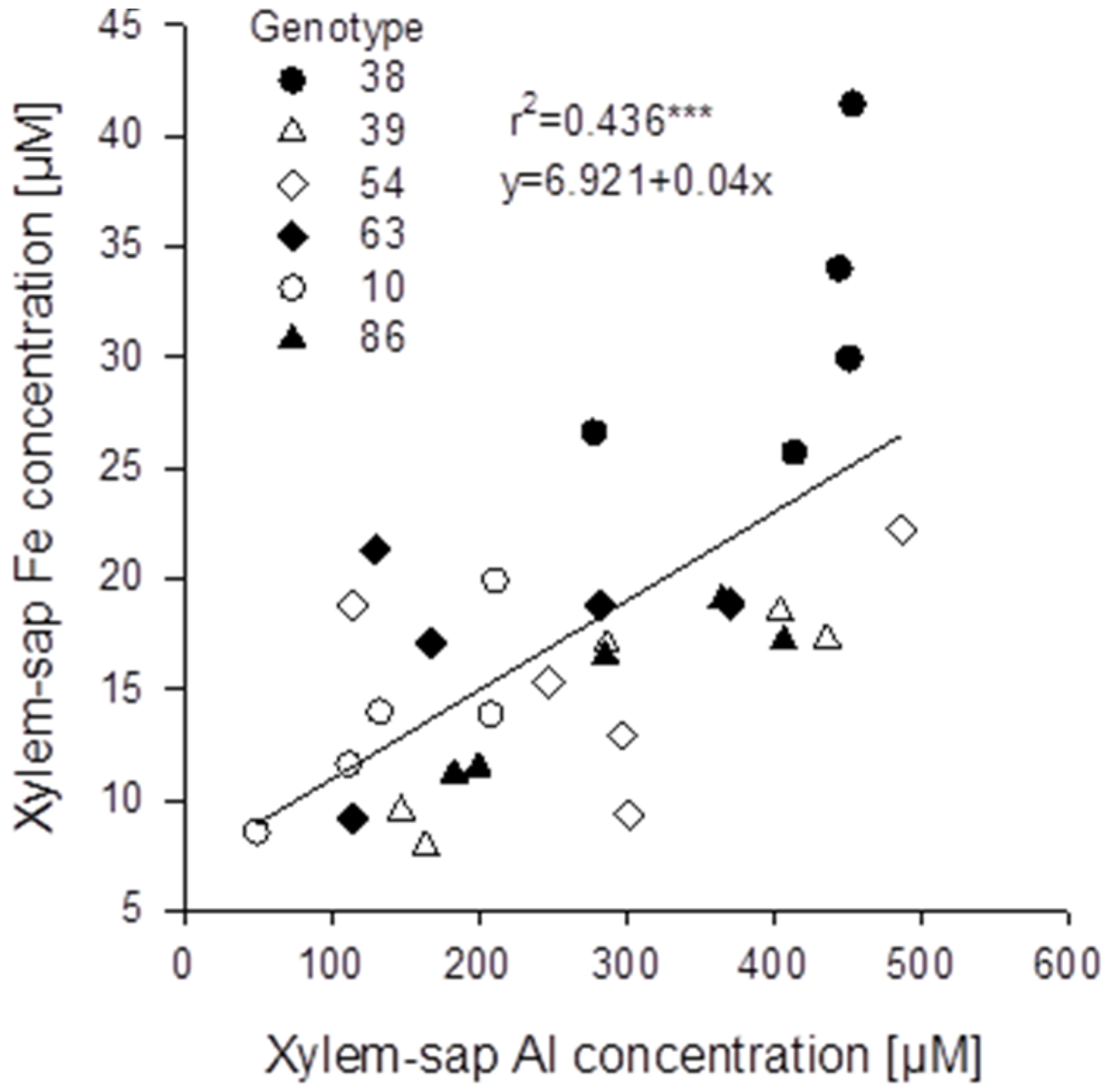
Figure 7B). The genotypes differed significantly in both, root and xylem-sap Fe concentrations, however, they responded comparably to Al treatment (no Al/genotype interaction). In spite of the clear negative effect of Al supply on the Fe root and xylem-sap concentrations the Al and Fe concentrations were highly significantly positively correlated across genotypes and Al treatments (
Figure 8) as in the substrate experiment (see above
Figure 5B).
Figure 7.
Iron concentrations of the bulk-root dry matter (A) and xylem-sap Fe concentrations (B) of six selected Fagopyrum esculentum genotypes as affected by Al supply. Plants were pre-cultured in complete nutrient solution containing at 60 μM Fe-EDDHA and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. Bars represent means ± SE, n = 3. For the ANOVA, *, **, and *** denote significant effects at p < 0.05, 0.01 and 0.001, respectively, n.s. non-significant.
Figure 7.
Iron concentrations of the bulk-root dry matter (A) and xylem-sap Fe concentrations (B) of six selected Fagopyrum esculentum genotypes as affected by Al supply. Plants were pre-cultured in complete nutrient solution containing at 60 μM Fe-EDDHA and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. Bars represent means ± SE, n = 3. For the ANOVA, *, **, and *** denote significant effects at p < 0.05, 0.01 and 0.001, respectively, n.s. non-significant.
Figure 8.
Relationship between xylem-sap Al and Fe concentrations of six selected Fagopyrum esculentum genotypes (n = 5). Plants were pre-cultured in complete nutrient solution containing 60 μM Fe-EDDHA and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. *** denote significance of the regression coefficient at p < 0.001.
Figure 8.
Relationship between xylem-sap Al and Fe concentrations of six selected Fagopyrum esculentum genotypes (n = 5). Plants were pre-cultured in complete nutrient solution containing 60 μM Fe-EDDHA and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. *** denote significance of the regression coefficient at p < 0.001.
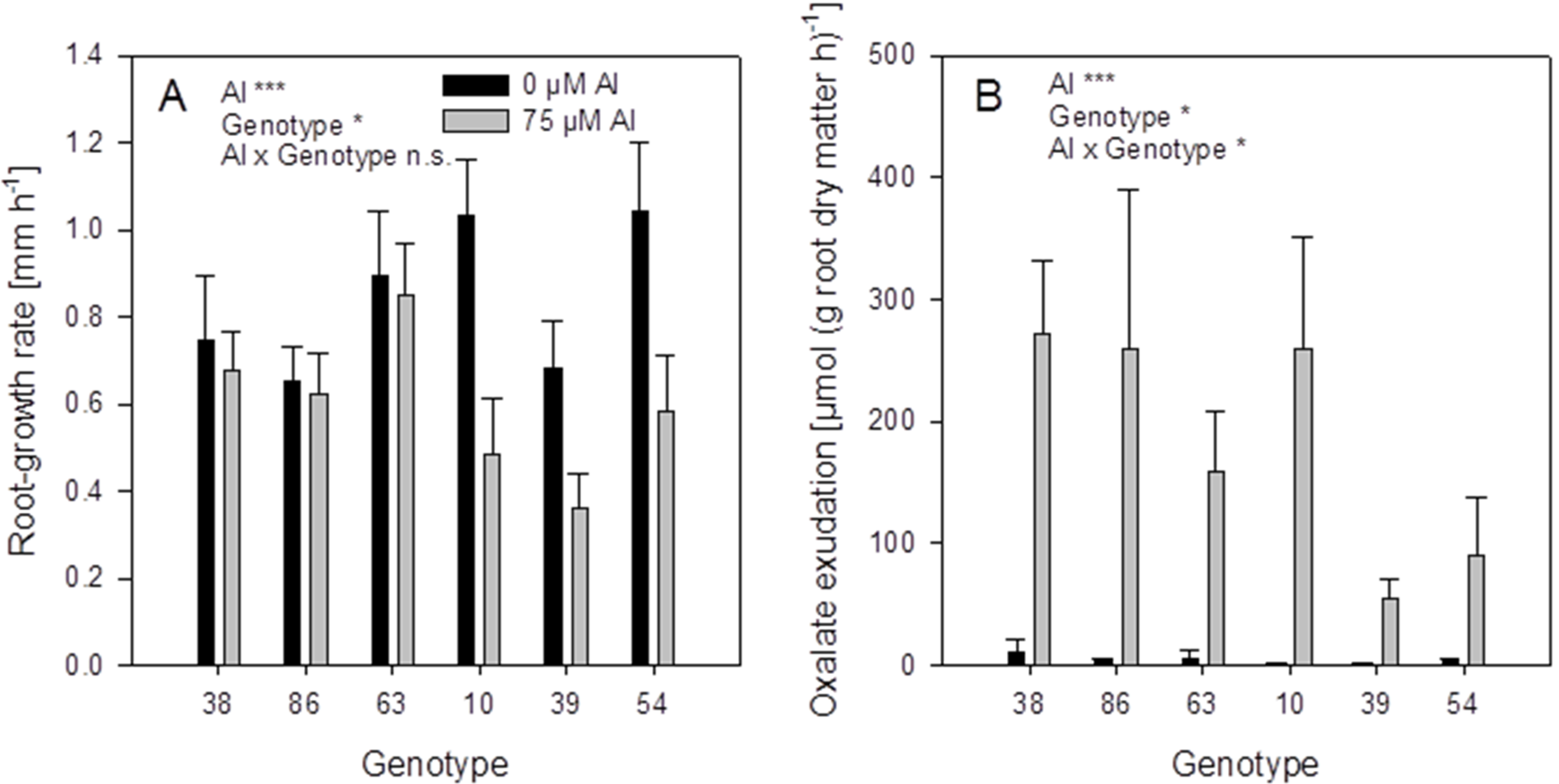
The root-growth rates of the controls not treated with Al were in the range of 0.7–1 mm·h
−1 (
Figure 9A). Aluminium supply (75 μM) decreased the root growth in some genotypes. The genotypes 38, 86, and 63 showed no root-growth inhibition (thus can be classified as Al-resistant), while the genotypes 10, 39, and 54 were inhibited by 30%–50% (thus can be classified as Al-sensitive).
Figure 9.
Root-growth rate (A) and oxalate exudation-rate (B) of six selected buckwheat cultivars. Plants were pre-cultured in complete nutrient solution and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. Root growth-rate was determined by marking the root 15 mm behind the tip before the treatment and measuring the distance again after 24 h. Root oxalate exudation rate was determined during the first 4 h of Al treatment. Bars represent means ± SE, n = 3. For the ANOVA, *, ** and *** denote significant effects at p < 0.05, 0.01 and 0.001, respectively.
Figure 9.
Root-growth rate (A) and oxalate exudation-rate (B) of six selected buckwheat cultivars. Plants were pre-cultured in complete nutrient solution and then transferred for 24 h to simplified nutrient solution without or with 75 μM Al. Root growth-rate was determined by marking the root 15 mm behind the tip before the treatment and measuring the distance again after 24 h. Root oxalate exudation rate was determined during the first 4 h of Al treatment. Bars represent means ± SE, n = 3. For the ANOVA, *, ** and *** denote significant effects at p < 0.05, 0.01 and 0.001, respectively.
Root oxalate exudation, was significantly activated by Al application in all genotypes (
Figure 9B). Citrate was exuded in minor amounts and showed no Al-activated exudation pattern). The Al-resistant genotypes 38 and 86 showed significantly higher oxalate exudation rates than the Al-sensitive genotypes 39 and 54. However, the genotypes 63 and 10 showed lower or higher oxalate exudation-rates, respectively, than could be expected from their Al resistance classification.
3. Discussion
The main focus of this study was to determine differences in Al accumulation in the shoots among a large number of genotypes across three
Fagopyrum species. The comparison revealed that all tested species and genotypes accumulated Al in the shoot, thus no qualitative differences were found. In a recent study comparing Al tolerance and Al accumulation of three
Fagopyrum species (
F. esculentum,
F. tataricum,
F. homotropicum) reference [
27] also concluded that Al accumulation is a conserved trait in
Fagopyrum. However, there were significant quantitative differences in Al accumulation capability. Genotypes differed in xylem Al concentration by a factor of about four (
Figure 4). The xylem-sap Al concentration was chosen as the primary parameter for the comparison of the genotypes for Al accumulation capacity. The xylem sap was sampled after only 0.5 h, because various studies showed that only shortly after cutting of the shoot representative data on the
in vivo xylem-sap composition can be assessed [
27]. Moreover, the same technique has been used for the characterization of heavy metal hyper-accumulation. Xylem-sap Cd and Zn concentrations were used to show a considerable scope for the selection of advanced-hyper-accumulating ecotypes with the objective of increasing phyto-extraction efficiency and remediation of metal-contaminated soils [
28]. The Al-accumulation trait could not be related to the geographic origin of the genotypes. The genotypes with the highest Al concentration in the xylem sap originate from different regions as for example Belarus (genotype 15), Iran (genotype 38), North Korea (genotype 73), and Italy (genotype 86).
A similar range of quantitative differences in Al accumulation has also been found within the family of
Melastomataceae [
29]. Species from this family accumulated Al in great amounts in the shoot. However, the variation within the family was shown to be in the range of 6–66 mg Al·g
−1 dry matter. In contrast, a comparison of members of the taxa
Polygonaceae, of which the genus
Fagopyrum is a member, showed that some species differing in Al resistance did not accumulate Al in their shoots [
30]. This might suggest that the trait of Al accumulation is not spread over the whole family of the
Polygonaceae and is rather typical for the genus
Fagopyrum. Here, this genus showed only quantitative but not qualitative differences in Al accumulation. Based on semi-quantitative tests reference [
29], mapped a recent angiosperm phylogeny for the trait of Al accumulation. As indicated by references [
15,
31] this classification might suggest that Al hyper-accumulation is a simple, primitive trait that has arisen independently several times during evolution but was lost independently in many derived taxa. These authors suggest that the trait of Al accumulation shows low incidence in evolutionary advanced groups which appeared to be correlated with the herbaceous habit. Therefore, the herbaceous genus
Fagopyrum might represent an exceptional case.
An unexpected result of the study was the confirmation of an interrelationship between Al and Fe transport in buckwheat. Across all
Fagopyrum genotypes studied xylem-sap Al and Fe concentrations were highly significantly positively correlated (
Figure 5B). A similar relationship could also be found in the hydroponic experiment with six selected
F. esculentum genotypes (
Figure 8). This was unexpected, because Al supply strongly reduced root Fe concentrations (
Figure 7A) and xylem-sap Fe concentrations (
Figure 7B). Since during the 24h Al treatment period in simplified nutrient solution no Fe was applied, this cannot be explained by inhibition of Fe uptake by Al. It rather appears that the Al-induced release of oxalate from the root ([
18],
Figure 9B) mobilizes apoplastically immobilized Fe. It has been reported that a large amount of Fe may be bound in the root apoplast which may be mobilized under conditions of Fe deficiency through Fe deficiency-induced release of Fe complexors [
32]. However, the mobilization of apoplastic Fe during the Al treatment period did not lead to higher Fe uptake and translocation in the xylem. It thus appears that Fe-oxalate is not readily taken up and loaded into the xylem, where, in fact, Fe is transported as citrate complex [
33,
34].
Using NMR, reference [
35] showed that in buckwheat also Al is complexed and detoxified by citrate anions in the xylem sap, and reference [
23] confirmed the dominance of Al-citrate as the main Al transport form not only in
F. esculentum but also in
F. tataricum and the wild buckwheat
F. homotropicum. In the study by reference [
35] the xylem citrate-concentration did not respond to externally applied or internally transported Al. These results are in agreement with the analysis of the effect of Al on the global transcriptome in
F. esculentum [
36] and
F. tataricum [
37] root tips which did not show major effects on genes involved in organic acid metabolism suggesting that citrate was not a rate-limiting step for Al transport into and in the xylem. However, in these studies a major difference in citrate concentration and synthesis between the root cortex and the central cylinder has not been taken into consideration. This assumption is supported by own data on the oxalate and citrate contents in surgically partitioned cortical and stele tissues [
25]. In contrast, in our study a significant positive correlation between xylem-sap citrate and Al concentrations existed when the Al supply to substrate grown buckwheat cultivar Lifago was increased (
Figure 3B) and across the
Fagopyrum genotypes (
Figure 5A) confirming previous results by references [
24,
25]. Also in the Al accumulator
H. macrophylla Al and citrate xylem-sap concentrations were positively correlated [
38]. However, only a low percentage of the variation of the citrate concentration could be explained by the Al concentration (
r2 = 0.23 ***), and citrate was more abundant than Al by a factor of ten (
Figure 5A). Iron is known to be transported in the xylem coupled to citrate which is loaded into the xylem through MATE/FRD-proteins coded by
FRD3 in Arabidopsis [
39],
OsFRDL1 in rice [
40] or
HvAACT1 in barley [
41]. The above mentioned recent global transcriptome analysis of Al-induced genes in root tips clearly revealed that among the strongly Al up-regulated genes were the homologues of these genes,
FtFRDL1 and
FtFRDL2 in
F. tataricum [
37] and
FeMATE1 and
FeMATE2 in
F. esculentum [
36]. Their role in citrate and Al loading into the xylem remains to be investigated in the future.
It is particularly important to clarify whether Al (and Fe) is loaded into the xylem in a co-transport or as the negatively charged Al-citrate complex as has been postulated by reference [
25]. It is intriguing that the genotypic differences in xylem-sap Al concentrations in the presence of Al are closely related to the Fe concentrations in the absence of Al (compare
Figure 6A and
Figure 7B). Together with the strong reduction of Fe xylem-sap concentrations by Al (
Figure 7B) this may indicate that both Al and Fe are transported through the same transporter, and Fe transport is competitively inhibited by the more abundant Al (factor ten). The positive correlation between xylem-sap Al and Fe concentrations may then reflect the Al-enhanced transporter abundance in agreement with the higher expression of the genes coding for these transporters [
36,
37].
The six
F. esculentum genotypes selected for differences in Al xylem-sap concentrations also showed great variability in Al-induced root-growth inhibition and thus Al resistance (
Figure 9A). The Al-induced root oxalate-exudation is a well-established Al resistance and Al exclusion mechanism (see Introduction). Among the six genotypes Al resistance was highly significantly positively correlated with Al-induced root oxalate-exudation (
Table 1). However, oxalate exudation explained only about 50% of the variation in Al resistance. Also shoot Al accumulation was highly significantly correlated with Al resistance indicating that Al accumulation and Al tolerance also contribute to overall Al resistance of buckwheat as has been suggested by reference [
25]. Evaluating the role of both oxalate exudation and Al accumulation in Al resistance using multiple regression analysis (
Table 1) revealed that more than 70% (
r2 = 0.725 ***) of the variation in Al-induced root-growth inhibition can be explained by these two variables. It thus appears that both Al exclusion by oxalate root-exudation and rapid oxalate-facilitated Al uptake and citrate-facilitated Al translocation to the shoot are involved in the protection of the root-tip apoplast, the main target of Al rhizotoxicity as elaborated by reference [
42]. The genes and proteins (particularly transporters involved in Al exclusion and Al tolerance/accumulation) have still not yet been identified. It is expected that recent transcriptomic studies will pave the way for a better understanding of the molecular basis of Al resistance of buckwheat [
36,
37,
43]. In the model plants Arabidopsis and rice, an interplay between Al exclusion by root exudation of organic acid anions and the rapid uptake and accumulation in the root vacuole has been widely established [
17,
44]. However, these plant species do not translocate Al to the shoots, a typical feature of Al accumulators such as
Fagopyrum.
Table 1.
Regression analysis of the relationships between relative root-growth rate (75 μM Al supply for 24 h in simplified nutrient solution compared to the 0 μM Al control) and root oxalate exudation or/and leaf Al concentration of six F. esculentum genotypes. *** denote the level of significance.
Table 1.
Regression analysis of the relationships between relative root-growth rate (75 μM Al supply for 24 h in simplified nutrient solution compared to the 0 μM Al control) and root oxalate exudation or/and leaf Al concentration of six F. esculentum genotypes. *** denote the level of significance.
| Variable parameters | Function of the regression | r2 | p value |
|---|
| Oxalate exudation vs. relative root-growth rate | y = 37.09 + 0.19x | 0.538 | <0.001 *** |
| Leaf Al accumulation vs. relative root-growth rate | y = −23.14 + 0.7x | 0.485 | <0.001 *** |
Multiple regression:
Oxalate exudation and leaf Al accumulation vs. relative root-growth rate | y = −18.534 + (0.139 · oxalate exudation) + (0.480 leaf Al accumulation) | 0.725 | <0.001 *** |