Candidate Gene Identification for a Lethal Chlorophyll-Deficient Mutant in Soybean
Abstract
:1. Introduction
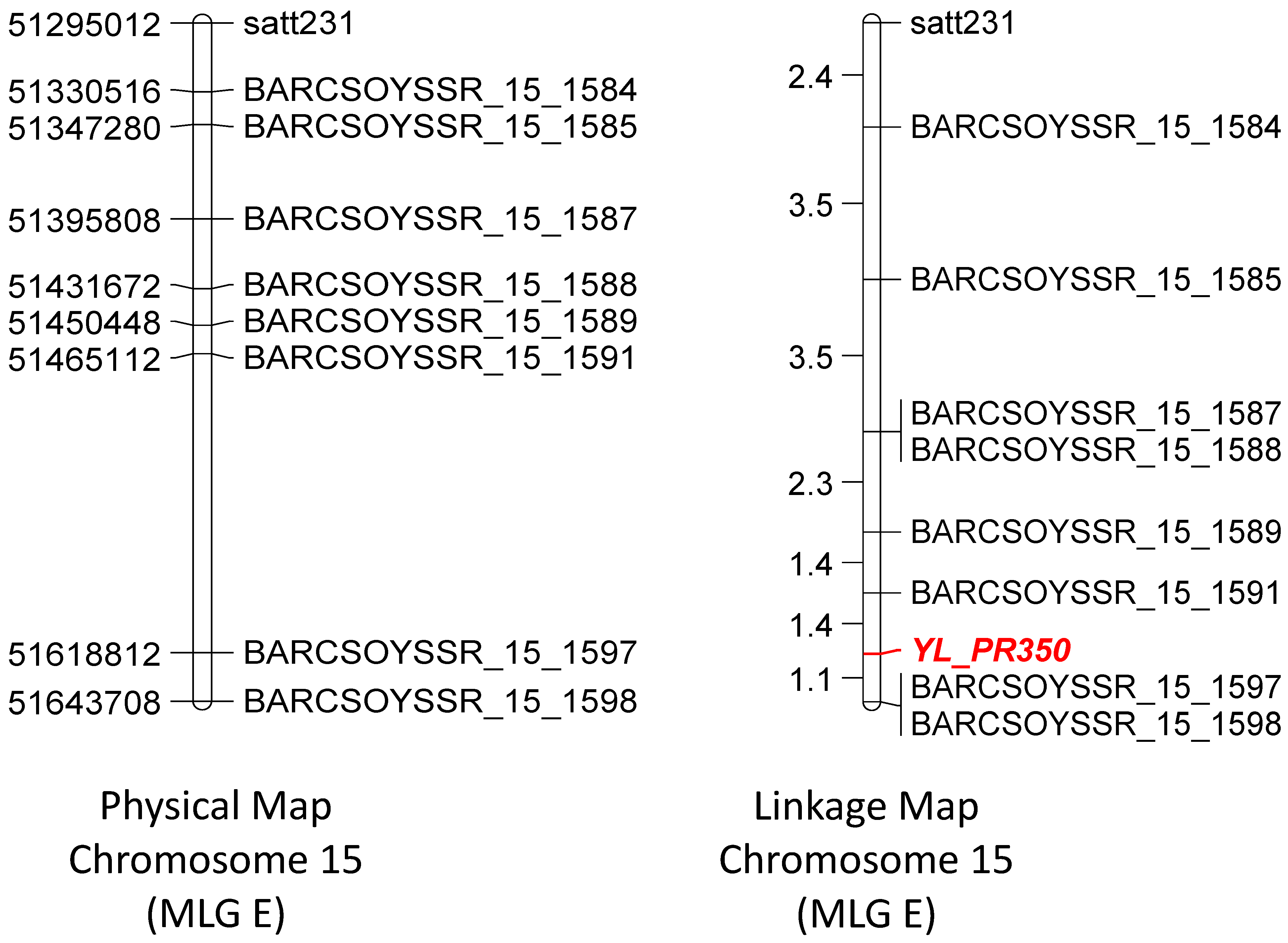
2. Results and Discussion
| Population | Gene | No. F2 plants | No. F2:3 families | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Green | Yellow | χ2 (3:1) | P | All Green | Segregating | χ2 (1:2) | P | ||
| PR350-1 | YL_PR350 | 137 | 36 | 1.62 | 0.20 | 38 | 94 | 1.23 | 0.27 |
| PR350-6 | YL_PR350 | 153 | 55 | 0.23 | 0.63 | 59 | 83 | 4.32 | 0.04 |
| Total | 290 | 91 | 0.25 | 0.62 | 97 | 177 | 0.53 | 0.47 | |
| Gene | Start Position (bp) | End Position (bp) | Predicted Protein/Function |
|---|---|---|---|
| Glyma.15g275700 | 51472288 | 51473210 | No functional annotation |
| Glyma.15g275800 | 51473359 | 51476124 | Unknown |
| Glyma.15g275900 | 51486036 | 51491942 | Biogenesis of Photosystem I and II and chloroplast ultrastructure |
| Glyma.15g276000 | 51495263 | 51502516 | DNA Gyrase/Topoisomerase IV, DNA binding, ATP binding |
| Glyma.15g276100 | 51510448 | 51513137 | Unknown (DUF642) |
| Glyma.15g276200 | 51533618 | 51534670 | Unknown |
| Glyma.15g276300 | 51541078 | 51546842 | Sterol methyltransferase C-terminal, steroid biosynthetic process |
| Glyma.15g276400 | 51549706 | 51555332 | Copper Amine oxidase |
| Glyma.15g276500 | 51564250 | 51573306 | Flavin containing amine oxidoreductase |
| Glyma.15g276600 | 51574389 | 51574676 | Wound induced protein |
| Glyma.15g276700 | 51578950 | 51593999 | Uncharacterized protein family, UPF0114 |
| Glyma.15g276800 | 51600225 | 51651653 | Mandelate racemase / muconate lactonizing enzyme, Thiamine pyrophosphate enzyme |
| Glyma.15g275600 | 51456041 | 51457063 | Unknown |
3. Experimental Section
3.1. Plant Material
3.2. DNA Isolation and Bulked Segregant Analysis (BSA)
3.3. Molecular Analysis
4. Conclusions
Acknowledgements
Author Contribution
Conflict of Interest
References and Notes
- Huang, X.Q.; Wang, P.R.; Zhao, H.X.; Deng, X.J. Genetic analysis and molecular mapping of a novel chlorophyll-deficit mutant gene in rice. Rice Sci. 2008, 15, 7–12. [Google Scholar] [CrossRef]
- Kato, K.K.; Palmer, R.G. Duplicate chlorophyll-deficient loci in soybean. Genome 2004, 47, 190–198. [Google Scholar] [CrossRef]
- Palmer, R.G.; Nelson, R.L.; Bernard, R.L.; Stelly, D.M. Genetics and linkage of three chlorophyll-deficient mutants in soybean: y19, y22, and y23. J. Hered. 1990, 81, 404–406. [Google Scholar]
- Palmer, R.G.; Burzlaff, J.D.; Shoemaker, R.C. Genetic analyses of two independent chlorophyll-deficient mutants identified among the progeny of a single chimeric foliage soybean plant. J. Hered. 2000, 91, 297–303. [Google Scholar] [CrossRef]
- Yue, B.; Cai, X.; Vick, B.; Hu, J. Genetic characterization and molecular mapping of a chlorophyll deficiency gene in sunflower (Helianthus annuus). J. Plant Physiol. 2009, 166, 644–651. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Han, S.; Zhang, X.; Yu, D. Identification and gene mapping of a soybean chlorophyll-deficient mutant. Plant Breed. 2011, 130, 133–138. [Google Scholar] [CrossRef]
- Zou, J.J.; Singh, R.J.; Hymowitz, T. Association of the yellow leaf (y10) mutant to soybean chromosome 3. J. Hered. 2003, 94, 352–355. [Google Scholar] [CrossRef]
- Eckhardt, U.; Grimm, B.; Hörtensteiner, S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 2004, 56, 1–14. [Google Scholar] [CrossRef]
- Fromme, P.; Melkozernov, A.; Jordan, P.; Krauss, N. Structure and function of photosystem I: Interaction with its soluble electron carriers and external antenna systems. FEBS Lett. 2003, 555, 40–44. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.; Hu, G.; Si, H.; Zhu, L.; Wu, C.; Sun, Z. Identification and fine mapping of a thermo-sensitive chlorophyll deficient mutant in rice (Oryza sativa L.). Planta 2007, 226, 785–795. [Google Scholar] [CrossRef]
- Jung, K.-H.; Hur, J.; Ryu, C.-H.; Choi, Y.; Chung, Y.-Y.; Miyao, A.; Hirochika, H.; An, G. Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol. 2003, 44, 463–472. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; He, B.; Diao, L.; Sheng, S.; Wang, J.; Guo, X.; Su, N.; Wang, L.; Jiang, L.; et al. A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol. 2007, 145, 29–40. [Google Scholar] [CrossRef]
- Palmer, R.G.; Pfeiffer, T.W.; Buss, G.R.; Kilen, T.C. Qualitative Genetics. In Soybeans: Improvement, Production, and Uses, 3rd ed, Agronomy Monograph no. 16; Boerma, H.R., Specht, J., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2004; pp. 137–233. [Google Scholar]
- Palmer, R.G.; Xu, M. Positioning 3 qualitative trait loci on soybean molecular linkage group E. J. Hered. 2008, 99, 674–678. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Available online: http://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Gmax (accessed on 28 October 2014).
- Barneche, F.; Winter, V.; Crevecoeur, M.; Rochaix, J.D. ATAB2 is a novel factor in the signalling pathway of light-controlled synthesis of photosystem proteins. EMBO J 2006, 25, 5907–5918. [Google Scholar] [CrossRef]
- Sandhu, D.; Gao, H.; Cianzio, S.; Bhattacharyya, M.K. Deletion of a disease resistance nucleotide-binding-site leucine-rich- repeat-like sequence is associated with the loss of the Phytophthora resistance gene Rps4 in soybean. Genetics 2004, 168, 2157–2167. [Google Scholar] [CrossRef]
- Michelmore, R.W.; Paran, I.; Kesseli, R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. USA 1991, 88, 9828–9832. [Google Scholar] [CrossRef]
- Song, Q.J.; Marek, L.F.; Shoemaker, R.C.; Lark, K.G.; Concibido, V.C.; Delannay, X.; Specht, J.E.; Cregan, P.B. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 2004, 109, 122–128. [Google Scholar] [CrossRef]
- Song, Q.J.; Jia, G.F.; Zhu, Y.L.; Grant, D.; Nelson, R.T.; Hwang, E.Y.; Hyten, D.L.; Cregan, P.B. Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop Sci. 2010, 50, 1950–1960. [Google Scholar] [CrossRef]
- Lander, E.S.; Green, P.; Abrahamson, J.; Barlow, A.; Daly, M.J.; Lincoln, S.E.; Newburg, L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1987, 1, 174–181. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distance from recombination values. Ann. Eugen. 1944, 12, 172–175. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reed, S.; Atkinson, T.; Gorecki, C.; Espinosa, K.; Przybylski, S.; Goggi, A.S.; Palmer, R.G.; Sandhu, D. Candidate Gene Identification for a Lethal Chlorophyll-Deficient Mutant in Soybean. Agronomy 2014, 4, 462-469. https://doi.org/10.3390/agronomy4040462
Reed S, Atkinson T, Gorecki C, Espinosa K, Przybylski S, Goggi AS, Palmer RG, Sandhu D. Candidate Gene Identification for a Lethal Chlorophyll-Deficient Mutant in Soybean. Agronomy. 2014; 4(4):462-469. https://doi.org/10.3390/agronomy4040462
Chicago/Turabian StyleReed, Sam, Taylor Atkinson, Carly Gorecki, Katherine Espinosa, Sarah Przybylski, Alcira Susana Goggi, Reid G. Palmer, and Devinder Sandhu. 2014. "Candidate Gene Identification for a Lethal Chlorophyll-Deficient Mutant in Soybean" Agronomy 4, no. 4: 462-469. https://doi.org/10.3390/agronomy4040462





