Directional Alignment of Polyfluorene Copolymers at Patterned Solid-Liquid Interfaces
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Measurement Methods
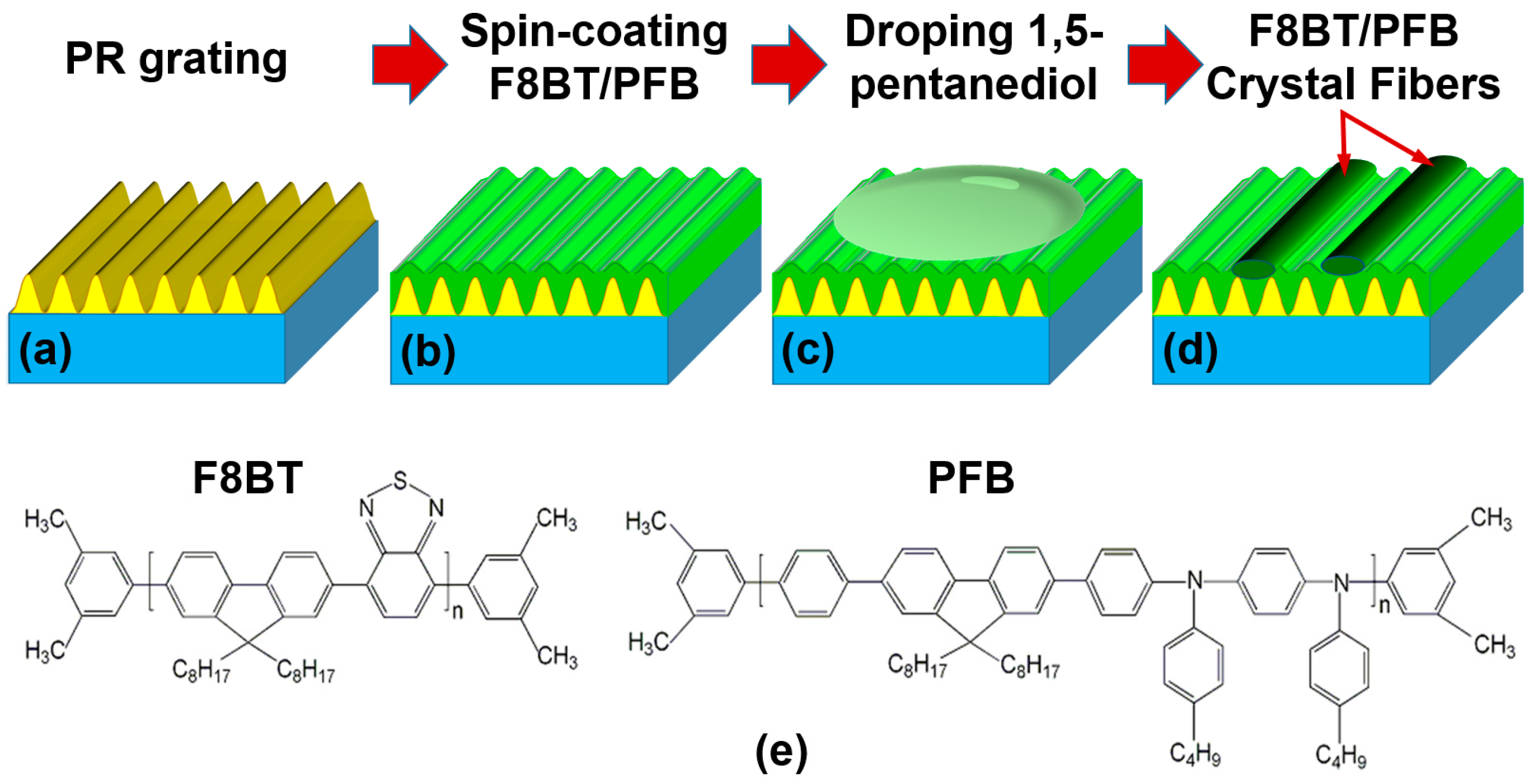
2.2. Preparation of the Directionally-Aligned Polymer Fibers
2.3. Device Fabrication and Characterization
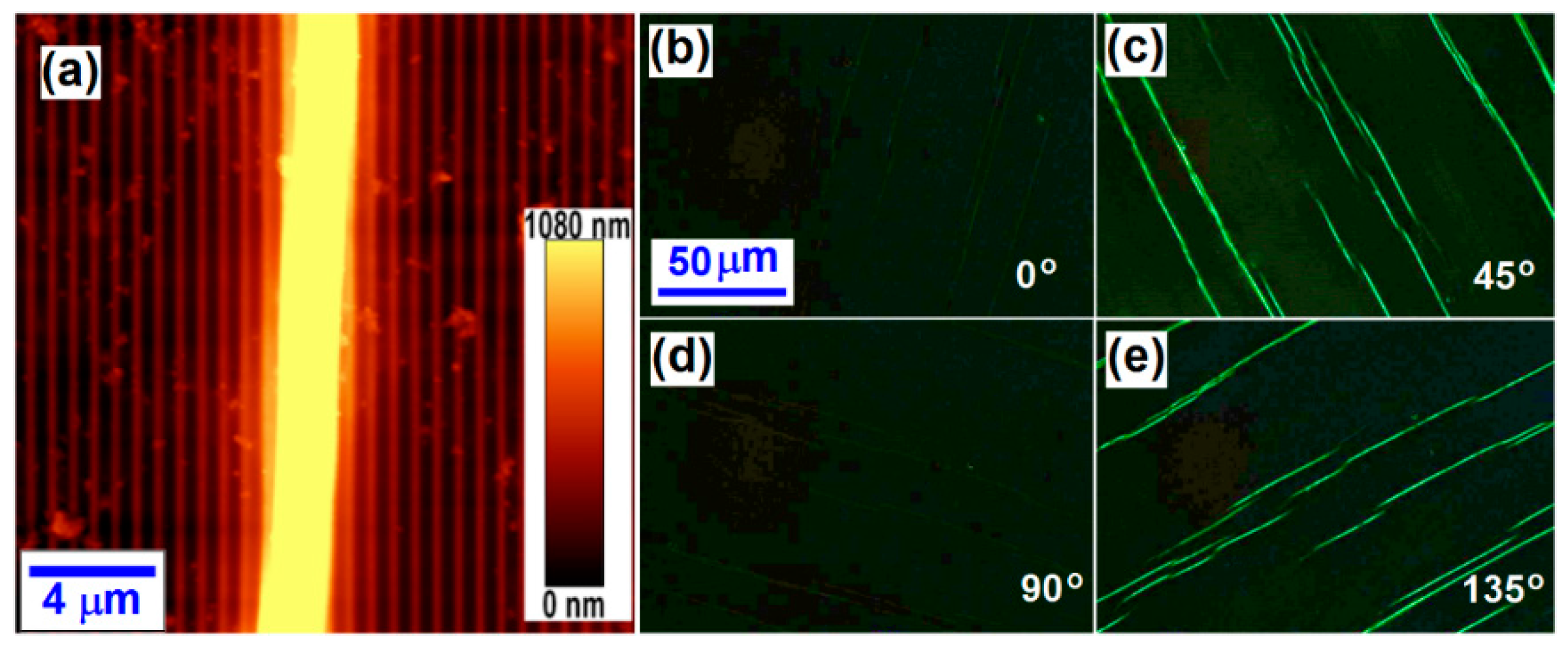
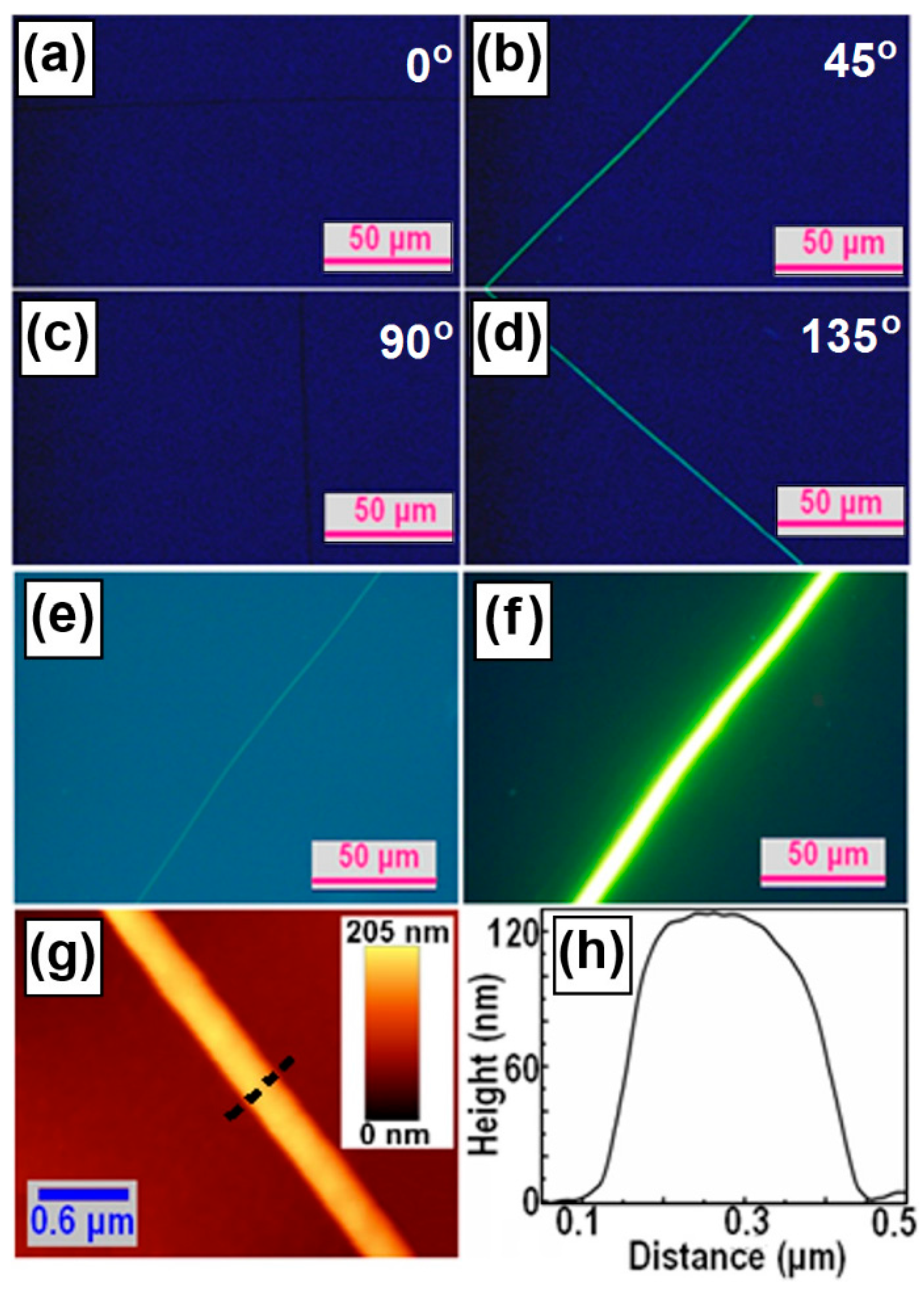
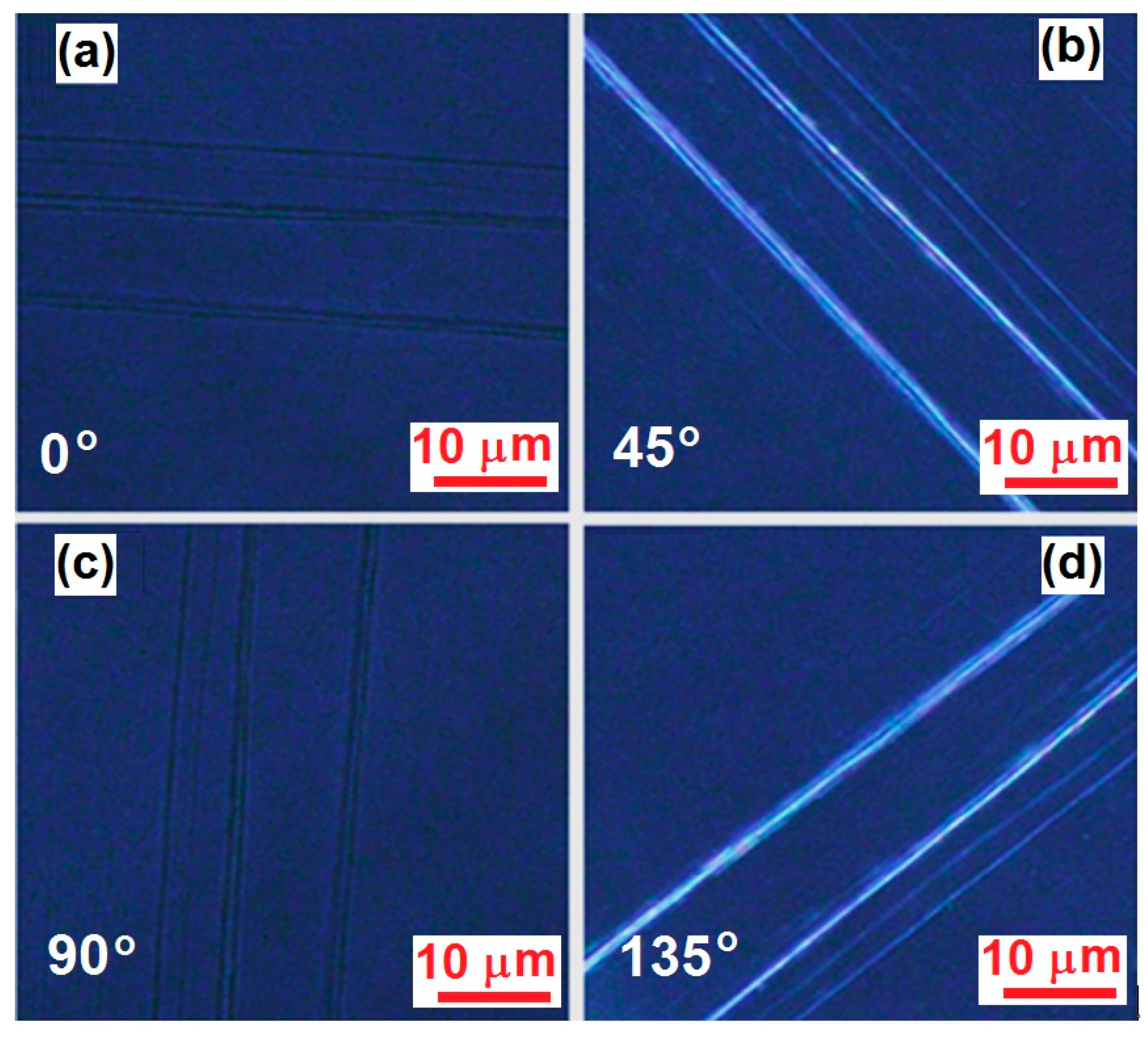
3. Microscopic Characterization
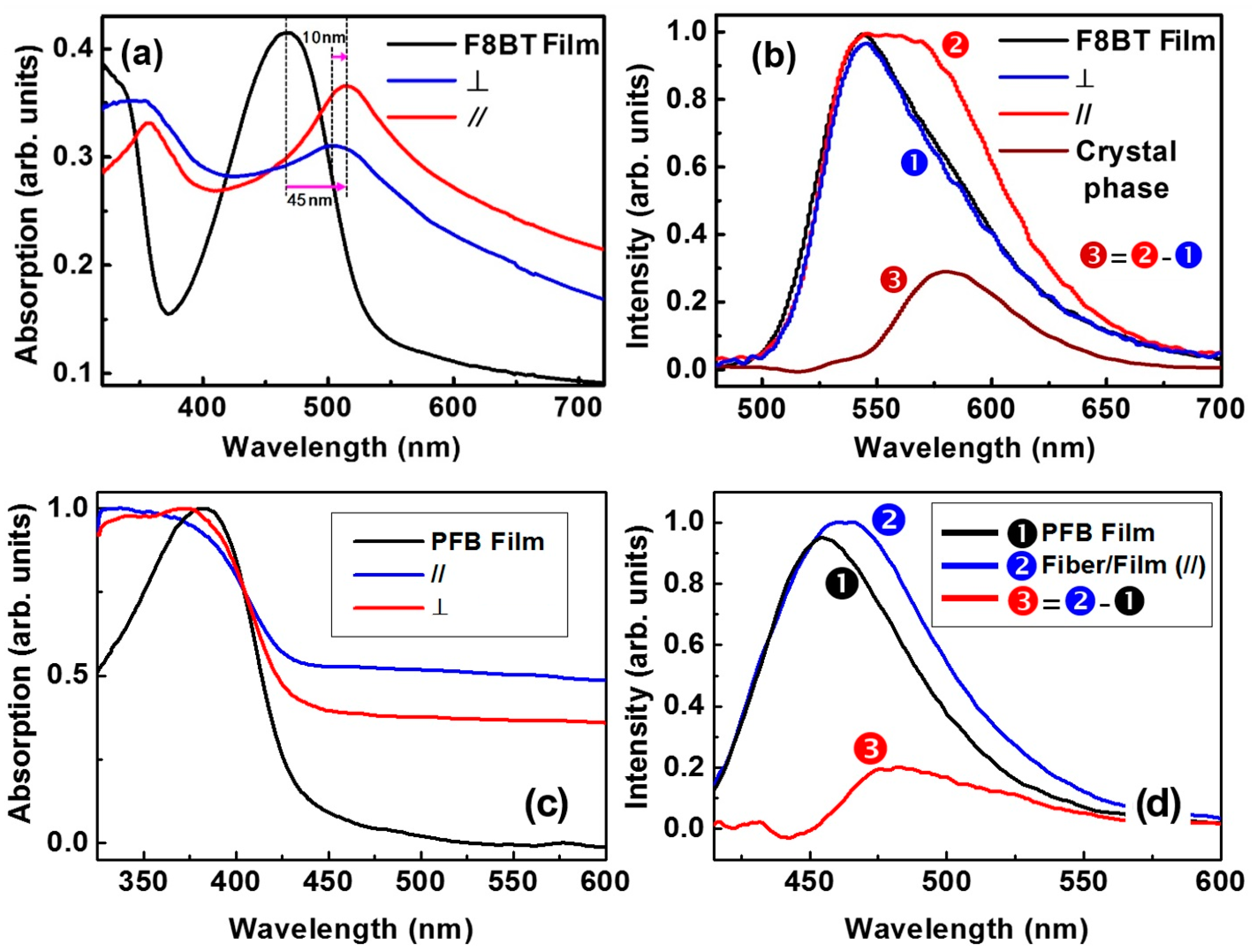
4. Spectroscopic Characterization
5. Photoconductivity Characterization
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Kumashiro, R.; Li, Z.F.; Nouchi, R.; Tanigaki, K. Light Emitting Ambipolar Field-effect Transistors of 2,5-bis(4-biphenyl)bithiophene Single Crystals with Anisotropic Carrier Mobilities. Appl. Phys. Lett. 2009, 95, 103306. [Google Scholar] [CrossRef]
- Singh, M.K.; Kumar, A.; Prakash, R. Self-assembly of Regioregular Poly (3, 3000-didodecylquarterthiophene) in Chloroform and Study of its Junction Properties. Mater. Sci. Eng. B 2017, 217, 12–17. [Google Scholar] [CrossRef]
- Li, X.J.; Xu, Y.X.; Li, F.; Ma, Y.G. Organic Light-emitting Diodes Based on an Ambipolar Single Crystal. Org. Electron. 2012, 13, 762–766. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Z.Q.; Shen, J.C.; Ma, Y.G. Progress on the Optoelectronic Functional Organic Crystals. Sci. China Ser. B 2007, 50, 433–452. [Google Scholar] [CrossRef]
- Shinde, D.B.; Salunke, J.K.; Candeias, N.R.; Tinti, F.; Gazzano, M.; Wadgaonkar, P.P.; Priimagi, A.; Camaioni, N.; Vivo, P. Crystallisation-enhanced Bulk Hole Mobility in Phenothiazine-based Organic Semiconductors. Sci. Rep. 2017, 7, 46268. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lim, B.T.; Yoon, S.B.; Choi, H.J.; Ha, J.U.; Chung, D.S.; Lee, S.G. A Small Molecule Composed of Anthracene and Thienothiophene Devised for High-performance Optoelectronic Applications. Dyes Pigments 2015, 120, 30–36. [Google Scholar] [CrossRef]
- Ozdemir, M.; Choi, D.; Kwon, G.; Zorlu, Y.; Cosut, B.; Kim, H.; Facchetti, A.; Kim, C.; Usta, H. Solution-Processable BODIPY-Based Small Molecules for Semiconducting Microfibers in Organic Thin-Film Transistors. Appl. Mater. Interfaces 2016, 8, 14077–14087. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, R.; Elschner, C.; Weil, M.; Uhrich, C.; Körner, C.; Riede, M.; Leo, K.; Pfeiffer, M.; Reinold, E.; Osteritz, E.M.; et al. Interrelation between Crystal Packing and Small-Molecule Organic Solar Cell Performance. Adv. Mater. 2012, 24, 675–680. [Google Scholar] [CrossRef]
- Chou, S.H.; Kang, H.W.; Chang, S.T.; Wu, K.Y.; Bazan, G.C.; Wang, C.L.; Lin, H.L.; Chang, J.H.; Lin, H.W.; Huang, Y.C.; et al. Cofacial Versus Coplanar Arrangement in Centrosymmetric Packing Dimers of Dipolar Small Molecules: Structural Effects on the Crystallization Behaviors and Optoelectronic Characteristics. Appl. Mater. Interfaces 2016, 8, 18266–18276. [Google Scholar] [CrossRef]
- Sun, J.P.; Hendsbee, A.D.; Eftaiha, A.F.; Macaulay, C.; Rutledge, L.R.; Welch, G.C.; Hill, I.G. Phthalimide–thiophene-based Conjugated Organic Dmall Molecules with High Electron Mobility. J. Mater. Chem. C 2014, 2, 2612–2621. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, G.H.; Lin, Y.; Su, Z.H.; Wang, Q. Self-assembly of Large-scale P3HT Patterns by Confined Evaporation in the Capillary Tube. RSC Adv. 2015, 5, 20491–20497. [Google Scholar] [CrossRef]
- Zhao, K.; Ding, Z.; Xue, L.; Han, Y. Crystallization-Induced Phase Segregation Based on Double-Crystalline Blends of Poly(3-hexylthiophene) and Poly(ethylene glycol)s. Macromol. Rapid Commun. 2010, 31, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Chang, M.; Reichmanis, E. Controlled Assembly of Poly (3-hexylthiophene): Managing the Disorder to Order Transition on the Nano- through Meso-Scales. Adv. Funct. Mater. 2015, 25, 920–927. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled Synthesis and Energy Applications of One-Dimensional Conducting Polymer Nanostructures: An Overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Wang, S.; Qu, Y.; Li, S.; Ye, F.; Chen, Z.; Yang, X. Improved Thermal Stability of Polymer Solar Cells by Incorporating Porphyrins. Adv. Funct. Mater. 2015, 25, 748–757. [Google Scholar] [CrossRef]
- Di, D.; Yang, L.; Richter, J.M.; Meraldi, L.; Altamimi, R.M.; Alyamani, A.Y.; Credgington, D.; Musselman, K.P.; MacManus-Driscoll, J.L.; Friend, R.H. Efficient Triplet Exciton Fusion in Molecularly Doped Polymer Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605987. [Google Scholar] [CrossRef] [PubMed]
- Nikolka, M.; Nasrallah, I.; Rose, B.; Ravva, M.K.; Broch, K.; Sadhanala, A.; Harkin, D.; Charmet, J.; Hurhangee, M.; Brown, A.; et al. High Operational and Environmental Stability of High-mobility Conjugated Polymer Field-effect Transistors through the Use of Molecular Additives. Nat. Mater. 2017, 16, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.P. Ultrafast Injection-locked Amplification in a Thin-film Distributed Feedback Microcavity. Nanoscale 2017, 9, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.R.; Xu, Z.Y.; Li, S.T.; Zhang, X.P. Red-green-blue Plasmonic Random Laser. Opt. Express 2017, 25, 2100–2106. [Google Scholar] [CrossRef]
- Liu, L.H.; Wu, K.Q.; Ding, J.Q.; Zhang, B.H.; Xie, Z.Y. Binary Solvent Mixture-induced Crystallization Enhancement for a White Emissive Polyfluorene Copolymer toward Improving Its Electroluminescence. Polymer 2013, 54, 6236–6241. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uda, H.; Nagase, T.; Watanabe, M.; Matsukawa, K.; Naito, H. Correlation between the Crystallization of Polyfluorene and the Surface Free Energy of Substrates. Thin Solid Films 2008, 517, 1340–1342. [Google Scholar] [CrossRef]
- Brinkmann, M. Directional Epitaxial Crystallization and Tentative Crystal Structure of Poly (9,9¢-di-n-octyl-2,7-fluorene). Macromolecules 2007, 40, 7532–7541. [Google Scholar] [CrossRef]
- Whitehead, K.S.; Grell, M.; Bradley, D.D.C.; Inbasekaran, M.; Woo, E.P. Polarized Emission from Liquid Crystal Polymers. Synth. Met. 2000, 111–112, 181–185. [Google Scholar] [CrossRef]
- Hinoue, T.; Shigenoi, Y.; Sugino, M.; Mizobe, Y.; Hisaki, I.; Miyata, M.; Tohnai, N. Regulation of π-Stacked Anthracene Arrangement for Fluorescence Modulation of Organic Solid from Monomer to Excited Oligomer Emission. Chem. Eur. J. 2012, 18, 4634–4643. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Hurkes, N.; Kaiser, A.; Wielandt, W. π-Stacking Modulates the Luminescence of [(dppz)Ni(Mes)Br] (dppz = dipyrido[3,2-a:2′,3′-c]phenazine, Mes = 2,4,6-trimethylphenyl). Zeitschrift für Anorganische und Allgemeine Chemie 2007, 633, 1659–1665. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kobayashi, N. Synthesis and Self-Association, Absorption, and Fluorescence Properties of Differentially Functionalized Hexakis(p-substituted phenylethynyl)benzenes. J. Org. Chem. 2004, 69, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.R.; Müller, P.; Tanski, J.M. Crystal Structure of 3-bromo-2-hydroxybenzonitrile. Acta Cryst. 2015, 71, 0523–0524. [Google Scholar] [CrossRef] [PubMed]
- Ko1nig, O.; Bü1rgi, H.B.; Armbruster, T.; Hulliger, J.; Weber, T. A Study in Crystal Engineering: Structure, Crystal Growth, and Physical Properties of a Polar Perhydrotriphenylene Inclusion Compound. J. Am. Chem. Soc. 1997, 119, 10632–10640. [Google Scholar] [CrossRef]
- Funasako, Y.; Mochida, T.; Yoza, K. Order-disorder Phase Transition with Associated Cell-tripling in the (octamethylferrocene)(2,3-dichloro-1,4-naphthoquinone)2 Charge-transfer Complex. J. Organomet. Chem. 2012, 698, 49–52. [Google Scholar] [CrossRef]
- Zhang, X.P.; Sun, B.Q. Organic Crystal Fibers Aligned into Oriented Bundles with Polarized Emission. J. Phys. Chem. B 2007, 111, 10881–10885. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Li, H.; Zhang, X. Directional Alignment of Polyfluorene Copolymers at Patterned Solid-Liquid Interfaces. Polymers 2017, 9, 356. https://doi.org/10.3390/polym9080356
Pan X, Li H, Zhang X. Directional Alignment of Polyfluorene Copolymers at Patterned Solid-Liquid Interfaces. Polymers. 2017; 9(8):356. https://doi.org/10.3390/polym9080356
Chicago/Turabian StylePan, Xiaolu, Hongwei Li, and Xinping Zhang. 2017. "Directional Alignment of Polyfluorene Copolymers at Patterned Solid-Liquid Interfaces" Polymers 9, no. 8: 356. https://doi.org/10.3390/polym9080356




