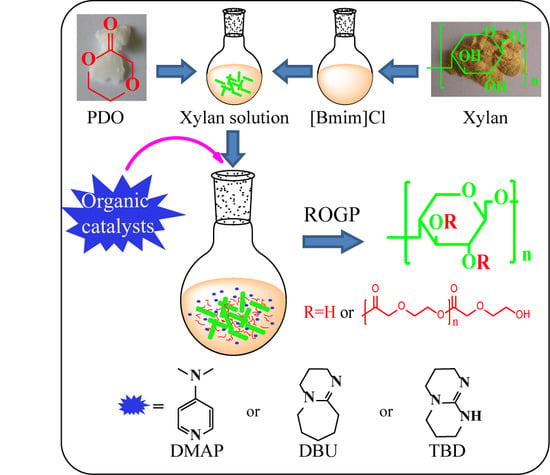
Organic Catalysis for Ring-Opening Graft Polymerization of p-Dioxanone with Xylan in Ionic liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
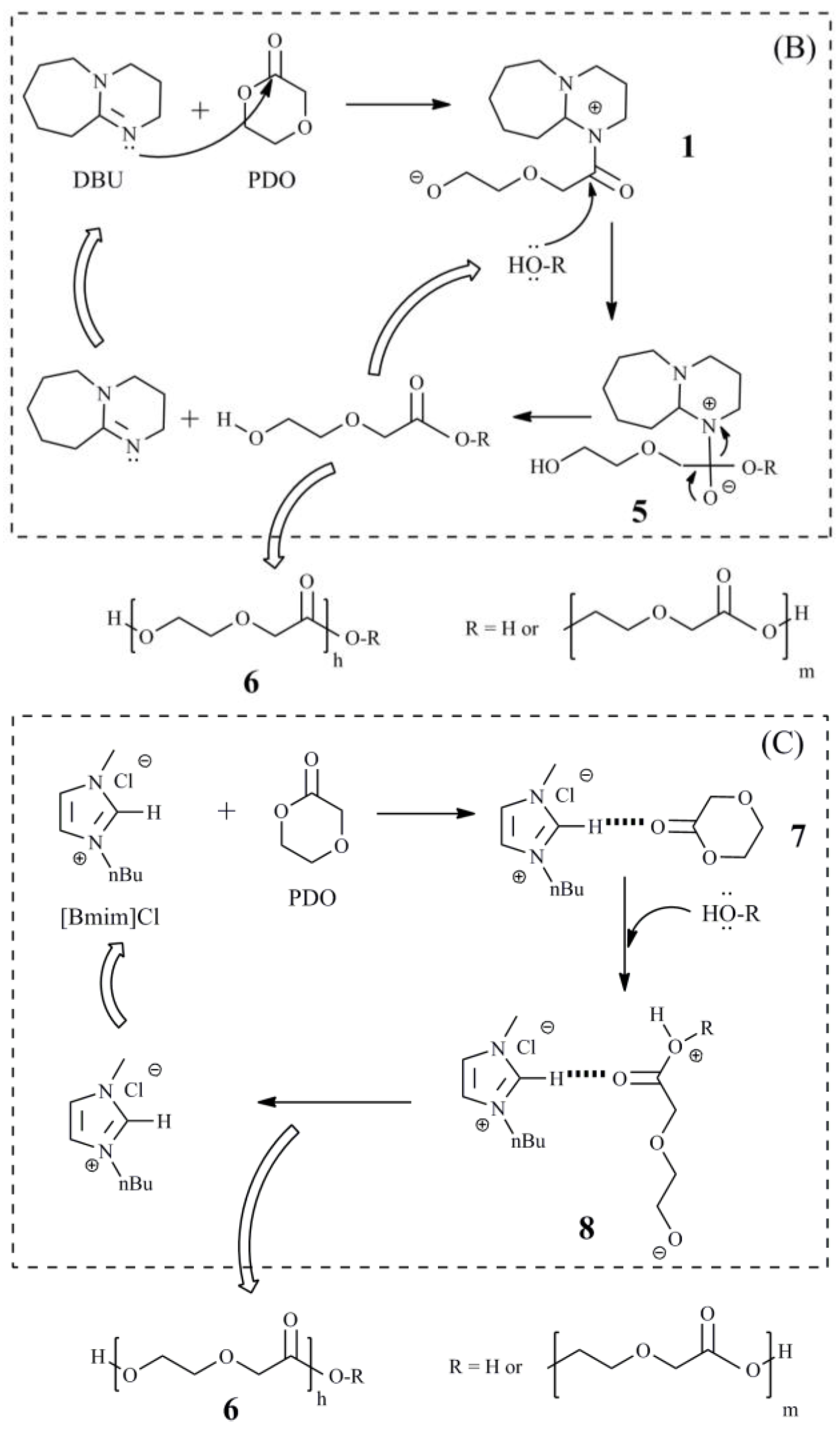
2.2. Synthesis of Xylan-g-PPDO Copolymers
2.3. Solubility Behavior
2.4. Characterization
3. Results
3.1. The Effects of Reaction Conditions on the Grafting Efficiency
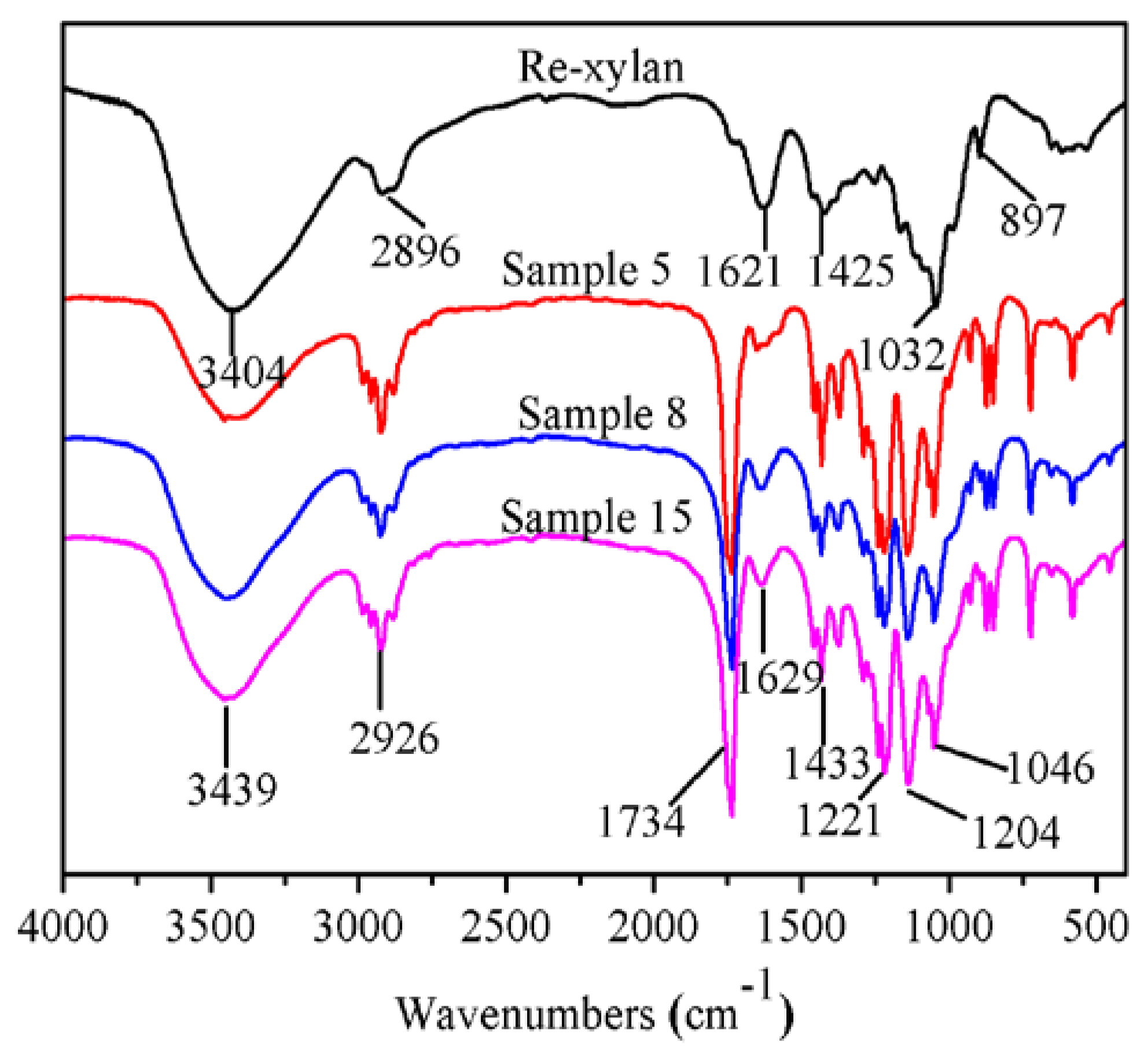
3.2. FT-IR Spectra
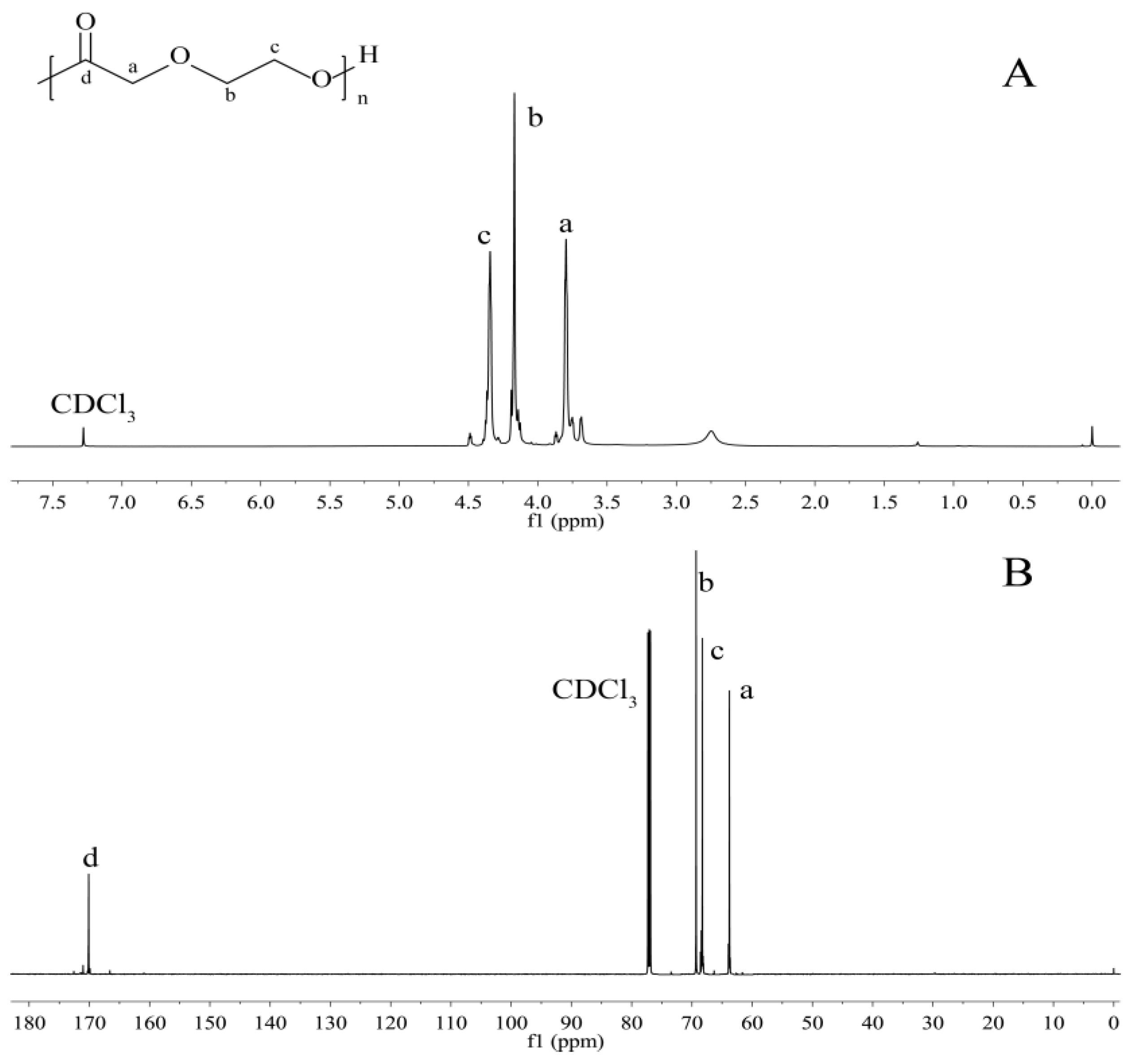
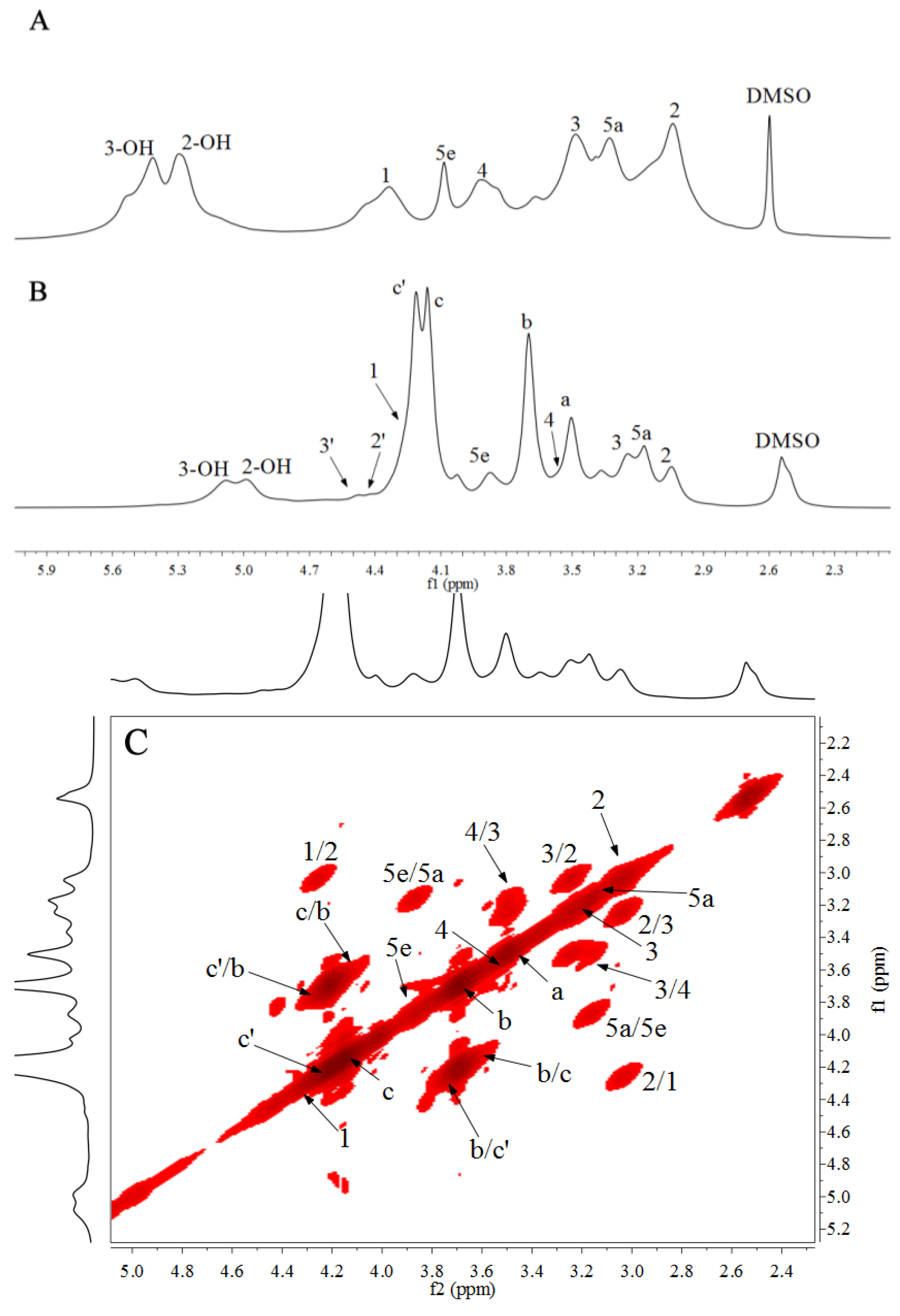
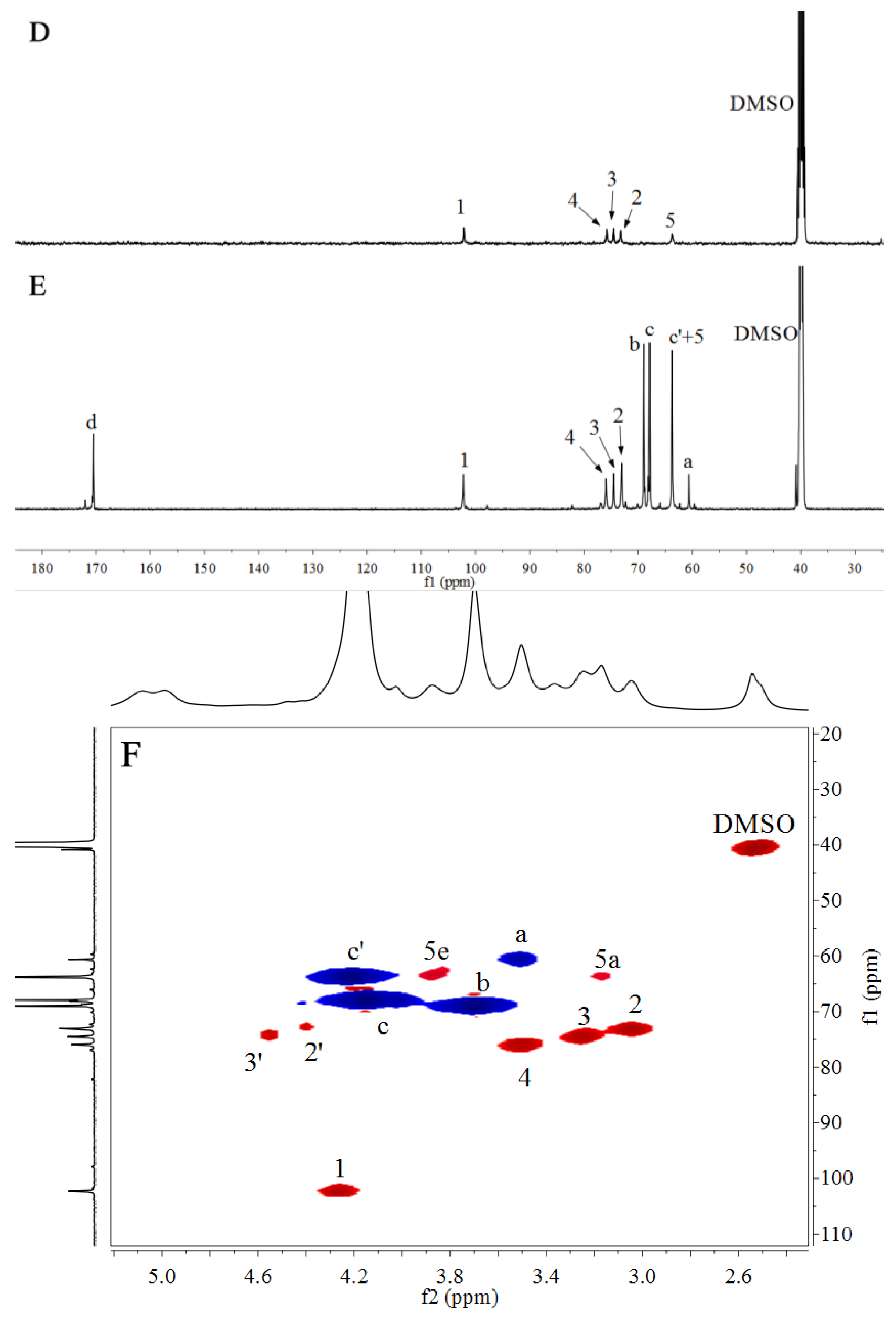
3.3. NMR Spectra
3.4. Thermal Stability of Xylan-g-PPDO Copolymers
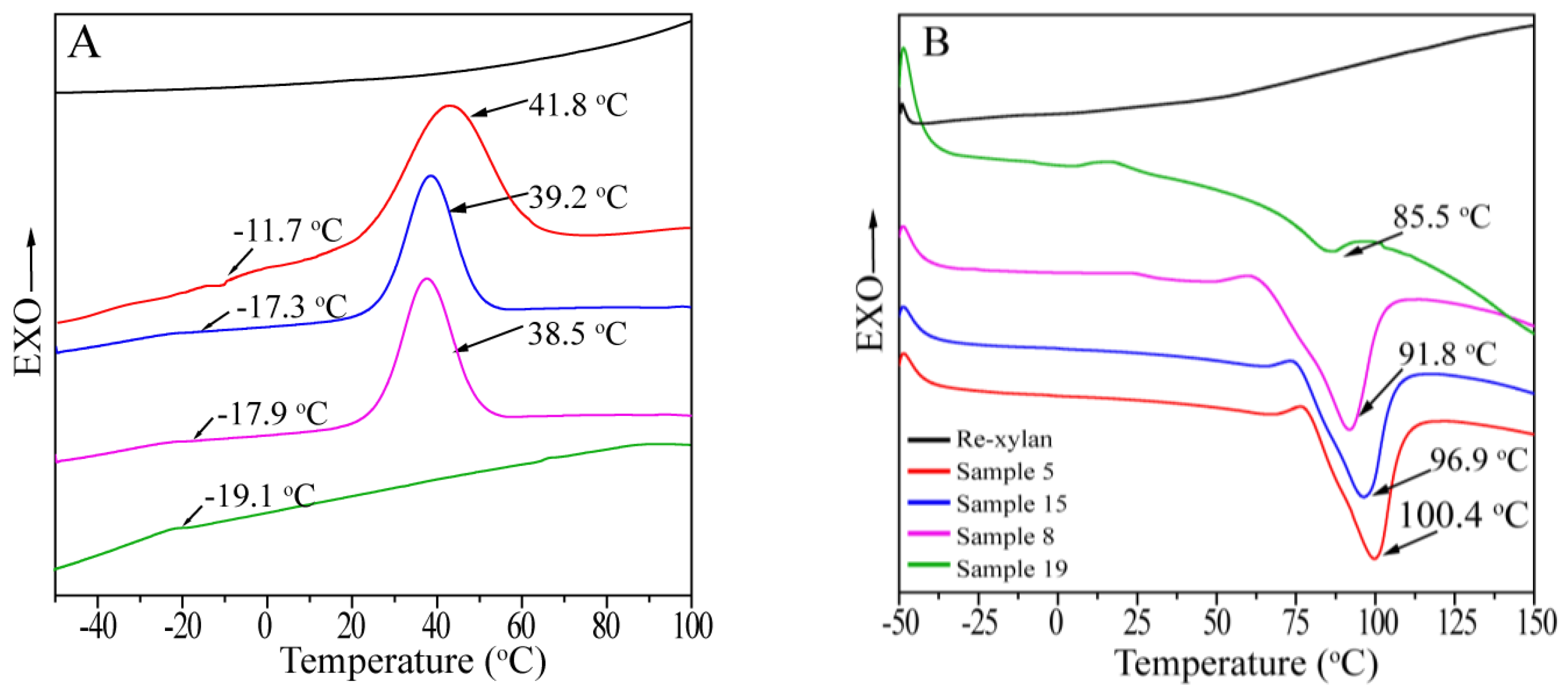
3.5. Thermal Transition Behavior of Xylan-g-PPDO Copolymers
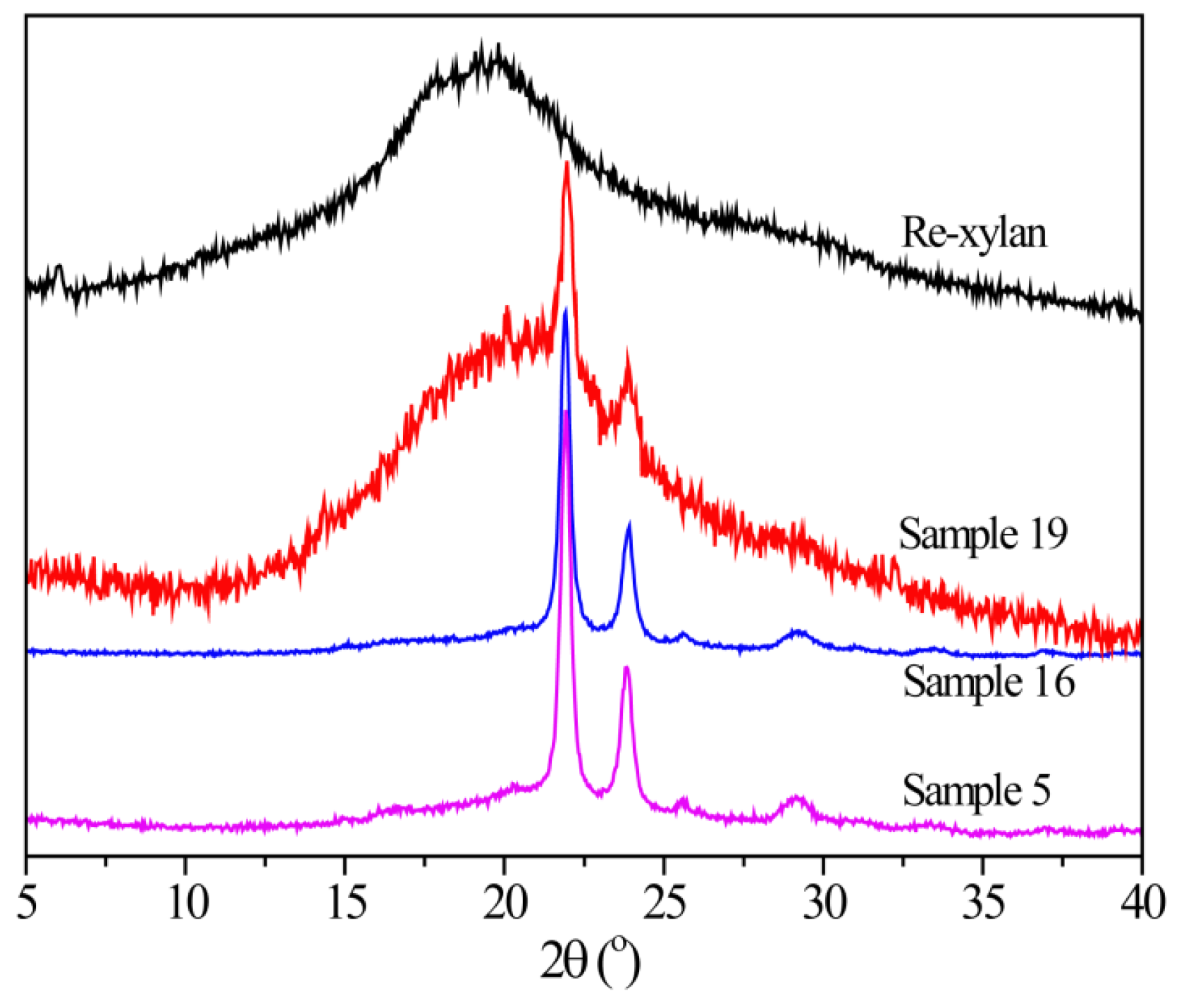
3.6. XRD Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lönnberg, H.; Larsson, K.; Lindström, T.; Hult, A.; Malmström, E. Synthesis of polycaprolactone-grafted microfibrillated cellulose for use in novel bionanocomposites—Influence of the graft length on the mechanical properties. ACS Appl. Mater. Interfaces 2011, 3, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A renewable lignin—Lactide copolymer and application in biobased composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Saha, B. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Yuan, T.Q.; Meng, L.J.; She, D.; Geng, Z.C.; Sun, R.C. Structural and thermal characterization of lauroylated hemicelluloses synthesized in an ionic liquid. Polym. Degrad. Stab. 2012, 97, 2323–2330. [Google Scholar] [CrossRef]
- Yang, D.; Zhong, L.X.; Yuan, T.Q.; Peng, X.W.; Sun, R.C. Studies on the structural characterization of lignin, hemicelluloses and cellulose fractionated by ionic liquid followed by alkaline extraction from bamboo. Ind. Crop. Prod. 2013, 43, 141–149. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Maleki, L.; Edlund, U.; Albertsson, A.-C. Synthesis of full interpenetrating hemicellulose hydrogel networks. Carbohydr. Polym. 2017, 170, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.U.; Oliveira, R.C.; Buckeridge, M.S. Seed storage hemicelluloses as wet-end additives in papermaking. Carbohydr. Polym. 2003, 52, 367–373. [Google Scholar] [CrossRef]
- Fundador, N.G.V.; Enomoto-Rogers, Y.; Takemura, A.; Iwata, T. Acetylation and characterization of xylan from hardwood kraft pulp. Carbohydr. Polym. 2012, 87, 170–176. [Google Scholar] [CrossRef]
- Ayoub, A.; Venditti, R.A.; Pawlak, J.J.; Sadeghifar, H.; Salam, A. Development of an acetylation reaction of switchgrass hemicellulose in ionic liquid without catalyst. Ind. Crop. Prod. 2013, 44, 306–314. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, M.J.; Liu, C.F.; Zhang, A.P.; Sun, R.C. Homogeneous ring opening graft polymerization of ε-caprolactone onto xylan in dual polar aprotic solvents. Carbohydr. Polym. 2015, 117, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Chen, M.J.; Liu, C.F.; Sun, R.C. Dual-component system dimethyl sulfoxide/licl as a solvent and catalyst for homogeneous ring-opening grafted polymerization of ε-caprolactone onto xylan. J. Agric. Food Chem. 2014, 62, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Enomoto Rogers, Y.; Iwata, T. Synthesis of xylan-g-poly(l-lactide) copolymers via click chemistry and their thermal properties. Carbohydr. Polym. 2012, 87, 1933–1940. [Google Scholar] [CrossRef]
- Fan, L.; Xiong, Y.B.; Xu, H.; Shen, Z.Q. l-lactide homopolymerization and l-lactide-ε-caprolactone block copolymerization by lanthanide tris(2,4,6-trimethylphenolate)s. Eur. Polym. J. 2005, 41, 1647–1653. [Google Scholar] [CrossRef]
- Pappalardo, D.; Annunziata, L.; Pellecchia, C. Living ring-opening homo- and copolymerization of ε-caprolactone and l- and d,l-lactides by dimethyl(salicylaldiminato)aluminum compounds. Macromolecules 2009, 42, 6056–6062. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, L.F.; Li, Q.; Chen, D.L.; Xiong, C.D. In vitro hydrolytic degradation of poly(para-dioxanone)/poly(d,l-lactide) blends. Mater. Chem. Phys. 2010, 122, 79–86. [Google Scholar] [CrossRef]
- Kaoukabi, A.; Guillen, F.; Qayouh, H.; Bouyahya, A.; Balieu, S.; Belachemi, L.; Gouhier, G.; Lahcini, M. The use of ionic liquids as an organocatalyst for controlled ring-opening polymerization of ϵ-caprolactone. Ind. Crops Prod. 2015, 72, 16–23. [Google Scholar] [CrossRef]
- Persson, J.; Dahlman, O.; Albertsson, A.C. Birch xylan grafted with pla branches of predictable length. Bioresources 2012, 7, 3640–3656. [Google Scholar]
- Zhang, X.Q.; Wang, H.H.; Liu, C.F.; Zhang, A.P.; Ren, J.L. Synthesis of thermoplastic xylan-lactide copolymer with amidine-mediated organocatalyst in ionic liquid. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Chen, M.J.; Liu, C.F.; Zhang, A.P.; Sun, R.C. Ring-opening graft polymerization of propylene carbonate onto xylan in an ionic liquid. Molecules 2015, 20, 6033–6047. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yang, K.K.; Chen, S.C.; Wang, X.L.; Zeng, J.B.; Wang, Y.Z. A new approach to prepare high molecular weight poly(p-dioxanone) by chain-extending from dihydroxyl terminated propolymers. Eur. Polym. J. 2008, 44, 465–474. [Google Scholar] [CrossRef]
- Giammanco, G.; Martínez de Ilarduya, A.; Alla, A.; Muñoz-Guerra, S. Hydrolyzable aromatic copolyesters of p-dioxanone. Biomacromolecules 2010, 11, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Ishikiriyama, K.; Pyda, M.; Zhang, G.; Forschner, T.; Grebowicz, J.; Wunderlich, B. Heat capacity of poly-p-dioxanone. J. Macromol. Sci., Part B: Phys. 1998, 37, 27–44. [Google Scholar] [CrossRef]
- Yang, K.K.; Wang, X.L.; Wang, Y.Z. Poly(p-dioxanone) and its copolymers. J. Macromol. Sci., Polym. Rev. 2002, C42, 373–398. [Google Scholar] [CrossRef]
- Libiszowski, J.; Kowalski, A.; Szymanski, R.; Duda, A.; Raquez, J.-M.; Degée, P.; Dubois, P. Monomer—Linear macromolecules—Cyclic oligomers equilibria in the polymerization of 1,4-dioxan-2-one. Macromolecules 2004, 37, 52–59. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, X.T.; Wang, X.L.; Wang, Y.Z. Preparation and properties of a novel biodegradable ethyl cellulose grafting copolymer with poly(p-dioxanone) side-chains. Carbohydr. Polym. 2010, 80, 350–359. [Google Scholar] [CrossRef]
- Lu, F.; Wang, X.L.; Chen, S.C.; Yang, K.K.; Wang, Y.Z. An efficient approach to synthesize polysaccharides-graft-poly(p-dioxanone) copolymers as potential drug carriers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5344–5353. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.W.; Wang, X.H.; Sun, R.C. Self-assembled conjugated polymer/carboxymethyl chitosan grafted poly(p-dioxanone) nanomicelles and their use in functionalized indicator paper for fast and visual detection of a banned food dye. Polym. Chem. 2014, 5, 4251–4258. [Google Scholar] [CrossRef]
- Wang, X.L.; Huang, Y.; Zhu, J.; Pan, Y.B.; He, R.; Wang, Y.Z. Chitosan-graft poly(p-dioxanone) copolymers: Preparation, characterization, and properties. Carbohydr. Res. 2009, 344, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, W.T.; Wang, X.L.; Li, B.; Wang, Y.Z. Green synthesis of a novel biodegradable copolymer base on cellulose and poly(p-dioxanone) in ionic liquid. Carbohydr. Polym. 2009, 76, 139–144. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhai, Y.L.; Wang, X.L.; Wang, W.T.; Pan, Y.B.; Dong, X.T.; Wang, Y.Z. Preparation, characterization, and in vitro drug release behavior of biodegradable chitosan-graft-poly(1,4-dioxan-2-one) copolymer. Carbohydr. Polym. 2008, 74, 862–867. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Wang, D.; Zhang, R.; Liu, G.; Cheng, G. Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour. Technol. 2014, 153, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Song, L.C.; Yang, Y.L.; Xie, H.B.; Liu, E.H. Cellulose dissolution and insitu grafting in a reversible system using an organocatalyst and carbon dioxide. ChemSusChem 2015, 8, 3217–3221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kennedy, J.F.; Liu, L.J. An ionic liquid as reaction media in the ring opening graft polymerization of ε-caprolactone onto starch granules. Carbohydr. Polym. 2008, 72, 113–121. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal catalysts for epsilon-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Kakuchi, R.; Tsuji, Y.; Chiba, K.; Fuchise, K.; Sakai, R.; Satoh, T.; Kakuchi, T. Controlled/living ring-opening polymerization of δ-valerolactone using triflylimide as an efficient cationic organocatalyst. Macromolecules 2010, 43, 7090–7094. [Google Scholar] [CrossRef]
- Nederberg, F.; Connor, E.F.; Möller, M.; Glauser, T.; Hedrick, J.L. New paradigms for organic catalysts: The first organocatalytic living polymerization. Angew. Chem. Int. Ed. 2001, 40, 2712–2715. [Google Scholar] [CrossRef]
- Nederberg, F.; Lohmeijer, B.G.G.; Leibfarth, F.; Pratt, R.C.; Choi, J.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic ring opening polymerization of trimethylene carbonate. Biomacromolecules 2006, 8, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.P. Organic catalysis for ring-opening polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Naumann, S.; Scholten, P.B.V.; Wilson, J.A.; Dove, A.P. Dual catalysis for selective ring-opening polymerization of lactones: Evolution toward simplicity. J. Am. Chem. Soc. 2015, 137, 14439–14445. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Zhang, J.M.; Lv, Y.X.; Yu, J.; Wu, J.; Zhang, J.; He, J.S. Thermoplastic cellulose-graft-poly(l-lactide) copolymers homogeneously synthesized in an ionic liquid with 4-dimethylaminopyridine catalyst. Biomacromolecules 2009, 10, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic ring-opening polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Bai, T.; Xue, X.Q.; Huang, W.Y.; Chen, J.H.; Jiang, B.B. Synthesis of metal-free poly(p-dioxanone) by phosphazene base catalyzed ring-opening polymerization. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Naumann, S.; Schmidt, F.G.; Frey, W.; Buchmeiser, M.R. Protected N-heterocyclic carbenes as latent pre-catalysts for the polymerization of ε-caprolactone. Polym. Chem. 2013, 4, 4172–4181. [Google Scholar] [CrossRef]
- Brown, H.A.; De Crisci, A.G.; Hedrick, J.L.; Waymouth, R.M. Amidine-mediated zwitterionic polymerization of lactide. ACS Macro Lett. 2012, 1, 1113–1115. [Google Scholar] [CrossRef]
- Hua, S.; Chen, F.; Liu, Z.Y.; Yang, W.; Yang, M.B. Preparation of cellulose-graft-polylactic acid via melt copolycondensation for use in polylactic acid based composites: Synthesis, characterization and properties. RSC Adv. 2016, 6, 1973–1983. [Google Scholar] [CrossRef]
- Chuma, A.; Horn, H.W.; Swope, W.C.; Pratt, R.C.; Zhang, L.; Lohmeijer, B.G.G.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L.; Rice, J.E. The reaction mechanism for the organocatalytic ring-opening polymerization of l-lactide using a guanidine-based catalyst: Hydrogen-bonded or covalently bound? J. Am. Chem. Soc. 2008, 130, 6749–6754. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Peruch, F.; Bibal, B. Ring-opening polymerization of lactones using supramolecular organocatalysts under simple conditions. RSC Adv. 2012, 2, 12851–12856. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, M.J.; Wang, H.H.; Liu, C.F.; Zhang, A.P.; Sun, R.C. Characterization of xylan-g-polycaprolactone copolymers prepared in ionic liquid. Ind. Eng. Chem. Res. 2015, 54, 6282–6290. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.Z.; Zhou, J.H.; Sun, G.W.; Han, Y.; Wang, X.H. Synthesis and characterization of cellulose-g-poly(p-dioxanone) copolymers via homogeneous ring-opening graft polymerization in ionic liquids. Bioresources 2016, 11, 2698–2711. [Google Scholar] [CrossRef]
- Howarth, J.; Hanlon, K.; Fayne, D.; McCormac, P. Moisture stable dialkylimidazolium salts as heterogeneous and homogeneous lewis acids in the diels-alder reaction. Tetrahedron Lett. 1997, 38, 3097–3100. [Google Scholar] [CrossRef]
- Aggarwal, A.; Lancaster, N.L.; Sethi, A.R.; Welton, T. The role of hydrogen bonding in controlling the selectivity of diels-alder reactions in room-temperature ionic liquids. Green Chem. 2002, 4, 517–520. [Google Scholar] [CrossRef]
- Fang, J.M.; Sun, R.C.; Tomkinson, J.; Fowler, P. Acetylation of wheat straw hemicellulose b in a new non-aqueous swelling system. Carbohydr. Polym. 2000, 41, 379–387. [Google Scholar] [CrossRef]
- Kac̆uráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Zhang, T.H.; Wen, Z.B.; Hui, Y.; Yang, M.N.; Yang, K.K.; Zhou, Q.; Wang, Y.Z. Facile fabrication of a well-defined poly(p-dioxanone) dynamic network from metallosupramolecular interactions to obtain an excellent shape-memory effect. Polym. Chem. 2015, 6, 4177–4184. [Google Scholar] [CrossRef]
- Daud, W.; Djuned, F. Cellulose acetate from oil palm empty fruit bunch via a one step heterogeneous acetylation. Carbohydr. Polym. 2015, 132, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Fundador, N.G.V.; Enomoto-Rogers, Y.; Takemura, A.; Iwata, T. Syntheses and characterization of xylan esters. Polymer 2012, 53, 3885–3893. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energ. Convers. Manage. 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Marcelino, H.; da Silva, A.; Gomes, M.; Oliveira, E.; Nagashima-Junior, T.; Pinheiro, G.; da Silva, A.; Timoteo, A.; Agnez-Lima, L.; Ayala, A.; et al. Leads from physical, chemical, and thermal characterization on cytotoxic effects of xylan-based microparticles. Polymers 2015, 7, 1515. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Wang, X.L.; Wang, Y.Z.; Yang, K.K.; Chen, S.C.; Wu, G.; Li, J. Thermal properties and non-isothermal crystallization behavior of biodegradable poly(p-dioxanone)/poly(vinyl alcohol) blends. Polym. Int. 2006, 55, 383–390. [Google Scholar] [CrossRef]



| Sample | Catalyst | Catalyst/AXU (mol/mol) | WPG a (%) | Solubility | |
|---|---|---|---|---|---|
| DMSO | DMF | ||||
| 1 | DMAP | 0.2:1 | 384.51 | ++ | + |
| 2 | DMAP | 0.5:1 | 394.63 | ++ | + |
| 3 | DMAP | 1:1 | 403.79 | ++ | + |
| 4 | DMAP | 1.5:1 | 403.96 | ++ | + |
| 5 | DMAP | 2:1 | 431.07 | ++ | + |
| 6 | DMAP | 3:1 | 419.98 | ++ | + |
| 7 | DMAP | 4:1 | 339.88 | ++ | + |
| 8 | DBU | 0.2:1 | 316.72 | ++ | + |
| 9 | DBU | 0.5:1 | 155.02 | + | + |
| 10 | DBU | 1:1 | 93.13 | + | - |
| 11 | DBU | 1.5:1 | 48.06 | + | - |
| 12 | DBU | 2:1 | 12.39 | - | - |
| 13 | DBU | 3:1 | 12.06 | - | - |
| 14 | DBU | 4:1 | 11.09 | - | - |
| 15 | TBD | 0.2:1 | 323.15 | ++ | + |
| 16 | TBD | 0.5:1 | 239.26 | ++ | + |
| 17 | TBD | 1:1 | 55.37 | + | - |
| 18 | TBD | 1.5:1 | 16.64 | - | - |
| 19 | TBD | 2:1 | 13.03 | - | - |
| 20 | TBD | 3:1 | 5.28 | - | - |
| 21 | TBD | 4:1 | 2.55 | - | - |
| Sample | WPG (%) | Tc (°C) | Tg (°C) | Tm (°C) | ∆Hm (J/g) |
|---|---|---|---|---|---|
| Re-xylan | - | - | - | - | - |
| PPDO a | - | 43.8 | −9.0 | 107.8 | 58.3 |
| 5 | 431.07 | 41.8 | −11.7 | 100.4 | 54.8 |
| 15 | 323.15 | 39.2 | −17.3 | 96.9 | 47.2 |
| 8 | 316.72 | 38.5 | −17.9 | 91.8 | 44.3 |
| 19 | 13.03 | - | −19.1 | 85.5 | 2.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, C.; Zhang, A.; Sun, R. Organic Catalysis for Ring-Opening Graft Polymerization of p-Dioxanone with Xylan in Ionic liquid. Polymers 2017, 9, 345. https://doi.org/10.3390/polym9080345
Zhang X, Liu C, Zhang A, Sun R. Organic Catalysis for Ring-Opening Graft Polymerization of p-Dioxanone with Xylan in Ionic liquid. Polymers. 2017; 9(8):345. https://doi.org/10.3390/polym9080345
Chicago/Turabian StyleZhang, Xueqin, Chuanfu Liu, Aiping Zhang, and Runcang Sun. 2017. "Organic Catalysis for Ring-Opening Graft Polymerization of p-Dioxanone with Xylan in Ionic liquid" Polymers 9, no. 8: 345. https://doi.org/10.3390/polym9080345