New Eco-Friendly Phosphorus Organic Polymers as Gas Storage Media
Abstract
:1. Introduction
2. Experimental Section
2.1. Instrumentation
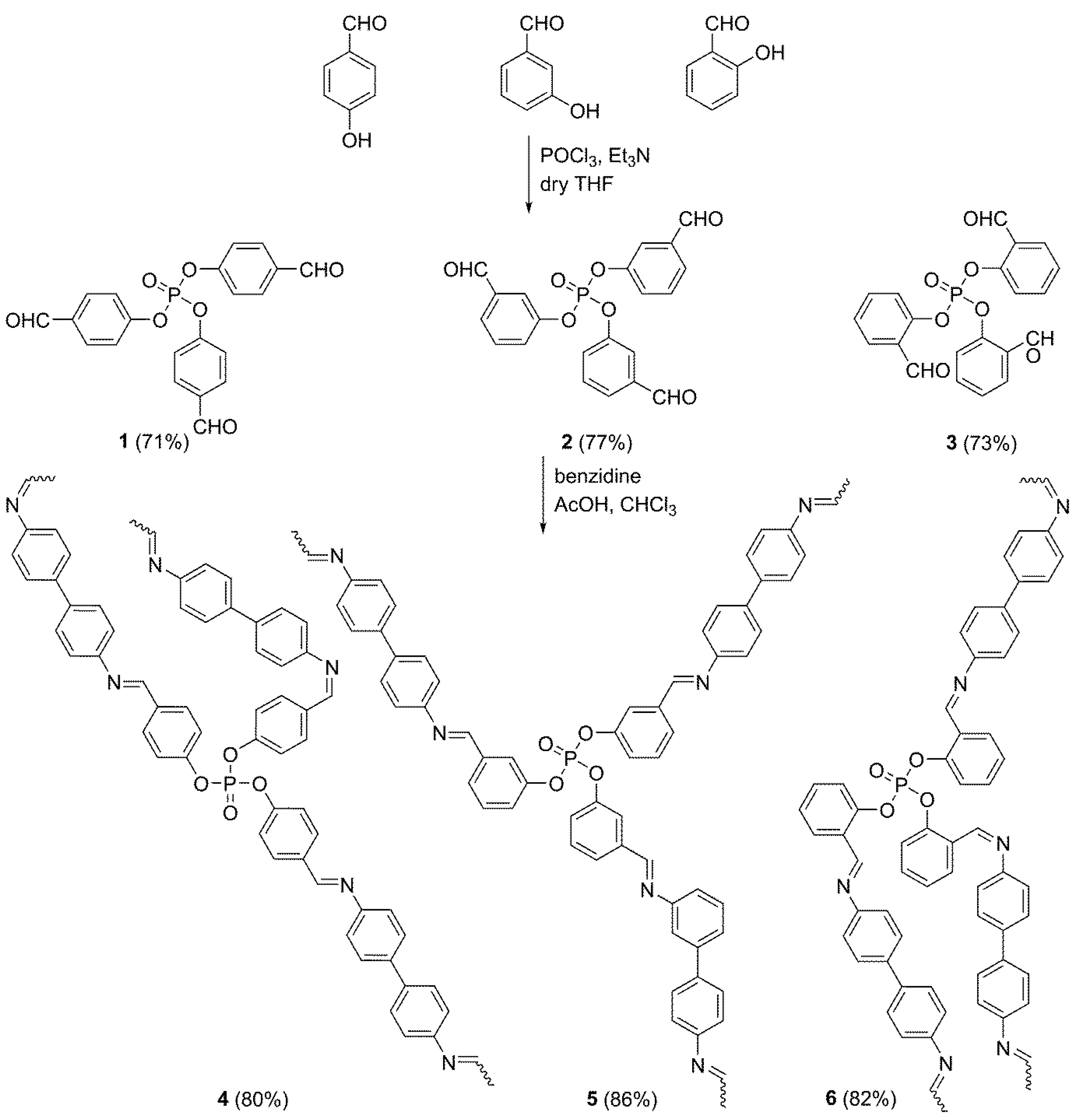
2.2. Synthesis of Phosphate Esters 1–3
2.3. Synthesis of Polymeric Schiff Bases 4–6
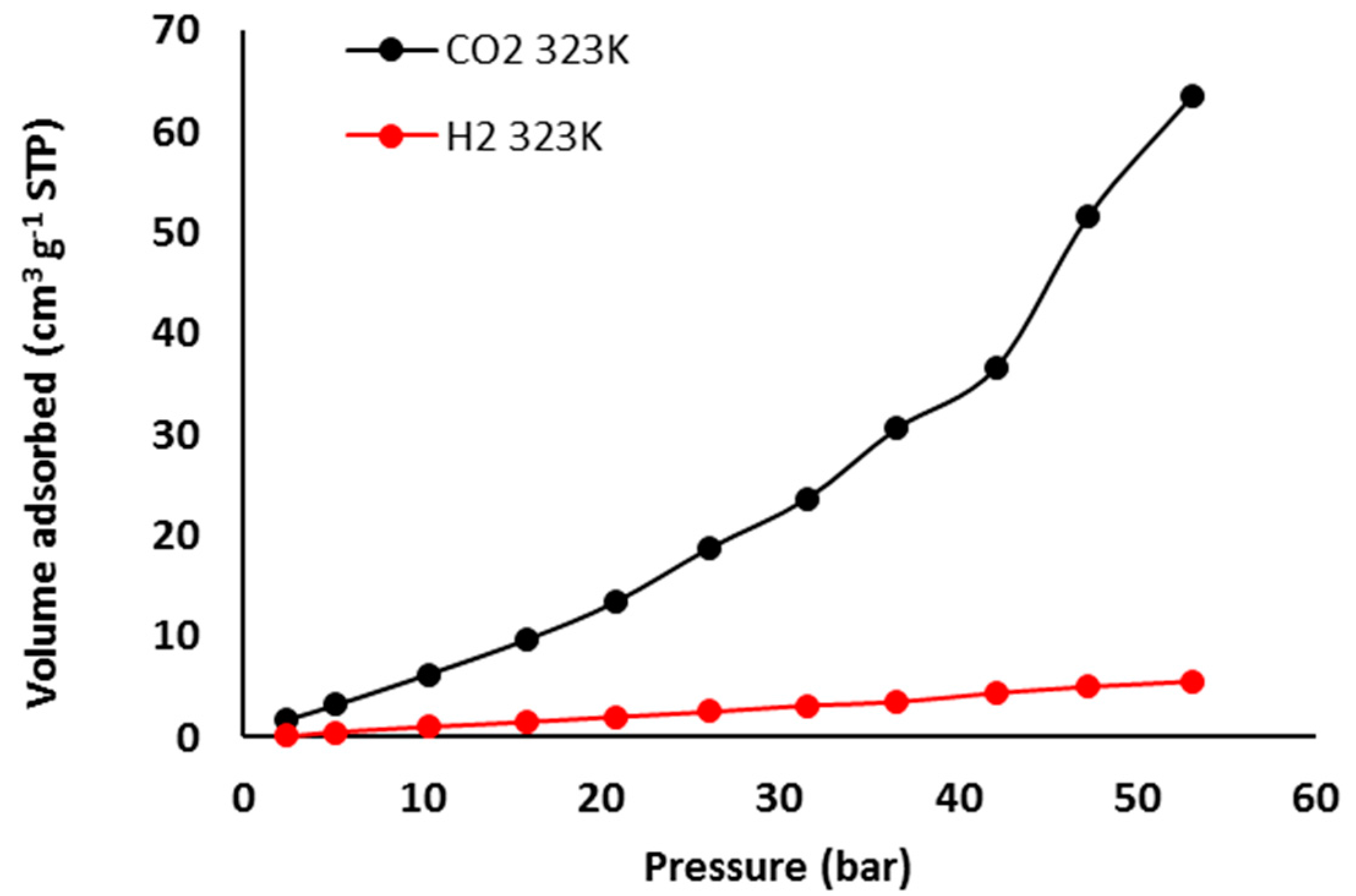
3. Results and Discussion
3.1. Synthesis of Phosphate Polymers 4–6
3.2. FT-IR Spectroscopy of Phosphate Esters 1–3
3.3. 1H NMR Spectroscopy of Phosphate Esters 1–3
3.4. FT-IR Spectroscopy of Phosphate Polymers 4–6
3.5. Molecular Weight and Molecular Distribution of Phosphate Polymers 4–6 Determined by Gel Permeation Chromatography (GPC)
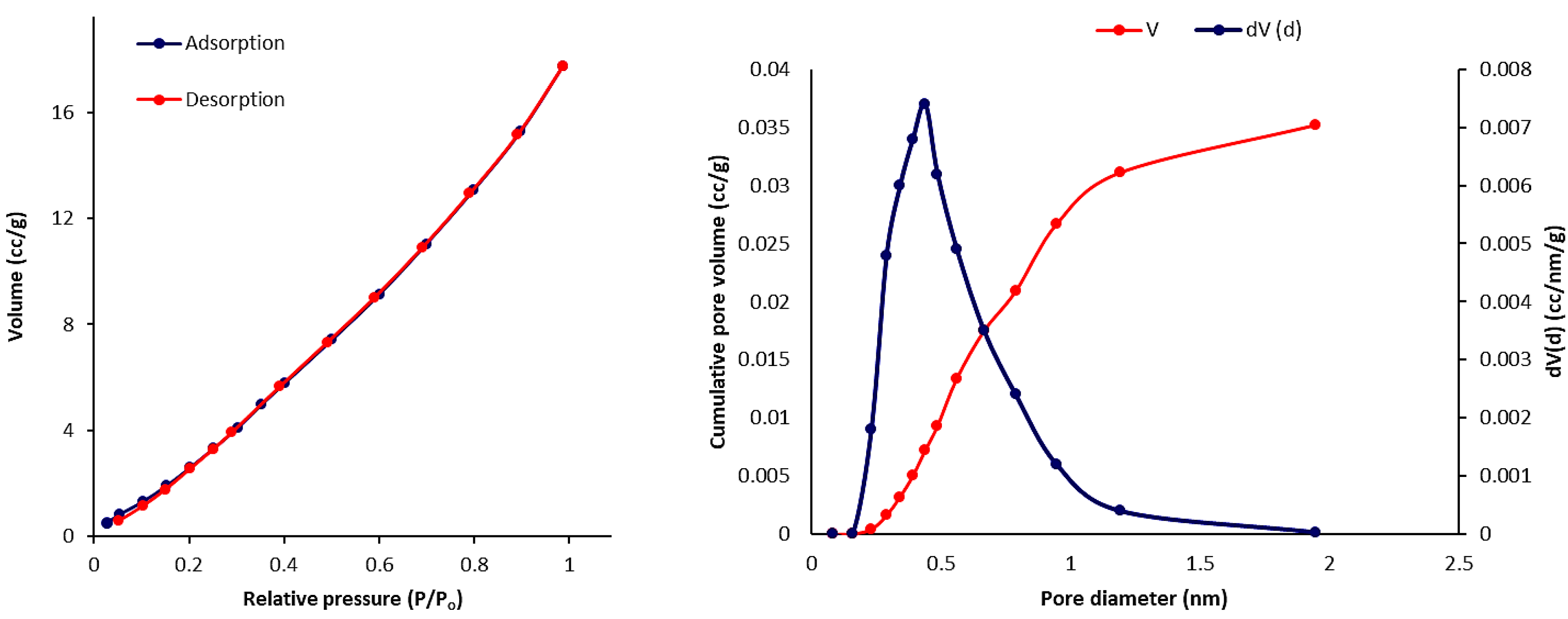
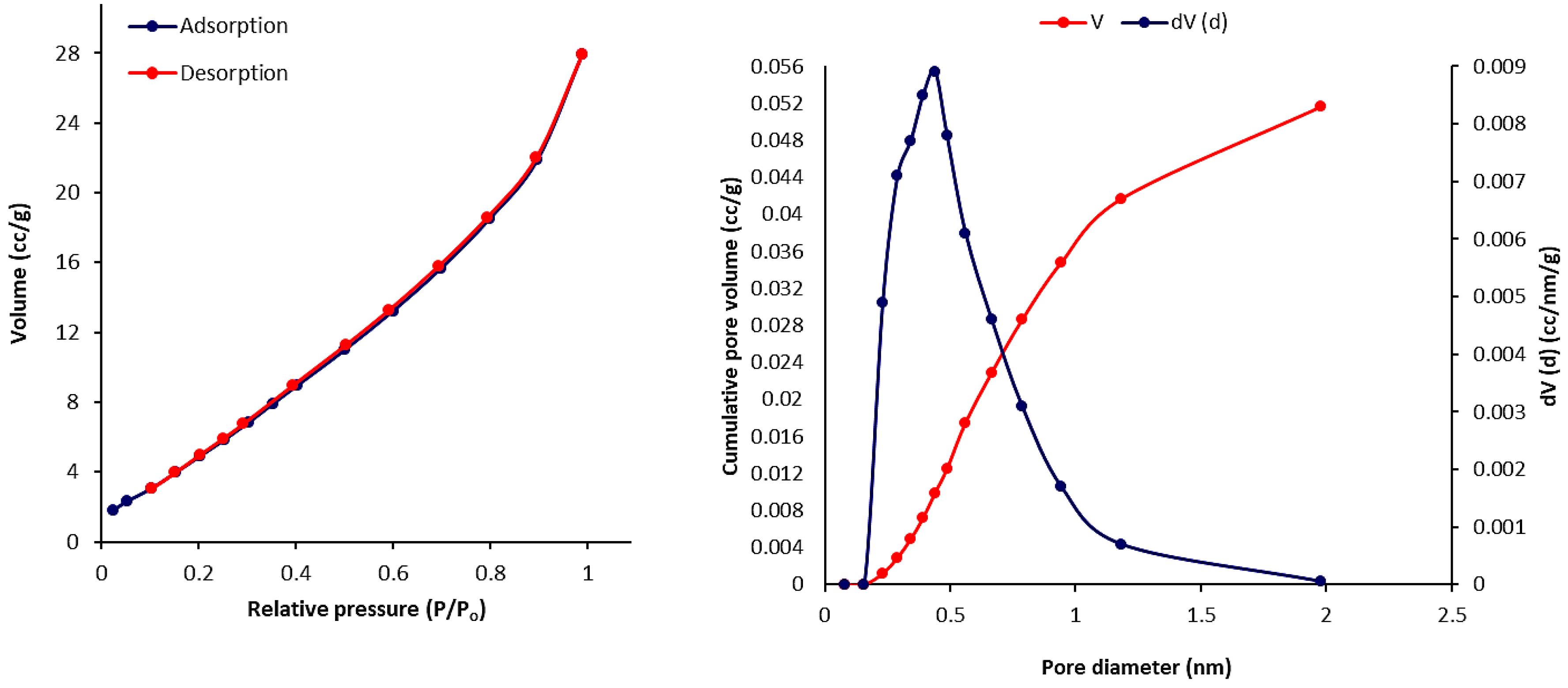
3.6. Scanning Electron Microscopy (SEM) of Phosphate Polymers 4–6
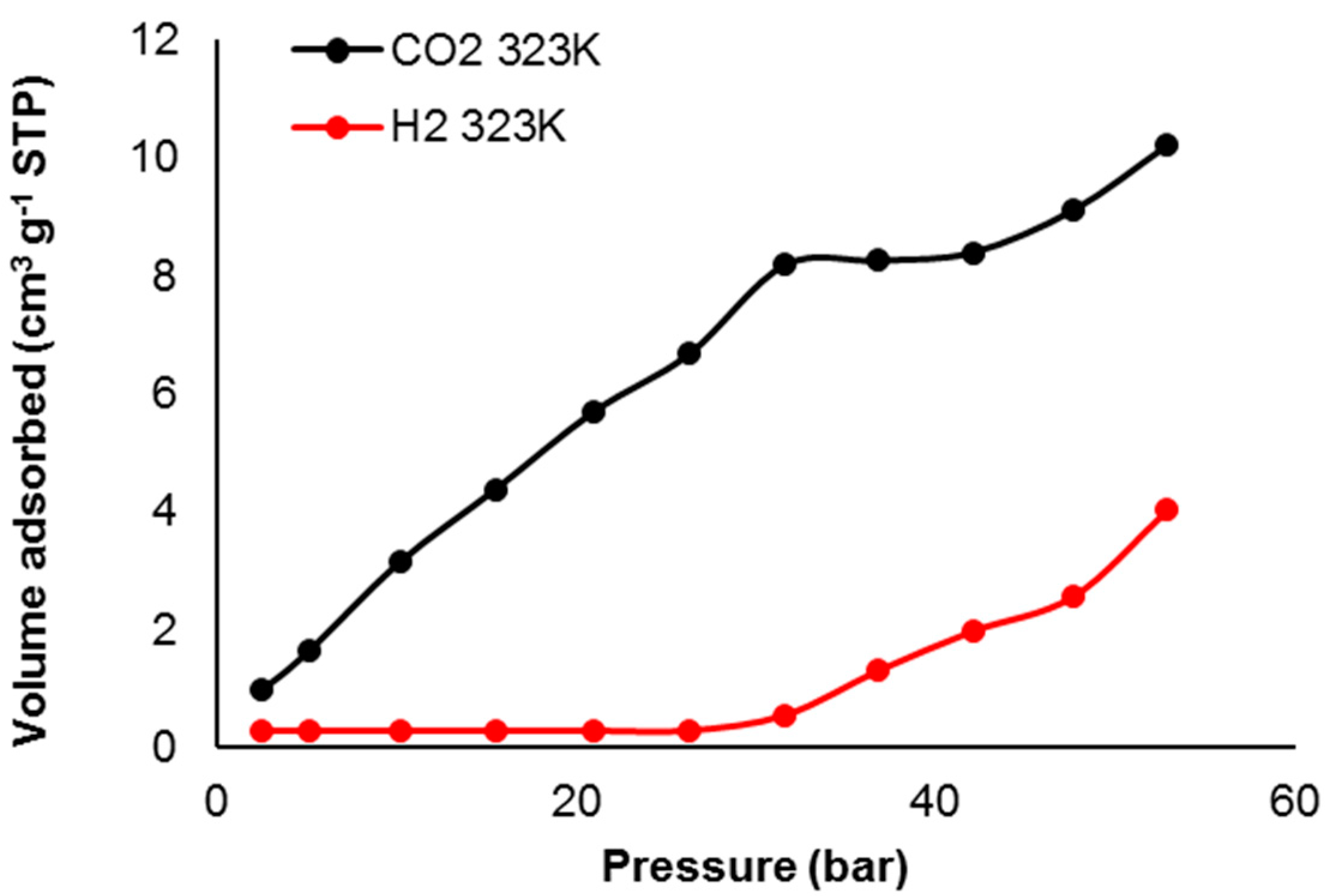
3.7. Pure Gas Adsorption of Phosphate Polymers 4–6
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dawson, R.; Adams, D.J.; Cooper, A.I. Chemical tuning of CO2 sorption in robust nanoporous organic polymers. Chem. Sci. 2011, 2, 1173–1177. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.Q.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H.-C. Sulfonate-grafted porous polymer networks for preferential CO2 adsorption at low pressure. J. Am. Chem. Soc. 2011, 133, 18126–18129. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Snurr, R.Q. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.Q.; Zhou, H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; van Heest, T.M.; Sholl, D.S. Can Metal–organic framework materials play a useful role in large-scale carbon dioxide separations? ChemSusChem 2010, 3, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Haszeldine, R.S. Carbon capture and storage: How green can black be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Mastalerz, M.; Schneider, M.W.; Oppel, I.M.; Presly, O. A Salicylbisimine cage compound with high surface area and selective CO2/CH4 adsorption. Angew. Chem. Int. Ed. 2011, 50, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Rabbani, M.G.; El-Kaderi, H.M. Synthesis and characterization of porous benzimidazole-linked polymers and their performance in small gas storage and selective uptake. Chem. Mater. 2012, 24, 1511–1517. [Google Scholar] [CrossRef]
- Kaliva, M.; Armatas, G.S.; Vamvakaki, M. Microporous polystyrene particles for selective carbon dioxide capture. Langmuir 2012, 28, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; McDonald, T. Toward carbon dioxide capture nanoporous materials. Pure Appl. Chem. 2011, 83, 57–66. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.W.C.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 668–681. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen adsorption in different carbon nanostructures. Carbon 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.A.; Hasell, T.; Wu, X.; Bacsa, J.; Jelfs, K.E.; Schmidtmann, M.; Chong, S.Y.; Adams, D.J.; Trewin, A.; Schiffman, F.; et al. Modular and predictable assembly of porous organic molecular crystals. Nature 2011, 474, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.-J.; Daniels, E.A.; Curtin, T.; Perry, J.J., IV; Zaworotko, M.J. Direct air capture of CO2 by physisorbent materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Mercado, R.; Huck, J.M.; Wang, H.; Guo, Z.; Wang, W.; Cao, D.; Haranczyk, M.; Smit, B. Systematic tuning and multifunctionalization of covalent organic polymers for enhanced carbon capture. J. Am. Chem. Soc. 2015, 137, 13301–13307. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.Y.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-separation membranes loaded with porous aromatic frameworks that improve with age. Angew. Chem. Int. Ed. 2015, 54, 2669–2673. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dai, Z.; Meng, X.; Wang, L.; Xiao, F.-S. Task-specific design of porous polymer heterogeneous catalysts beyond homogeneous counterparts. ACS Catal. 2015, 5, 4556–4567. [Google Scholar] [CrossRef]
- Patra, A.; Scherf, U. Fluorescent microporous organic polymers: Potential testbed for optical applications. Chem. Eur. J. 2012, 18, 10074–10080. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, K.M.; Jeon, H.J.; Choi, Y.J.; Lee, Y.; Kang, J.K. Acetylene gas mediated conjugated microporous polymers (ACMPs): First use of acetylene gas as a building unit. Macromolecules 2010, 43, 5508–5511. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Trewin, A.; Adams, D.J.; Cooper, A.I. Band gap engineering in fluorescent conjugated microporous polymers. Chem. Sci. 2011, 2, 1777–1781. [Google Scholar] [CrossRef]
- Yuan, D.; Lu, W.; Zhao, D.; Zhou, H.-C. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar]
- Wang, D.; Xue, L.; Li, L.; Deng, B.; Feng, S.; Liu, H.; Zhao, X. Rational design and synthesis of hybrid porous polymers derived from polyhedral oligomeric silsesquioxanes via Heck coupling reactions. Macromol. Rapid Commun. 2013, 34, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal–organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Zhou, W.; Chen, B. Porous metal–organic frameworks for gas storage and separation: What, how, and why? Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Corbridge, D.E.C. Phosphorus: Chemistry, Biochemistry and Technology, 6th ed.; CRC Press: New York, NY, USA, 2013. [Google Scholar]
- Iliescu, S.; Zubizarreta, L.; Plesu, N.; Macarie, L.; Popa, A.; Ilia, G. Polymers containing phosphorus groups and polyethers: From synthesis to application. Chem. Cent. J. 2012, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Sun, J.; Wu, B.; Zhou, Q. Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar] [CrossRef]
- Petreus, O.; Vlad-Bubulac, T.; Hamciuc, C. Synthesis and characterization of new polyesters with enhanced phosphorus content. Eur. Polym. J. 2005, 41, 2663–2670. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Wang, Y.-Z.; Ban, D.-M.; Yang, B.; Zhao, G.-M. A novel phosphorus-containing polymer as a highly effective flame retardant. Macromol. Mater. Eng. 2004, 289, 703–707. [Google Scholar] [CrossRef]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Dillard, A.; Pocius, A.V. Adhesion Science and Engineering, The Mechanism of Adhesion; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Lin-Gibson, S.; Glass, T.E.; Shultz, A.R.; Riffle, J.S. Controlled molecular weight cresol-formaldehyde oligomers. Polymer 2002, 43, 2017–2029. [Google Scholar] [CrossRef]
- Wu, C. Handbook of Size Exclusion Chromatography; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ma, Q.-Y.; Yang, B.-X.; Li, J.-Q. Porous organic polymers derived from tetrahedral silicon-centered monomers and a stereocontorted spirobifluorene-based precursor: Synthesis, porosity and carbon dioxide sorption. RSC Adv. 2015, 5, 64163–64169. [Google Scholar] [CrossRef]
- Muller, T.; Bräse, S. Tetrahedral organic molecules as components in supramolecular architectures and in covalent assemblies, networks and polymers. RSC Adv. 2014, 4, 6886–6907. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Li, L.; Zhao, X.; Feng, S.; Liu, H. Hybrid networks constructed from tetrahedral silicon-centered precursors and cubic POSS-based building blocks via Heck reaction: Porosity, gas sorption, and luminescence. J. Mater. Chem. A 2013, 1, 13549–13558. [Google Scholar] [CrossRef]
- Holst, J.R.; Stöckel, E.; Adams, D.J.; Cooper, A.I. High surface area networks from tetrahedral monomers: Metal-catalyzed coupling, thermal polymerization, and “click” chemistry. Macromolecules 2010, 43, 8531–8538. [Google Scholar] [CrossRef]
- Stöckel, E.; Wu, X.; Trewin, A.; Wood, C.D.; Clowes, R.; Campbell, N.L.; Jones, J.T.A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. High surface area amorphous microporous poly(aryleneethynylene) networks using tetrahedral carbon- and silicon-centred monomersw. Chem. Commun. 2009, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yuan, D.; Zhao, D.; Schilling, C.I.; Plietzsch, O.; Muller, T.; Bräse, S.; Guenther, J.; Blümel, J.; Krishna, R.; et al. Porous polymer networks: Synthesis, porosity, and applications in gas storage/separation. Chem. Mater. 2010, 22, 5964–5972. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Voss, B.A.; McCaffrey, R.; Baggett, C.T.; Noble, R.D.; Zhang, W. Microwave-assisted syntheses of highly CO2-selective organic cage frameworks (OCFs). Chem. Sci. 2012, 3, 874–877. [Google Scholar] [CrossRef]
- Yu, H.; Tian, M.; Shen, C.; Wang, Z. Facile preparation of porous polybenzimidazole networks and adsorption behavior of CO2 gas, organic and water vapors. Polym. Chem. 2013, 4, 961–968. [Google Scholar] [CrossRef]
- Katsoulidis, A.P.; Dyar, S.M.; Carmieli, R.; Malliakas, C.D.; Wasielewski, M.R.; Kanatzidis, M.G. Copolymerization of terephthalaldehyde with pyrrole, indole and carbazole gives microporous POFs functionalized with unpaired electrons. J. Mater. Chem. A 2013, 1, 10465–10473. [Google Scholar] [CrossRef]
- Ding, X.; Li, H.; Zhao, Y.-C.; Han, B.-H. Mannitol-based acetal-linked porous organic polymers for selective capture of carbon dioxide over methane. Polym. Chem. 2015, 6, 5305–5312. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M. Toxicity of nanoscale metal organic frameworks: A perspective. Environ. Sci. Pollut. Res. 2016, 23, 14805–14807. [Google Scholar] [CrossRef] [PubMed]




| Ester | FR-IR (υ, cm−1) | Elemental Analyses (%) Found (Calcd) | ||||
|---|---|---|---|---|---|---|
| P–O–C | P=O | C=C | C=O | C | H | |
| 1 | 1161 | 1219 | 1600 | 1681 | 61.64 (61.47) | 3.74 (3.68) |
| 2 | 1188 | 1276 | 1585 | 1693 | 61.57 (61.47) | 3.81 (3.68) |
| 3 | 1172 | 1284 | 1581 | 1687 | 61.61 (61.47) | 3.85 (3.68) |
| Ester | 1H NMR (400 MHz: DMSO-d6, δ, ppm, J in Hz) |
|---|---|
| 1 | 9.79 (s, 3 H, CHO), 7.74 (d, J = 8.5 Hz, 6 H, Ar), 6.93 (d, J = 8.5 Hz, 6 H, Ar) |
| 2 | 9.91 (s, 3 H, CHO), 7.41 (t, J = 8.2 Hz, 3 H, Ar), 7.36 (d, J = 8.2 Hz, 3 H, Ar), 7.24 (s, 3 H, Ar), 7.08 (d, J = 8.2 Hz, 3 H, Ar) |
| 3 | 10.30 (s, 3 H, CHO), 7.71 (d, J = 8.3 Hz, 3 H, Ar), 7.58 (t, J = 8.3 Hz, 3 H, Ar), 7.05 (d, J = 8.3 Hz, 3 H, Ar), 7.02 (t, J = 8.3 Hz, 3 H, Ar) |
| Polymer | FR-IR (υ, cm−1) | |||
|---|---|---|---|---|
| P–O–C | P=O | C=C | CH=N | |
| 4 | 1219 | 1172 | 1600 | 1635 |
| 5 | 1219 | 1087 | 1604 | 1635 |
| 6 | 1280 | 1172 | 1570 | 1616 |
| Polymer | Mw | Mn | Dp | Rt (min) |
|---|---|---|---|---|
| 4 | 12,700 | 12,000 | 1.05 | 17.20 |
| 5 | 12,400 | 11,100 | 1.11 | 17.19 |
| 6 | 26,800 | 25,900 | 1.03 | 16.61 |
| Polymers | SBET (m2·g–1) a | Vtotal (cm3·g–1) b | Pore Size (nm) c |
|---|---|---|---|
| 4 | 27.514 | 0.036 | 2.851 |
| 5 | 30.021 | 0.052 | 2.435 |
| 6 | 24.840 | 0.040 | 2.856 |
| Polymer | H2 Uptake (cm3·g–1) b | H2 Uptake (wt %) | CO2 Uptake (cm3·g–1) | CO2 Uptake (wt %) c |
|---|---|---|---|---|
| 4 | 4.0 | <0.050 | 10.2 | 1.8 |
| 5 | 7.4 | 0.066 | 82.1 | 14 |
| 6 | 5.5 | 0.050 | 63.4 | 11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New Eco-Friendly Phosphorus Organic Polymers as Gas Storage Media. Polymers 2017, 9, 336. https://doi.org/10.3390/polym9080336
Ahmed DS, El-Hiti GA, Yousif E, Hameed AS, Abdalla M. New Eco-Friendly Phosphorus Organic Polymers as Gas Storage Media. Polymers. 2017; 9(8):336. https://doi.org/10.3390/polym9080336
Chicago/Turabian StyleAhmed, Dina S., Gamal A. El-Hiti, Emad Yousif, Ayad S. Hameed, and Mustafa Abdalla. 2017. "New Eco-Friendly Phosphorus Organic Polymers as Gas Storage Media" Polymers 9, no. 8: 336. https://doi.org/10.3390/polym9080336









