Exceptionally High Negative Electro-Caloric Effects of Poly(VDF–co–TrFE) Based Nanocomposites Tuned by the Geometries of Barium Titanate Nanofillers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
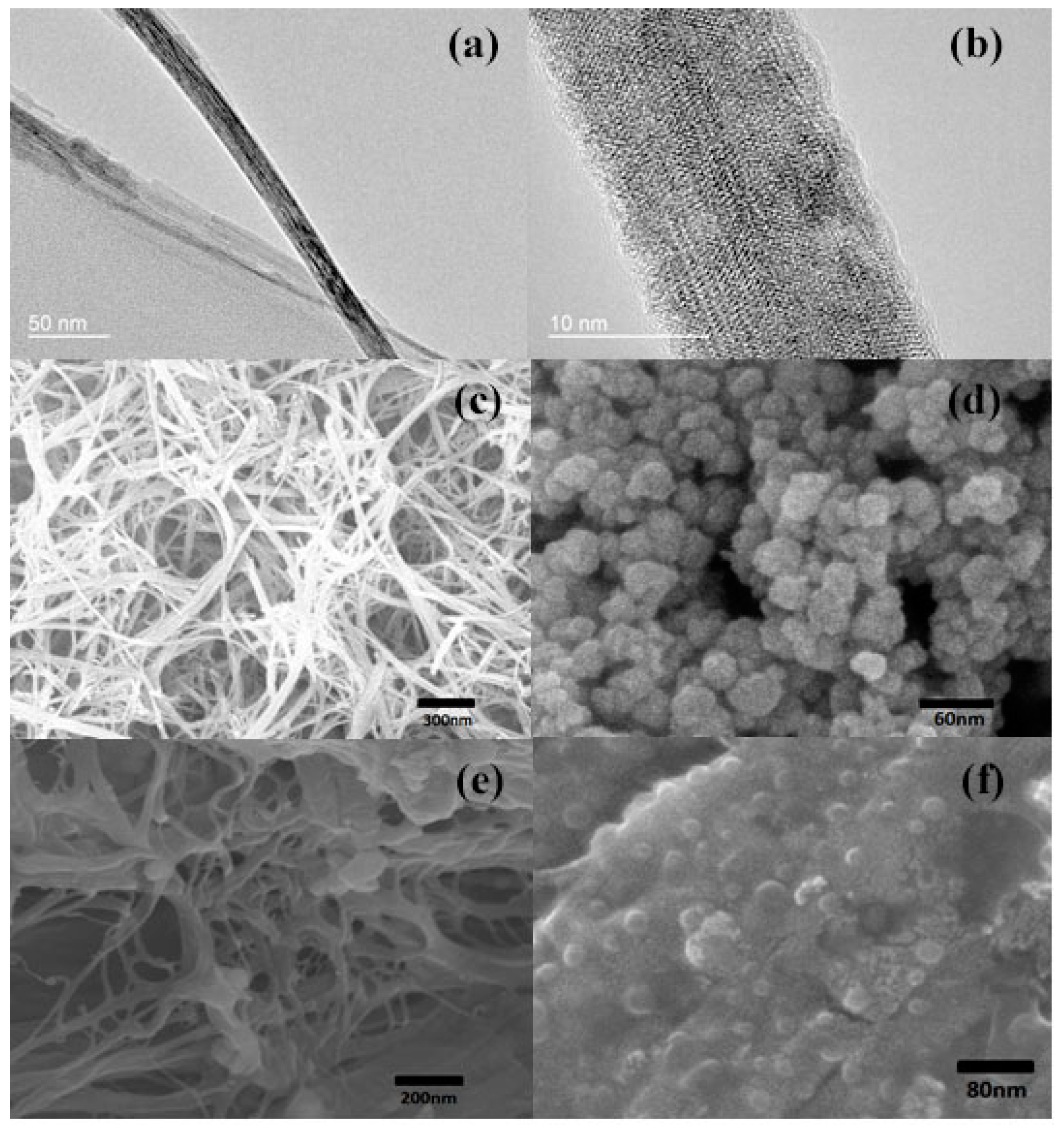
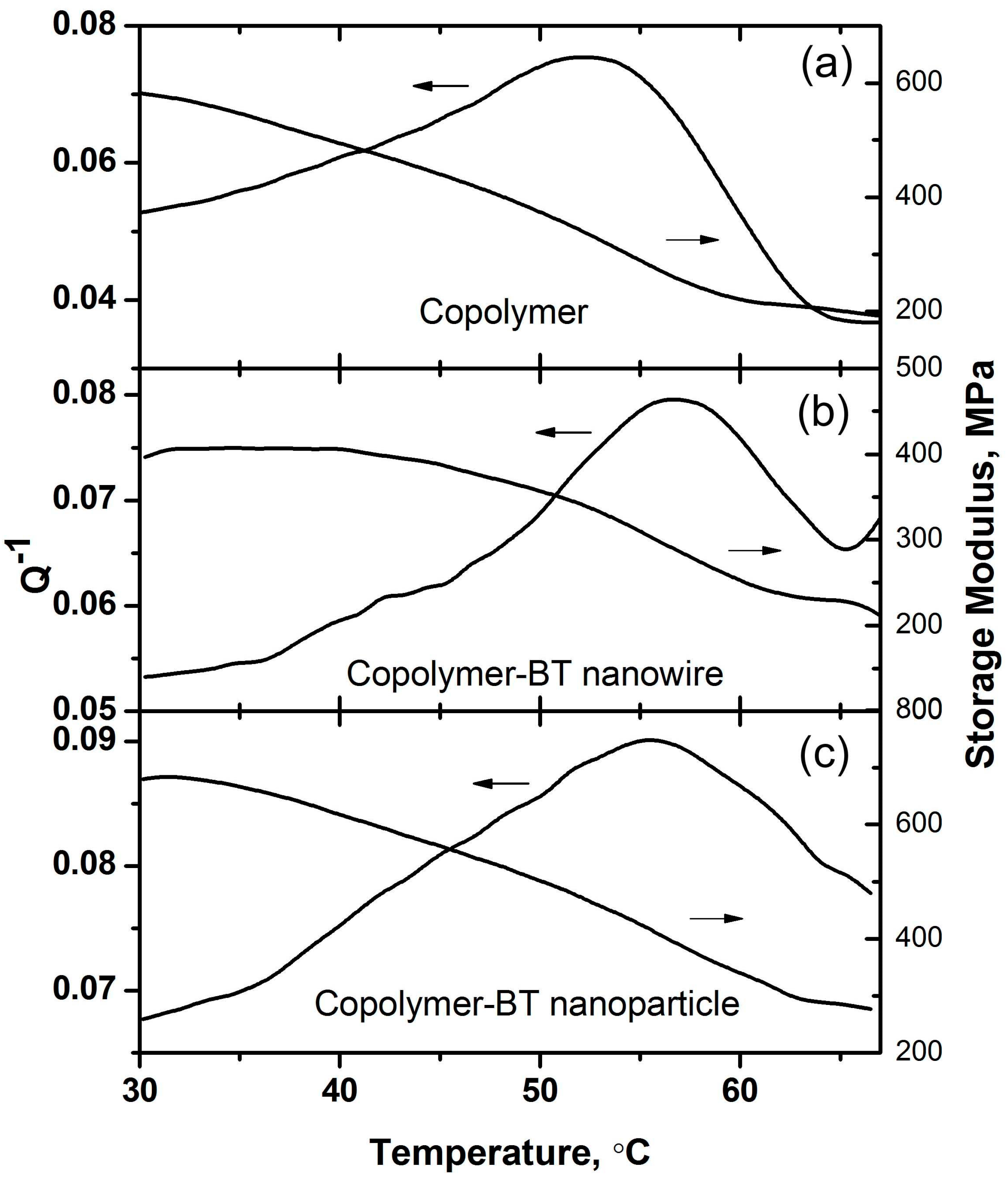
3.1. Characterizations on the P(VDF–TrFE)–BT Nanocomposites
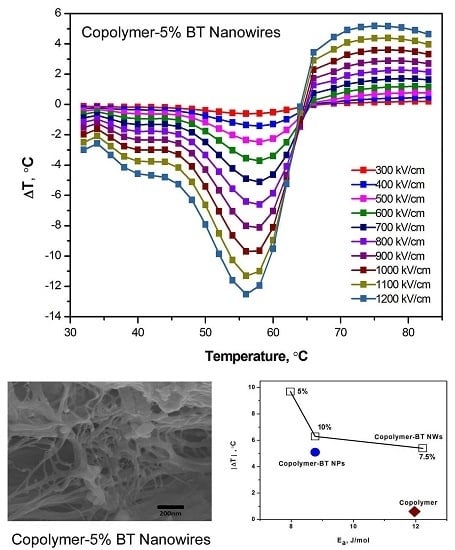
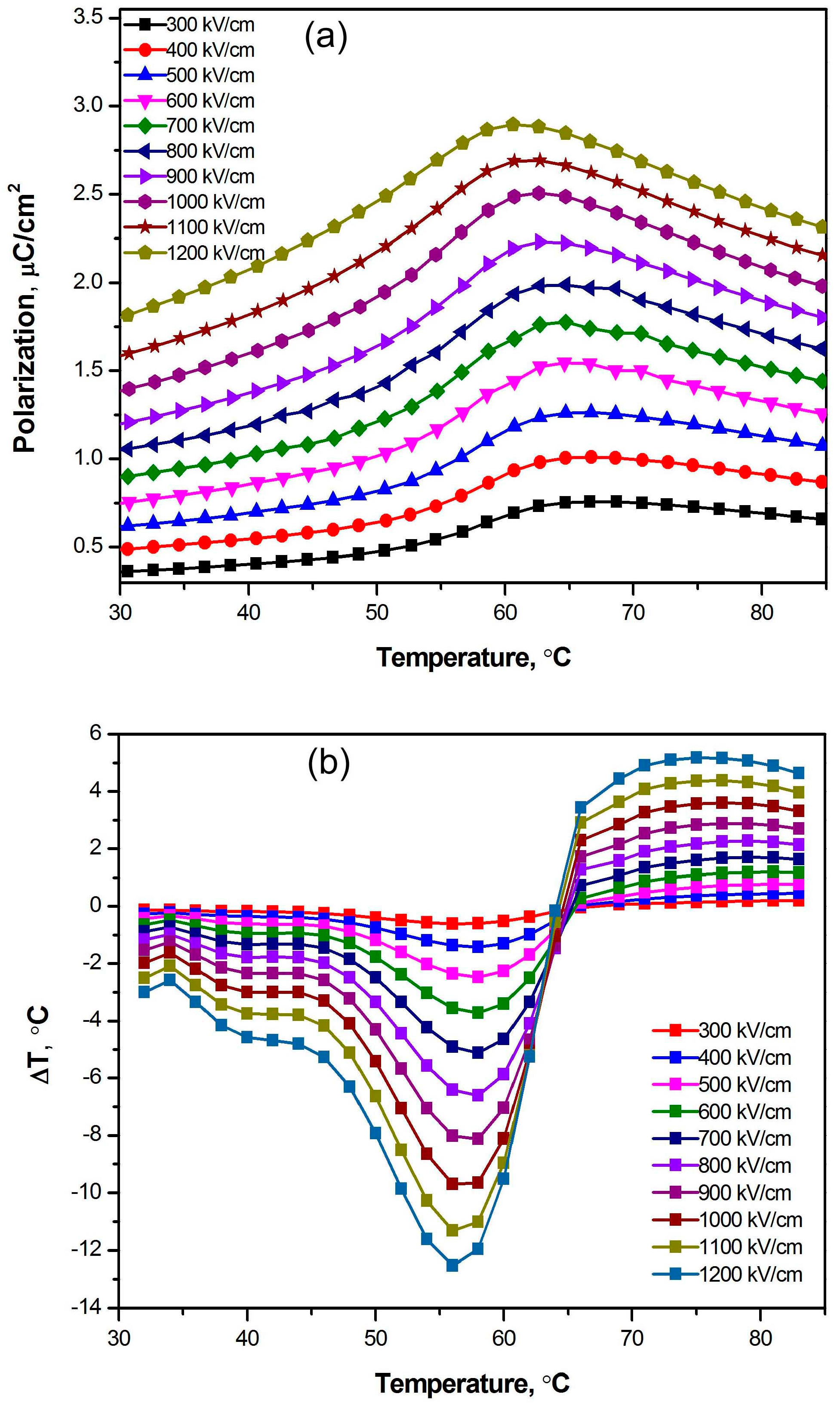
3.2. Exceptionally High Negative ECEs of Nanocomposites Containing BT Nanowires
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kobeko, P.; Kurtschatov, J.Z. Dielektrische Eigenschaften der Seignettesalzkristalle. Physik 1930, 66, 192. [Google Scholar] [CrossRef]
- Kutnjak, Z.; Rožič, B.; Pirc, R. Electrocaloric Effect: Theory, Measurements, and Applications. Wiley Encycl. Electr. Electron. Eng. 2015, 1–9. [Google Scholar] [CrossRef]
- Mischenko, A.S.; Zhang, Q.; Scott, J.F.; Whatmore, R.W.; Mathur, N.D. Giant Electrocaloric Effect in Thin-Film PbZr0.95Ti0.05O3. Science 2006, 311, 1270–1271. [Google Scholar] [CrossRef] [Green Version]
- Neese, B.; Chu, B.; Lu, S.G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large Electrocaloric Effect in Ferroelectric Polymers Near Room Temperature. Science 2008, 321, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ding, K.; Zheng, G.P.; Shi, S.Q.; Qiao, L. Entropy-change Measurement of Electrocaloric Effect of BaTiO3 Single Crystal. Phys. Status Solidi A 2012, 209, 941–944. [Google Scholar] [CrossRef]
- Scott, J.F. Electrocaloric Materials. Annu. Rev. Mater. Res. 2011, 41, 229–240. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Zhang, X.; Jiang, S.; Zeng, Y.; Wang, Q. Relaxor Ferroelectric-Based Electrocaloric Polymer Nanocomposites with a Broad Operating Temperature Range and High Cooling Energy. Adv. Mater. 2015, 27, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Q.; Gu, H.; Jiang, S.; Han, K.; Gadinski, M. R.; Haque, M.A.; Zhang, Q.; Wang, Q. Ferroelectric Polymer Nanocomposites for Room-Temperature Electrocaloric Refrigeration. Adv. Mater. 2015, 27, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Zhang, X.S.; Yang, T.N.; Li, Q.; Chen, L.Q.; Jiang, S.L.; Wang, Q. Colossal Room-Temperature Electrocaloric Effect in Ferroelectric Polymer Nanocomposites Using Nanostructured Barium Strontium Titanates. ACS Nano 2015, 9, 7164–7174. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zheng, G.P.; Shi, S.Q. Abnormal Electrocaloric Effect of Na0.5Bi0.5TiO3-BaTiO3 Lead-Free Ferroelectric Ceramics above Room Temperature. Mater. Res. Bull. 2011, 46, 1866–1869. [Google Scholar] [CrossRef]
- Zheng, X.C.; Zheng, G.P.; Lin, Z.; Jiang, Z.Y. Thermo-Electrical Energy Conversions in Bi0.5Na0.5TiO3-BaTiO3 Thin Films Prepared by Sol-Gel Method. Thin Solid Films 2012, 522, 125–128. [Google Scholar] [CrossRef]
- Goupil, F.L.; Berenov, A.; Axelsson, A.K.; Valant, M. Direct and Indirect Electrocaloric Measurements on <001>-PbMg1/3Nb2/3O3-30PbTiO3 Single Crystals. J. Appl. Phys. 2012, 111, 124109. [Google Scholar] [CrossRef]
- Jiang, X.J.; Luo, L.H.; Wang, B.Y.; Li, W.P.; Chen, H.B. Electrocaloric Effect Based on the Depolarization Transition in (1−x)Bi0.5Na0.5TiO3–xKNbO3Lead-free Ceramics. Ceram. Int. 2014, 40, 2627–2634. [Google Scholar] [CrossRef]
- Geng, W.; Liu, Y.; Meng, X.; Bellaiche, L.; Scott, J.F.; Dkhil, B.; Jiang, A. Giant Negative Electrocaloric Effect in Antiferroelectric La-Doped Pb(ZrTi)O3 Thin Films Near Room Temperature. Adv. Mater. 2015, 27, 3165. [Google Scholar] [CrossRef] [PubMed]
- Pirc, R.; Rožič, B.; Koruza, J.; Malič, B.; Kutnjak, Z. Negative Electrocaloric Effect in Antiferroelectric PbZrO3. Europhys. Lett. 2014, 107, 17002. [Google Scholar] [CrossRef]
- Vats, G.; Kumar, A.; Ortega, N.; Bowen, C.R.; Katiyar, R.S. Giant Pyroelectric Energy Harvesting and aNegative Electrocaloric Effect in Multilayered Nanostructures. Energy Environ. Sci. 2016, 9, 1335. [Google Scholar] [CrossRef] [Green Version]
- Soulestin, T.; Ladmiral, V.; Santos, F.D.D.; Améduri, B. Vinylidene Fluoride and Trifluoroethylene-containing Fluorinated Electroactive Copolymers. How Does Chemistry Impact Properties? Prog. Polym. Sci. 2017. [CrossRef]
- Tang, H.X.; Lin, Y.R.; Sodano, H.A. Synthesis of High Aspect Ratio BaTiO3 Nanowires for High Energy Density Nanocomposite Capacitors. Adv. Energy Mater. 2013, 3, 451–456. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liang, C.; Wang, M.; Zhao, P.; Liu, M.; Liu, J.; Che, R. Ultrathin BaTiO3 Nanowires with High Aspect Ratio: A Simple One-Step Hydrothermal Synthesis and Their Strong Microwave Absorption. ACS Appl. Mater. Interfaces 2013, 5, 7146–7151. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ueda, T.; Kitayama, T. Ferroelectric-to-paraelectric Phase Transition of Vinylidene Fluoride-Trifluoroethylene Copolymer. J. Appl. Phys. 1981, 52, 948–952. [Google Scholar] [CrossRef]
- Ozkazanc, E.; Guney, H.Y.; Oskay, T.; Tarcan, E. The Effect of Uniaxial Orientation on the Dielectric Relaxation Behavior of α-PVDF. J. Appl. Polym. Sci. 2008, 109, 3878–3886. [Google Scholar] [CrossRef]
- Kauzmann, W. The Nature of the Glassy State and the Behavior of Liquids at Low Temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Richert, R.; Angell, C.A. Dynamics of Glass-forming Liquids. V. On the Link between Molecular Dynamics and Configurational Entropy. J. Chem. Phys. 1998, 108, 9016–9026. [Google Scholar] [CrossRef]
- Zheng, X.C.; Zheng, G.P.; Lin, Z.; Jiang, Z.Y. Thermal and Dynamic Mechanical Analyses on Bi0.5Na0.5TiO3-BaTiO3 Ceramics Synthesized with Citrate Method. Ceram. Int. 2013, 39, 1233–1240. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.-Y.; Zheng, G.-P.; Zheng, X.-C.; Wang, H. Exceptionally High Negative Electro-Caloric Effects of Poly(VDF–co–TrFE) Based Nanocomposites Tuned by the Geometries of Barium Titanate Nanofillers. Polymers 2017, 9, 315. https://doi.org/10.3390/polym9080315
Jiang Z-Y, Zheng G-P, Zheng X-C, Wang H. Exceptionally High Negative Electro-Caloric Effects of Poly(VDF–co–TrFE) Based Nanocomposites Tuned by the Geometries of Barium Titanate Nanofillers. Polymers. 2017; 9(8):315. https://doi.org/10.3390/polym9080315
Chicago/Turabian StyleJiang, Zhi-Yuan, Guang-Ping Zheng, Xiu-Cheng Zheng, and Hao Wang. 2017. "Exceptionally High Negative Electro-Caloric Effects of Poly(VDF–co–TrFE) Based Nanocomposites Tuned by the Geometries of Barium Titanate Nanofillers" Polymers 9, no. 8: 315. https://doi.org/10.3390/polym9080315