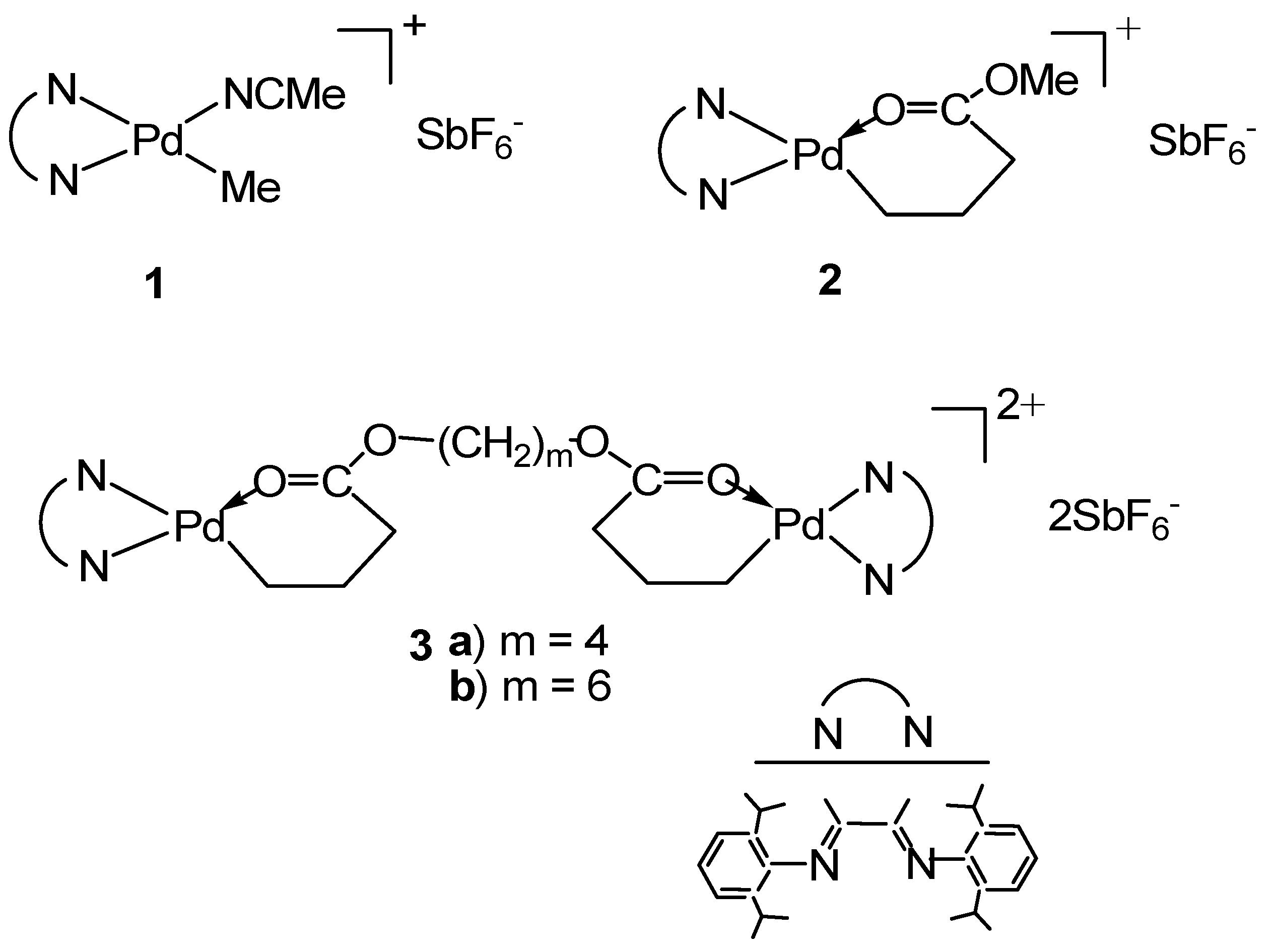
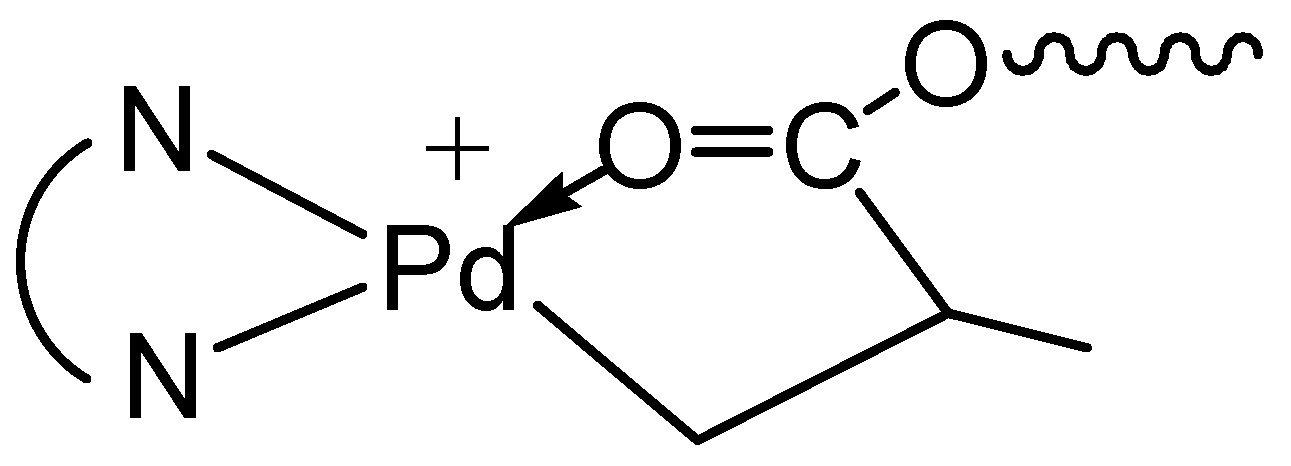3.1. Synthesis of Binuclear Pd–Diimine Chelate Complexes
The acetonitrile adduct
1 and acrylate chelate complex
2 are commonly used Pd–diimine catalysts for “living” polymerization of ethylene and α-olefins [
15,
16]. The acrylate chelate complexes (such as
2 as a typical example) can be easily synthesized by reaction of
1 with various acrylate species (Equation (1)).
In this reaction, the acrylate vinyl bond is inserted into the Pd–Me bond of
1 via a 2,1-insertion mechanism followed by rearrangement (via β-hydride elimination and reinsertion) to form the six-membered chelates, which does not allow further insertion of acrylates [
35,
36]. In olefin “living” polymerization catalyzed with the Pd–diimine acrylate chelate complexes, chain propagation starts by monomer insertion into the Pd–CH
2 bond and this yields uniquely polymer chains end-capped with a ester group (Equation (2)), which is introduced at the beginning of the chain [
16].
![Polymers 09 00282 i002]()
On the contrary, the polymers synthesized by “living” polymerization using
1 are fully saturated and unfunctionalized. With the unique synthesis and polymerization chemistry, our group has synthesized various acrylate chelate complexes [
17,
18,
19,
20,
21,
22,
28], including chelate complexes containing functional groups [
18,
19,
28], and trinuclear [
21] and multinuclear Pd–diimine complexes [
22], which facilitate the design of branched polyethylenes of various new chain architectures.
Taking advantage of the chemistry of the acrylate chelate, we synthesized in this work two binuclear Pd–diimine chelate complexes, 3a and 3b, by reacting 1 with two commercially available diacrylates, 1,4-butanediol diacrylate and 1,6-hexanediol diacrylate, respectively, using a 2:1 molar ratio between 1 and the diacrylates (Equation (3)).
![Polymers 09 00282 i003]()
In this reaction, both acrylate groups of the diacrylate monomers reacted with
1 leading to the binuclear complexes with two Pd–diimine acrylate chelates connected together through the ester linkage. The binuclear structure of the two complexes was confirmed by using
1H and
13C NMR spectroscopy.
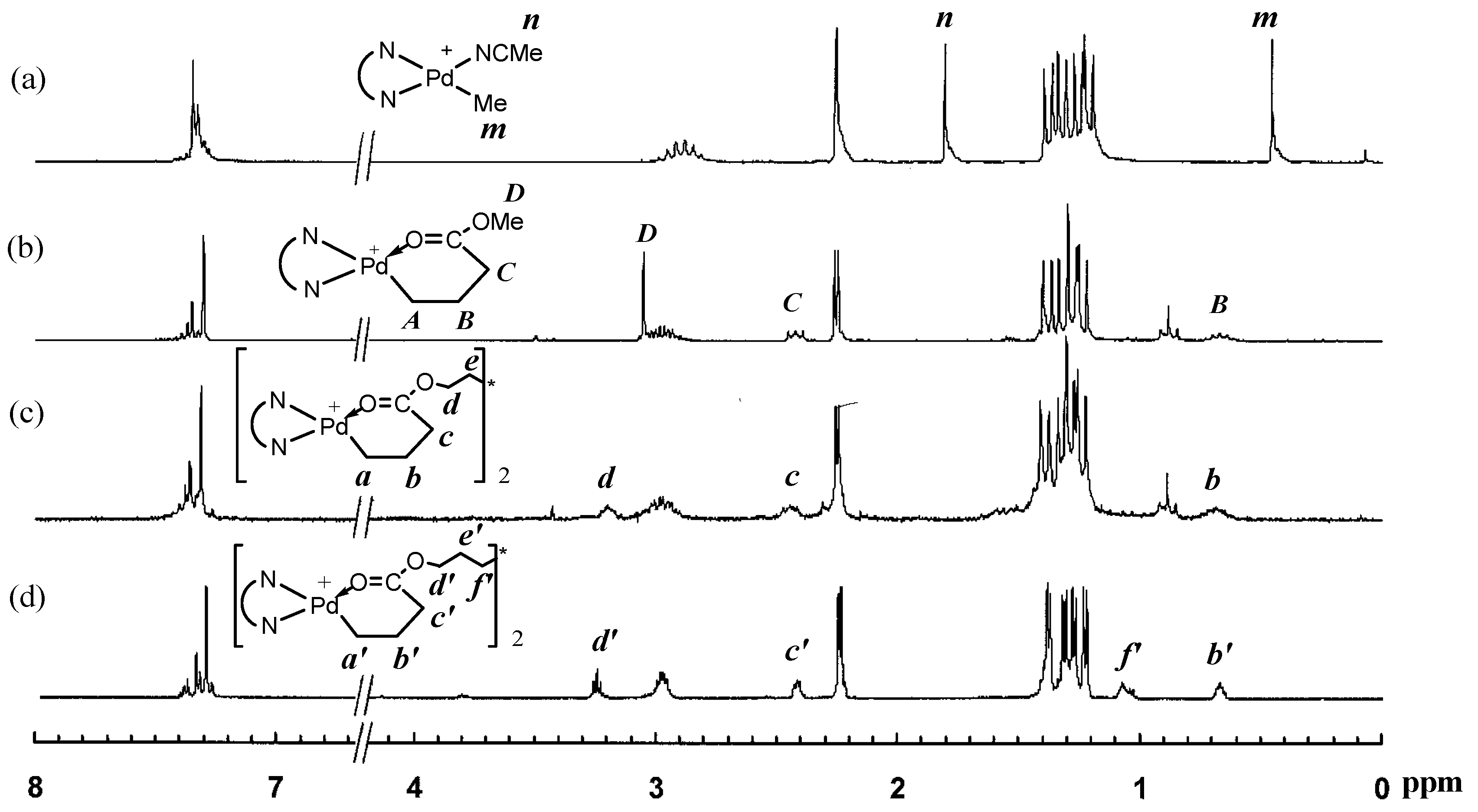
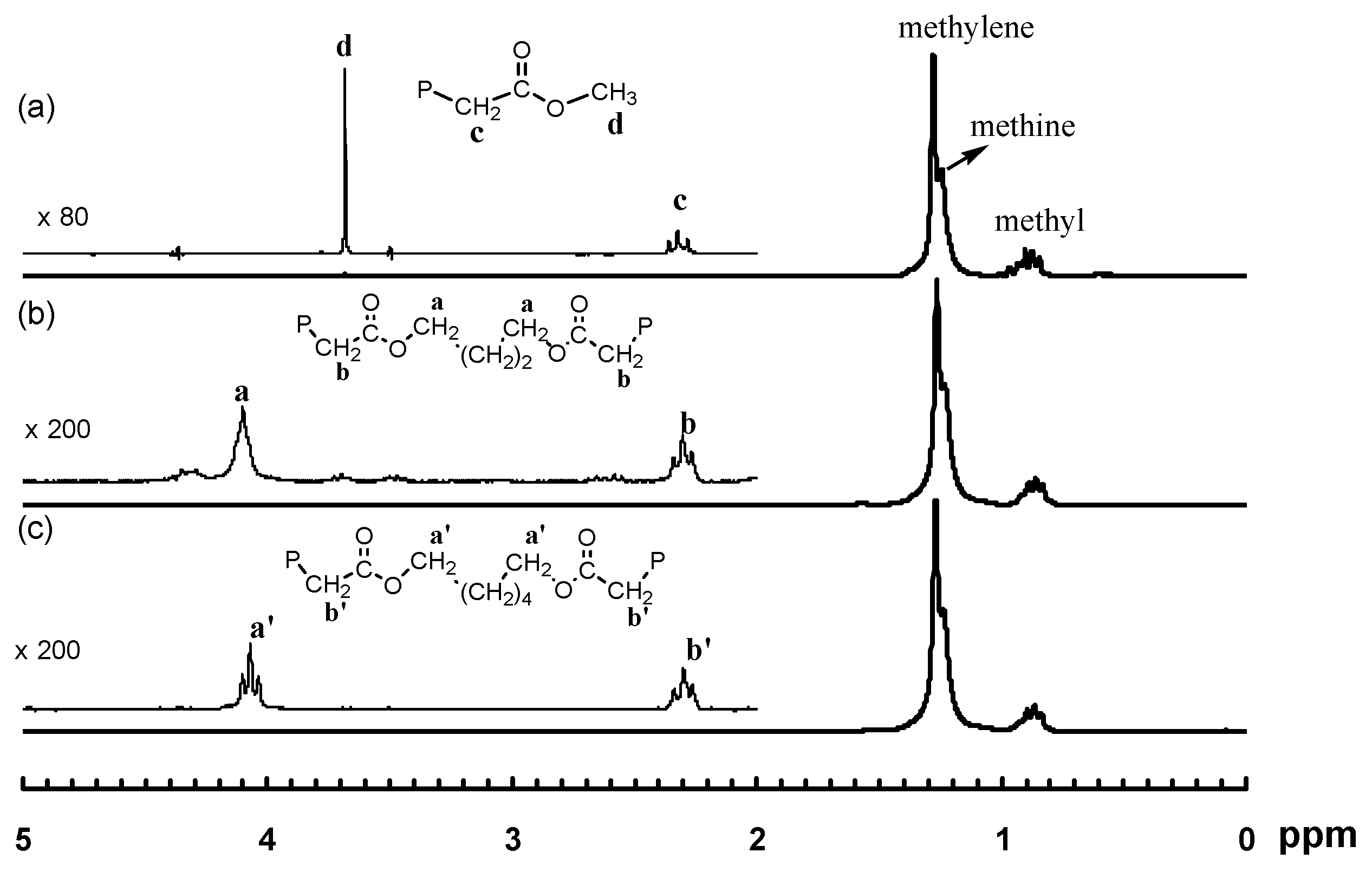
Figure 1 shows the
1H NMR spectra of the two binuclear complexes along with those of
1 and
2 for comparison. The resonance peaks at 0.46 ppm for PdMe and 1.81 ppm for PdNCMe in
1 (
m and
n in
Figure 1a, respectively) are absent in the two binuclear complexes (
Figure 1c,d)), showing the absence of unreacted
1 in
3a and
3b. Moreover, there are no peaks observed in the vinyl double bond region, which indicates the complete reaction of the acrylate groups in the diacrylates. The six-membered chelate structure in
3a and
3b is validated from the peaks at 2.42 ppm for CH
2C(O) (
c and
c’ in
Figure 1c,d, respectively) and 0.67 ppm for PdCH
2CH
2CH
2C(O) (
b and
b’ in
Figure 1c,d, respectively) [
35].
As shown by Brookhart et al., four-membered or five-membered chelate isomers are often found in the acrylate chelated Pd–diimine complexes though the six-membered chelate is always predominant [
35]. In the
13C NMR spectra of both binuclear complexes (see
Figures S1 and S2 in Supplementary Materials), a resonance peak at 194.3 ppm for C(O), typically for the five-membered chelate isomer (
Scheme 2) resulting from 1,2-insertion of the acrylate group into the Pd–Me of
1 followed by rearrangement [
35], is found. For the six-membered chelate, the peak for C(O) locates at 183.0 ppm. Integration shows that the five-membered chelate takes a percentage of about 13% in both binuclear complexes. Other chelate isomers were not found.
In particular, the binuclear structure and purity of complex
3b were also confirmed by characterization using electrospray ionization mass spectrometry (see
Figure S3 in Supplementary Materials) and elemental analysis, in addition to NMR spectra. The structure was further confirmed by using single crystal X-ray diffraction (XRD) analysis. Single crystals of
3b were obtained by layering pentane and diethyl ether into a dichloromethane solution of
3b. X-ray diffraction measurement was conducted to elucidate the molecular structure of
3b.
Figure 2 shows the thermal ellipsoid plot (30% probability) of the complex, which shows its binuclear structure with two Pd chelates joined together through the ester linkage (see
Figures S4 and S5, and Tables S1–S6 in Supplementary Materials for detailed crystallographic and molecular structure data). Owing to the structural symmetry, atoms on half of the binuclear complex are labeled in the plot. Two dichloromethane molecules are incorporated in the crystal lattice. The SbF
6− anions are far apart and do not show any interactions with the metal centers. A perusal of the bond angles around the metal center of complexes shows that the coordination geometry around the palladium center is distorted square-planar. The sterically bulky isopropyl substituted aryl rings are nearly perpendicular to the plane of the butandiimino moiety with the dihedral angles of 85.95° and 84.22°, respectively. The five-membered chelate isomer was not found in the single crystal analyzed. All the evidences above confirm the binuclear structure present in both
3a and
3b, as well as their high purity with no residual mononuclear
1 or the singly chelated complex with an unreacted pendant acrylate group observed.
3.2. Ethylene “Living” Polymerization with 2, 3a, and 3b at 400 psi and 5 °C
Ethylene “living” polymerization was conducted using the two binuclear catalysts,
3a and
3b, respectively. For comparison purpose, polymerization with the mononuclear catalyst
2 was also carried out as a control run. A polymerization condition with an ethylene pressure of 400 psi and a temperature of 5 °C, typical for ethylene “living” polymerization with Pd–diimine catalysts [
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30], was used. A catalyst concentration with [Pd] = 3.3 × 10
−4 M was used for all the polymerization runs. During the polymerizations, aliquots of the polymerization solution in chlorobenzene were removed every 1 h for 6 h and quenched with Et
3SiH prior to polymer isolation. The polymers obtained were analyzed using GPC to determine the average molecular weight and molecular weight distribution and
1H NMR to elucidate chain microstructure.
Table 1 summarizes the results for ethylene polymerization using catalysts
2.
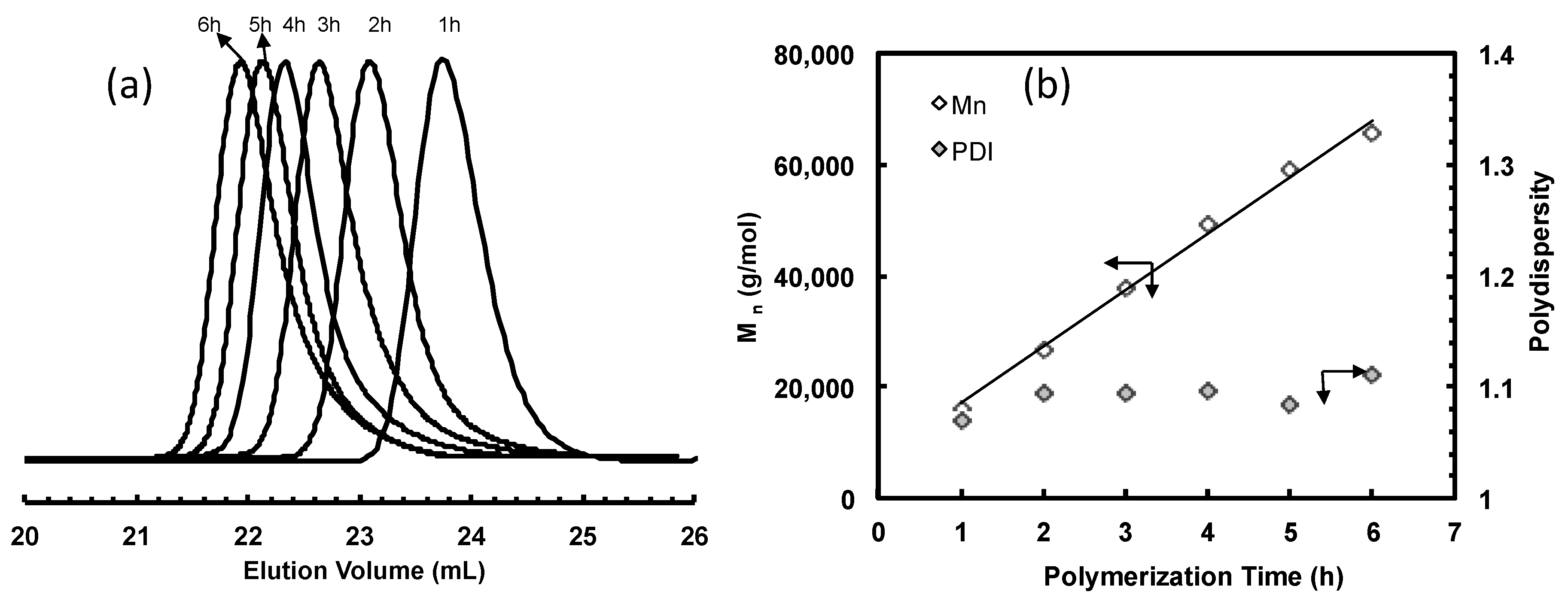
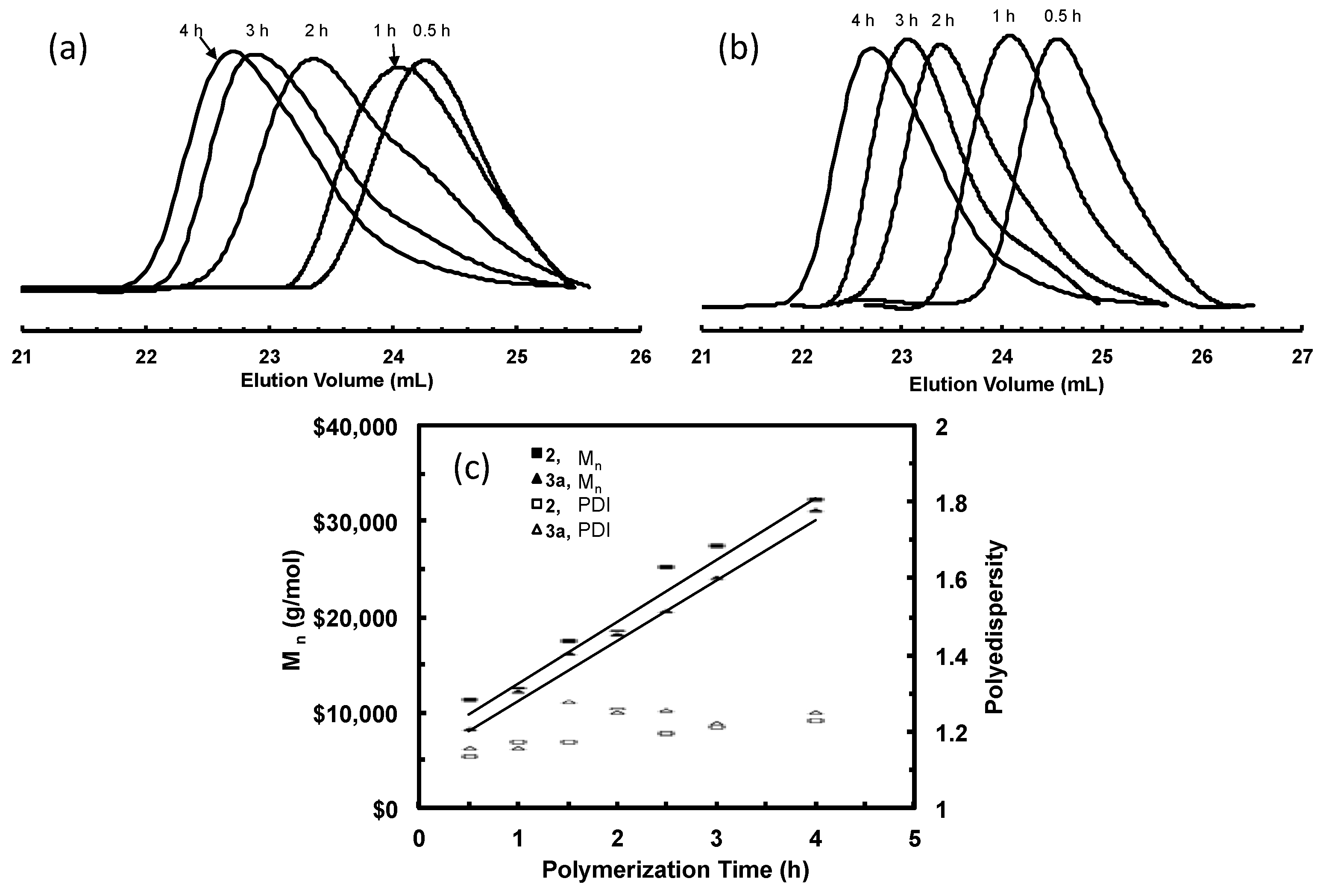
Figure 3a shows the GPC elution traces of polymer samples taken at different polymerization time during this polymerization and
Figure 3b plots the
Mn and
PDI vs. time. Characteristics of “livingness” of this polymerization system with
2 can be corroborated by the linear increase of
Mn with polymerization time. Monomodal molecular weight distribution with polydispersity below 1.11 is observed with all the samples. However, with the increase of polymerization time,
PDI increases slightly, and a low molecular weight tail in the GPC elution trace appears and becomes more obvious, indicating slight catalyst deactivation during polymerization. The
Mn values shown in
Table 2, which are based on polystyrene standards, are very close to those reported in the literature [
15] with ethylene polymerization using
2 under the identical condition. From
1H NMR analyses, the polymers are all highly branched with ca. 100 branches/1000 carbons, which was resulted from the characteristic chain walking mechanism of Pd–diimine catalysts [
13,
31,
32,
33,
34,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46]. The end-capping ester group –C(O)OCH
3 were evidenced in the
1H NMR spectra of all the samples. Based on the fact that each polymer chain contains one end-capping ester group, the turnover frequency (TOF) was calculated to be 309–367/h from the
1H NMR results of the three samples obtained within the first three hours (2-E400-1, 2-E400-2, and 2-E400-3). This TOF result is much higher compared to the literature reported data [
15,
16] for the same polymerization system, 216/h, which was calculated based on the weight of polymers produced per mole of catalysts employed. This difference indicates incomplete initiation or decomposition of some catalyst centers during the polymerization.
With the binding of two acrylate chelates on one molecule through the ester linkage, the binuclear catalysts 3a and 3b are supposed to initiate the bifunctional ethylene “living” polymerization with the simultaneous chain growth in two directions (Equation (4)). Such bifunctional “living” polymerization should theoretically yield polymers having a molecular weight twice the value of corresponding polymers obtained with the mononuclear catalyst 2 after the same polymerization time.
![Polymers 09 00282 i004]()
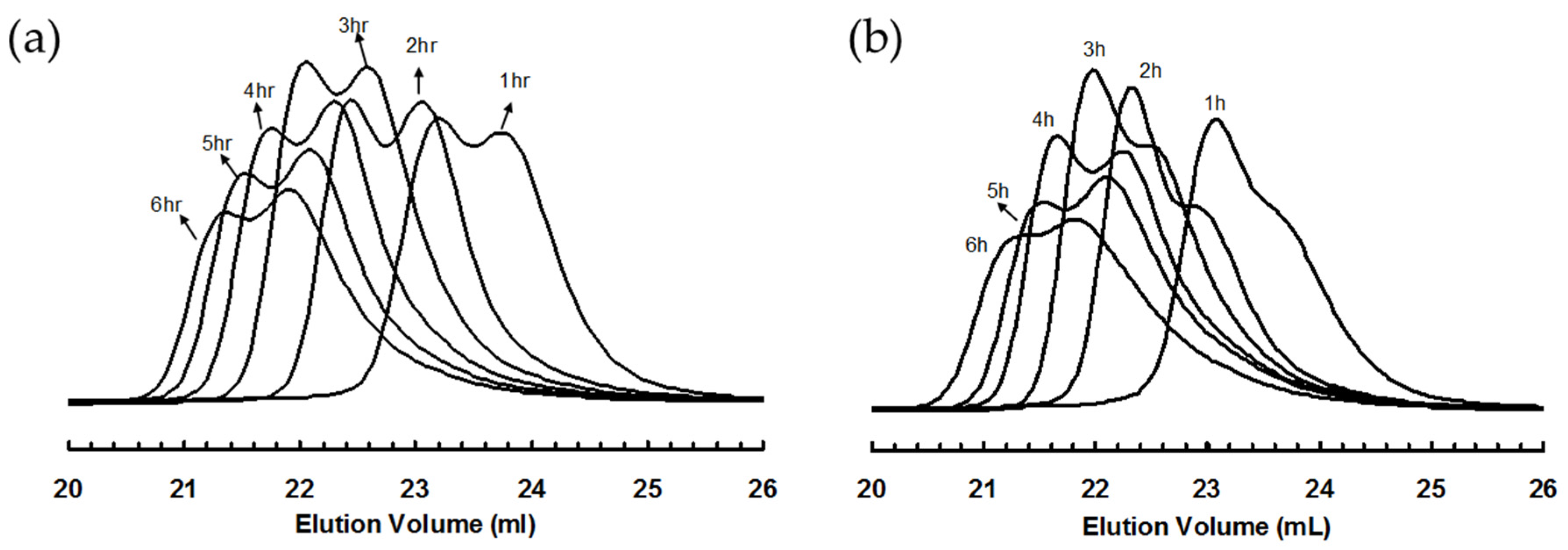
Figure 4a,b shows the GPC elution curves for the polymers obtained with catalysts
3a and
3b, respectively, at different polymerization times at 400 psi and 5 °C. Surprisingly, bimodal molecular weight distribution is observed for all the polymer samples obtained in polymerizations with both catalysts. Moreover, with the increase of polymerization time, the elution curves shift towards left with reduced elution volume, indicating the increase of average molecular weight with polymerization time. However, the polymers obtained have a
PDI below 1.41 regardless of their bimodal nature. From their
1H NMR spectra, the polymer samples possess branching density of ca. 100 branches/1000 C, which is almost identical to the polymers synthesized with
2 at the same condition.
Table 2 and
Table 3 summarize
Mn and
PDI values together with branching density data for these two batches of polymers synthesized with
3a and
3b, respectively.
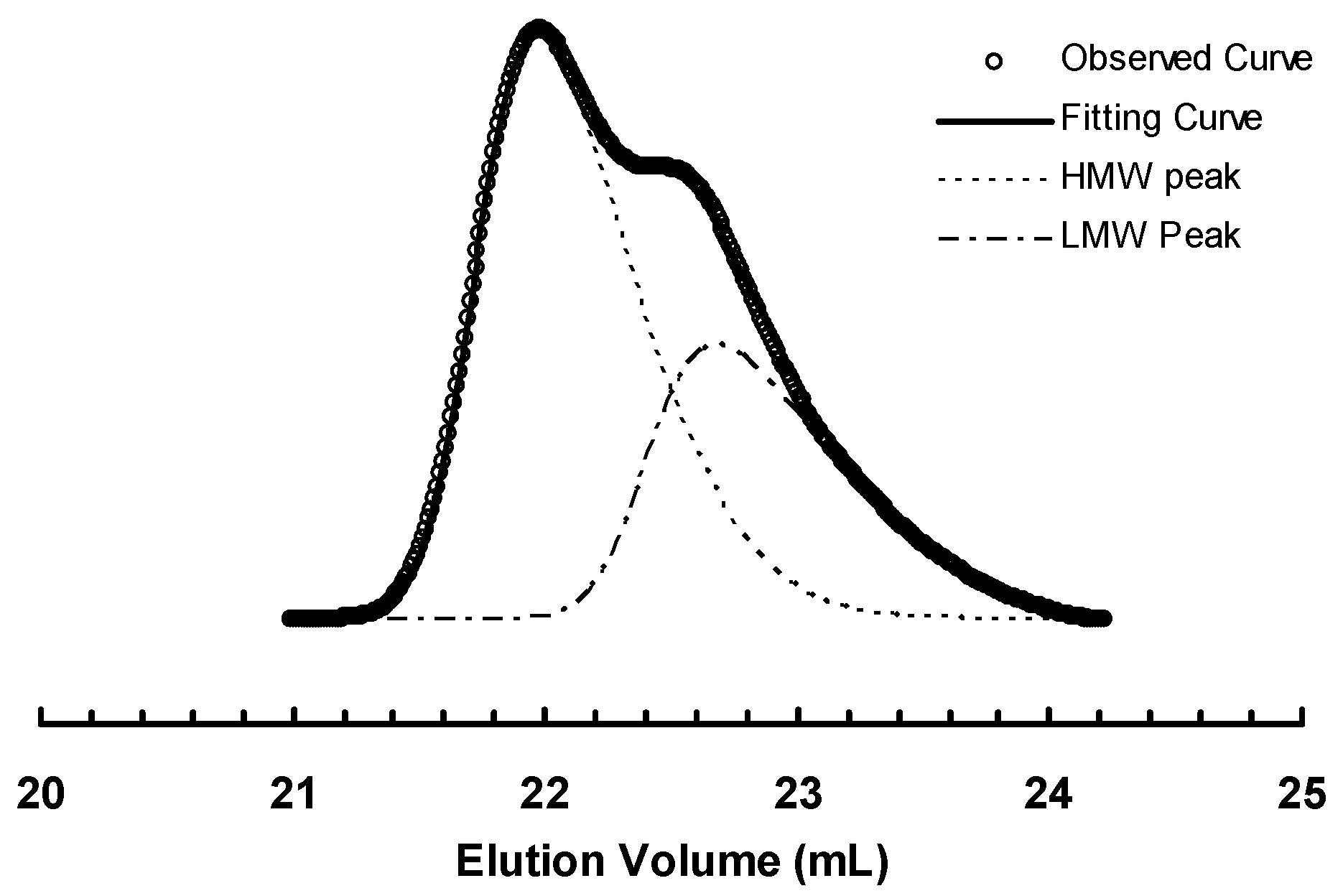
The bimodal molecular weight distribution behavior indicates the presence of two different types of catalytic growing species in the polymerization systems. Deconvolutions of GPC elution curves were conducted using a PeakFit software (v. 4.12; Systat Software, San Jose, CA, USA) to retrieve the information and relationship between the molecular weight values of polymers generated by these two types of species. The empirical half-Gaussian modified Gaussian (GMG) model was applied for all the deconvolutions. All the bimodal GPC elution traces were deconvoluted into two peaks, one high molecular weight (HMW) peak and the other low molecular weight (LMW) peak. To illustrate the effectiveness of the deconvolution procedure,
Figure 5 shows the two deconvoluted peaks for polymer sample 3b-E400-3, produced with
3b after 3 h of polymerization time, and compares the resulting fitting curve with the originally observed GPC elution curve. The chromatogram is well fitted within the whole peak range from the figure.
Deconvolution of GPC elution curve was performed on all the bimodal polymers obtained in polymerizations with
3a and
3b. One unique similarity is observed with the two batches of polymers obtained with the two catalysts. Comparing any pair of polymer samples obtained with
3a and
3b, respectively, at the same polymerization time, it is found that their LMW peaks exhibit almost identical peak positions and so do their HMW peaks. Moreover, the peak positions for the LMW peaks are also very close to that of corresponding polymer obtained with catalyst
2 after the same polymerization time.
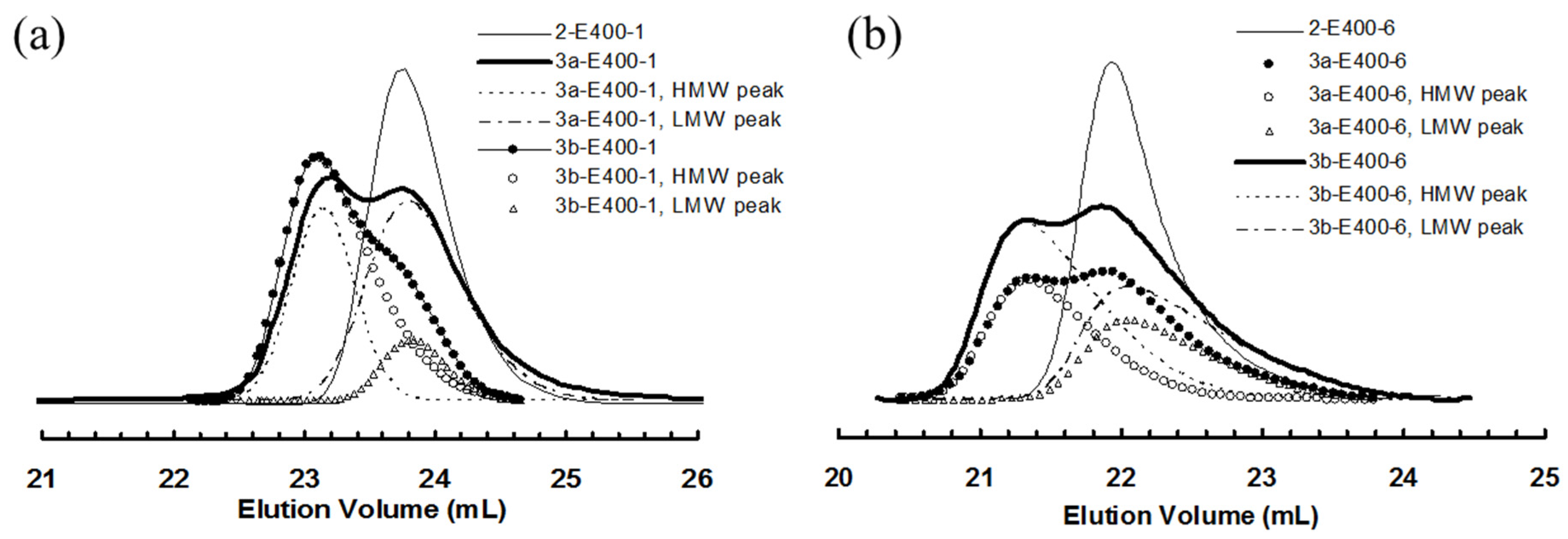
Figure 6a,b demonstrates this similarity by comparing the elution curves and deconvoluted peaks for the three polymers obtained using the three catalysts with a polymerization time of 1 h and 6 h, respectively.
The
Mn and
PDI values together with the area percentages for the fitted LMW and HMW peaks of all the bimodal polymers were calculated. These data are listed in
Table 2 and
Table 3. For both batches of polymers obtained with
3a and
3b, it is found from
Table 2 and
Table 3 that the
Mn values for both LMW and HMW peaks (
Mn,l and
Mn,h, respectively) increase with polymerization time.
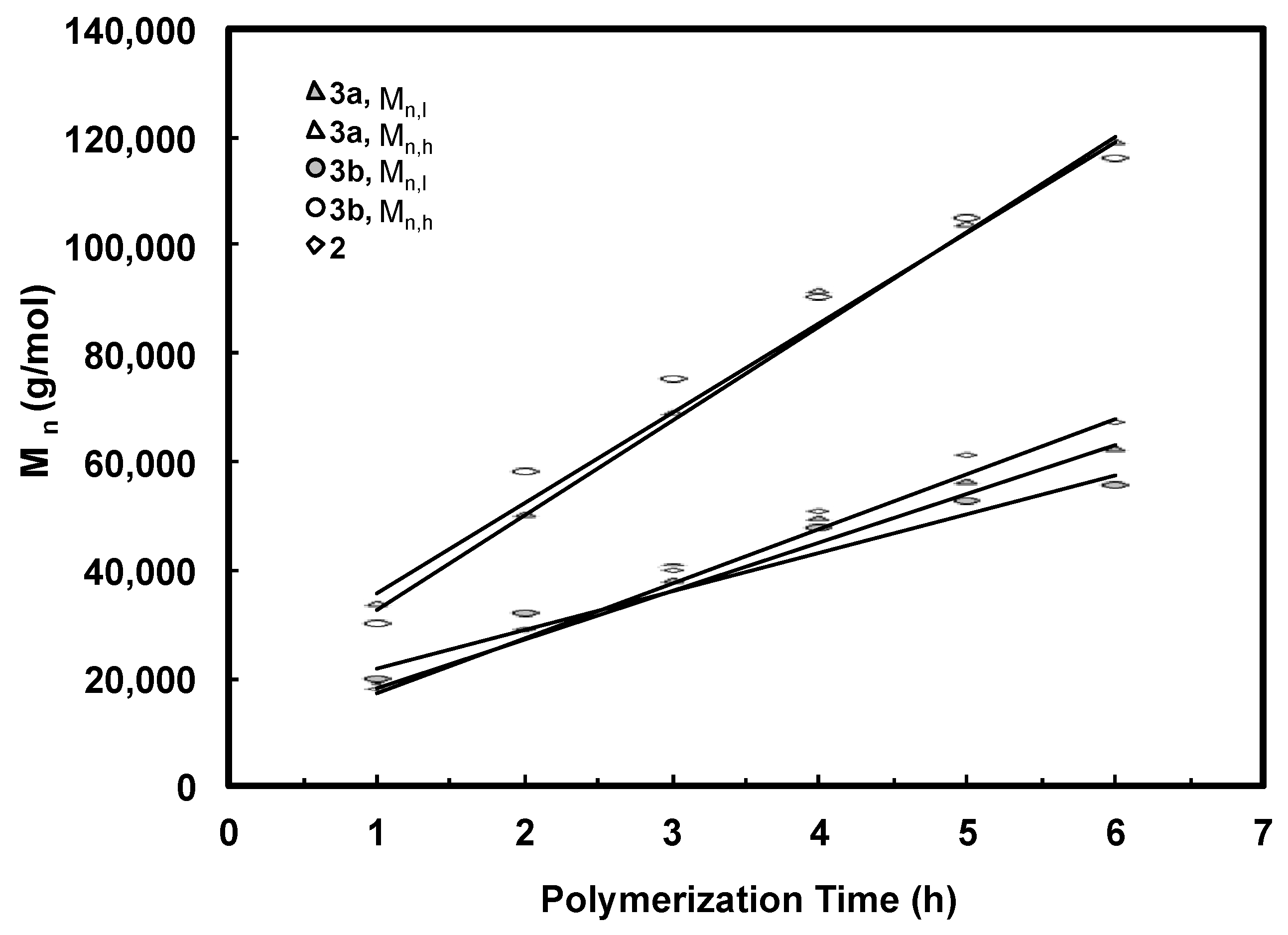
Figure 7 plots
Mn,l and
Mn,h as functions of the polymerization time for the two batches of polymers along with the data for polymers synthesized with catalyst
2 for comparison. Strikingly, linear dependencies of both
Mn,l and
Mn,h with time are evidenced. Moreover, the
Mn,l and
Mn,h values are almost identical for the polymers obtained with
3a and
3b after the same polymerization time. The
Mn,l values are also very close to those of polymers synthesized with catalyst
2 with the same polymerization time. More importantly, it is also found that the ratio of
Mn,h/
Mn,l for all the polymer samples obtained with
3a and
3b is always kept at around 2.0 (
Table 2 and
Table 3). Based on these evidences, we conclude that the LMW peaks correspond to polymers generated through monofunctional chain growth by monofunctional chain growing species resembling catalyst
2 and the HMW peaks represent polymers with twice molecular weight through bifunctional chain growth by the bifunctional species. Both these types of species exhibit “living” characteristics, as can be evidenced from the linear increases of both
Mn,l and
Mn,h with time. For all the polymers, the
PDI values of their HMW and LMW peaks are low and are generally below 1.20 (
Table 2 and
Table 3). However, the
PDI values of both peaks in each set of polymers increase with polymerization time. Low molecular weight tails are observed in their GPC elution traces and they increase proportionally with the polymerization time (
Figure 4), indicating the occurrence of some catalyst deactivation during polymerization.
Given the high purity of
3a and
3b with no residual mononuclear precursor
1 or singly-chelated complexes with one unreacted pendant acrylate group observed in our characterizations above, the presence of the monofunctional species in the polymerization systems with binuclear catalysts
3a and
3b indicates the possible incomplete initiation/activation of some metal centers of the binuclear chelate complexes and/or deactivation/chain transfer reactions of one active site in the bifunctional species during the polymerization. Similar LMW polymer fractions resulting from mononuclear catalytic species were also observed in ethylene “living” polymerization catalyzed with trinuclear and multinuclear Pd–diimine chelate complexes reported in the earlier studies by our group [
21,
22].
Owing to having the identical metal center structure, all the active growing sites, regardless of the catalysts, should possess the same TOF. The monofunctional species containing only one active site should resemble the polymerization behavior of the mononuclear catalyst
2, thus producing polymers with similar molecular weight. This also leads to the similarity observed above with
Mn,l and
Mn,h being close in the two groups of polymers obtained with
3a and
3b. The concentration ratio between these two types of species can be approximately reflected by the area percentages of the fitted LMW and HMW peaks, which are listed in
Table 2 and
Table 3 as well. For polymerization with catalyst
3a, the area percentage of the HMW peak first increases from 39% at 1 h to 61% at 2 h, and then slowly decreases. This trend of change reflects the initial increase (showing continuous chain initiation) and the late decrease (showing catalyst deactivation) in the concentration of bifunctional growing species during the course of polymerization. However, for polymerization with
3b, a continuous decrease in the area percentage of the LMW peak is found, indicating the chain initiation is faster with
3b. Based on this phenomenon, we hypothesize that chain initiation might be related to the linkage length between the two Pd chelates bound together in the same binuclear complex. The longer linkage length in
3b (two methylenes longer than
3a) might help reduce interactions between the two metal centers and improve chain initiation. The presence of both monofunctional and bifunctional chain growing species was also observed in bifunctional propylene “living” polymerization using V(acac)
3/AlEt
3Cl/diene catalyst system reported by Murata et al [
10].
Figure 8 shows the
1H NMR spectra of three polymers, 2-E400-2, 3a-E400-2 and 3b-E400-2, synthesized with
2,
3a and
3b, respectively, with 2 h of polymerization time. The end-capping methyl ester functionality is evidenced in 2-E400-2 (
Figure 8a). In both 3a-E400-2 and 3b-E400-2, an ester linkage is found with two triplet methylene resonances (a and b for 3a-E400-2 in
Figure 8b); a’ and b’ for 3b-E400-2 in
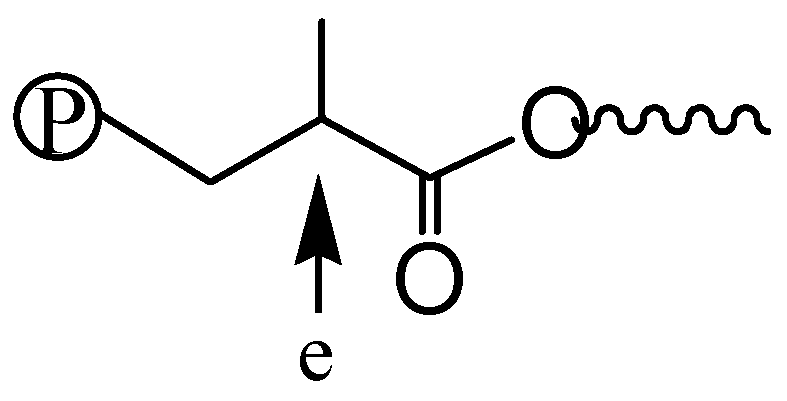
Figure 8c centered at 4.1 and 2.3 ppm, respectively. We have shown above that the isomeric five-membered chelate structure (
Scheme 2) exists in both
3a and
3b at a significant percentage of ~13%. If this chelate could also initiate ethylene “living” polymerization, the resulting polymer would possess the unique microstructure shown in
Scheme 3, where there is a methine group next to the ester functionality. The proton of this methine group (e in
Scheme 3) in this microstructure should have a multiplet centered at 2.5 ppm in the
1H NMR spectrum. However, this resonance was not found in the
1H NMR spectra (with at least 10,000 scans) of all the polymers analyzed. This microstructure was also not reported in the literature [
15,
16] with polymers obtained in ethylene “living” polymerization with catalyst
2, which was reported to have ~11% of the above five-membered chelate isomer [
36]. These results indicate that the five-membered chelates are possibly incapable of initiating ethylene “living” polymerization. If this is the case, it is another factor contributing to the presence of monofunctional chain growth in the polymerizations with
3a and
3b.
It is envisaged that, if the ester linkage is cleaved, the molecular weight of the polymers obtained by bifunctional chain growth with
3a and
3b will be halved considering the linkage being centered in the middle of the chain. Differently, for polymers obtained by monofunctional chain growth, their molecular weights should only be negligibly affected by cleavage due to the location of the ester linkage close to the chain end. It is thus expected that, after cleavage, the original bimodal polymers by
3a and
3b should exhibit a single GPC peak, which should be almost identical to the original LMW peak and to the GPC peak of corresponding polymer by
2 after the same polymerization time. This hypothesis is verified by conducting cleavage experiments on some selected polymer samples by hydrolysis of the ester linkages using KOH in a mixture of THF and methanol [
53]. The resulting polymers after hydrolysis were characterized by GPC.
Table 4 lists these samples and their molecular weights before and after the cleavage.
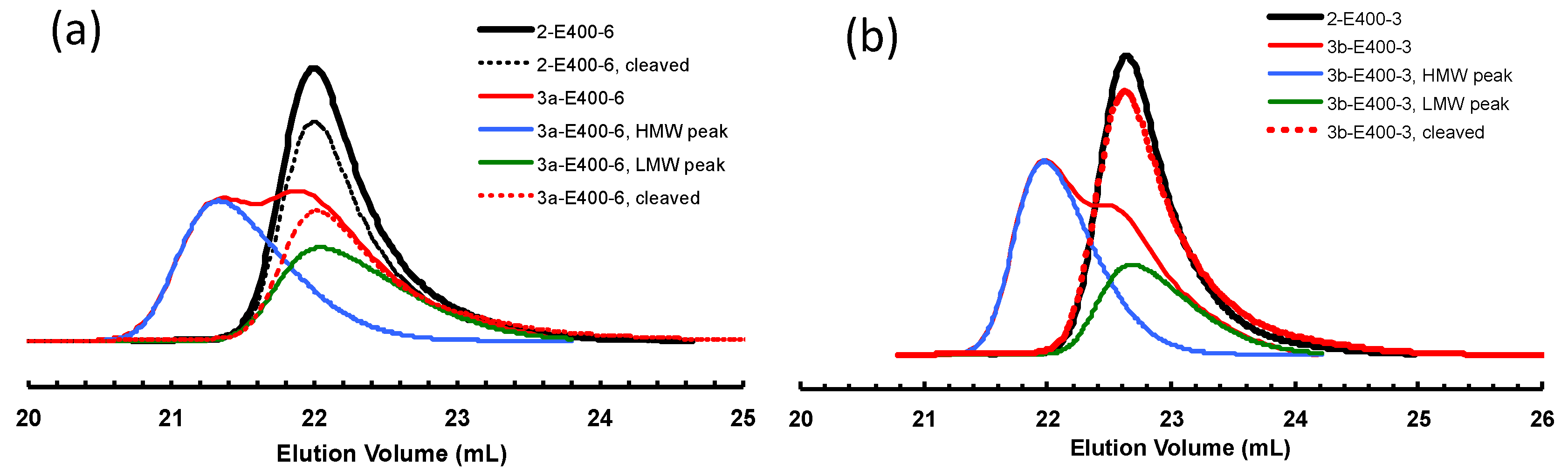
Figure 9a shows the effects of hydrolysis on the GPC elution curves of two polymers, 2-E400-6 (as a control sample) and 3a-E400-6, synthesized with
2 and
3a, respectively, with 6 h of polymerization time. For polymer 2-E400-6 grown from the monofunctional catalyst
2, no obvious change in the position and width of GPC elution curves can be found before and after the cleavage, and the changes in
Mn and
PDI are very small (
Table 4). This confirms that the polymer having a hydrocarbon backbone is stable with negligible degradation in the basic hydrolysis condition. Differently, for polymer 3a-E400-6, the cleaved sample exhibits a monomodal GPC curve in sharp contrast to the original bimodal curve. A significant 30% drop in
Mn is found after the cleavage. Moreover, the
Mn and
PDI values for the cleaved sample are very similar to those of the LMW peak and polymer 2-E400-6. This evidence further proves our conclusion above that the HMW peaks correspond to polymers obtained by bifunctional chain growth while the LMW peaks represent polymers from monofunctional chain growth.
Figure 9b shows the effect of cleavage on polymer 3b-E400-3. Similarly, the cleavage resulted in a monomodal GPC curve with
Mn and
PDI similar to those of corresponding LMW peak and polymer 2-E400-3.