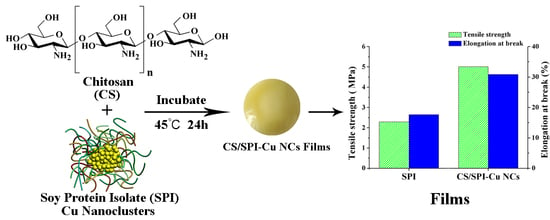
Preparation and Characterization of Chitosan/Soy Protein Isolate Nanocomposite Film Reinforced by Cu Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPI–Cu NCs
2.3. Preparation of CS/SPI–Cu NCs Composite Films
2.4. Characterization of SPI/CS Nanocomposite Films
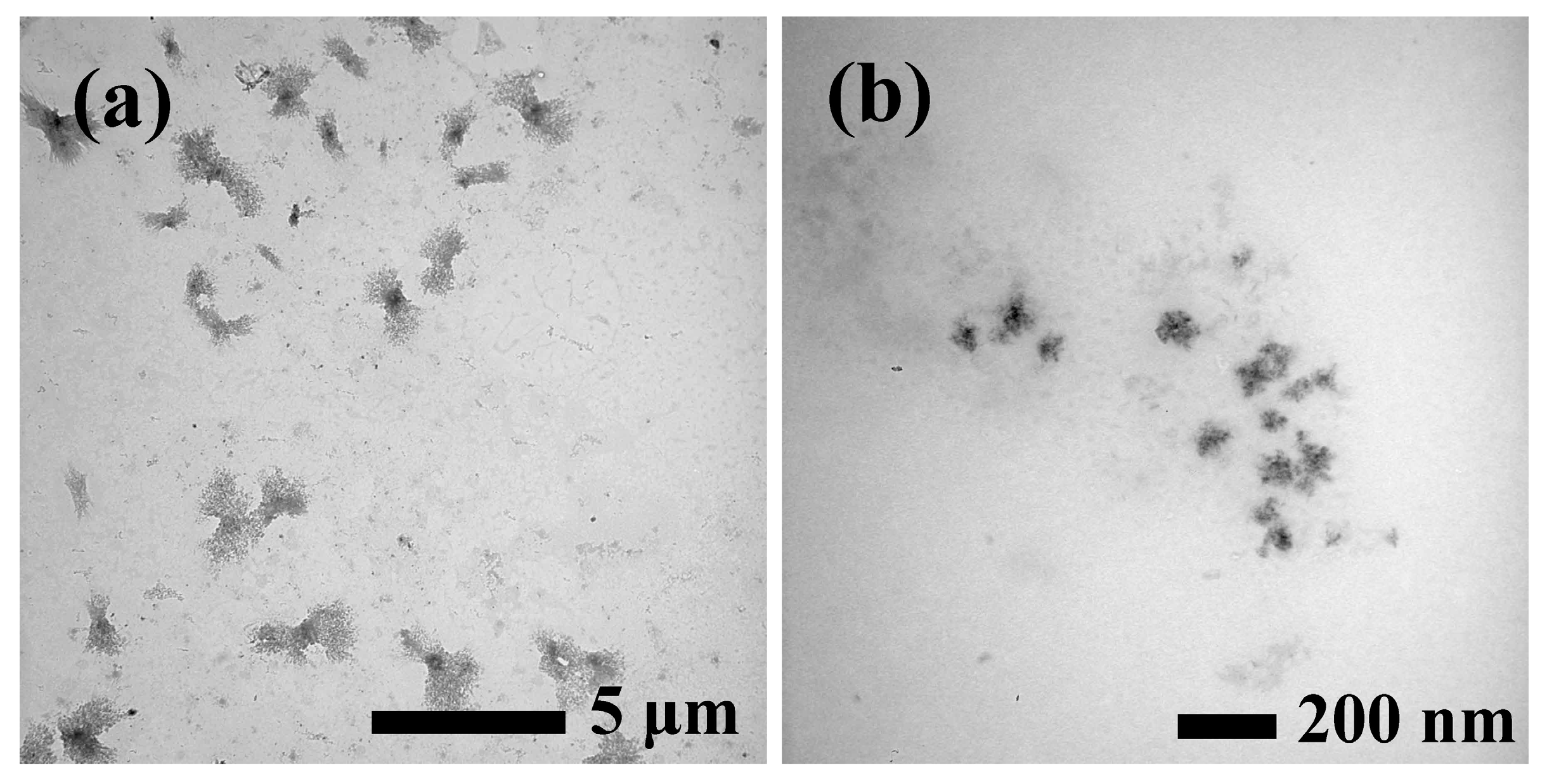
2.4.1. Transmission Electron Microscopy (TEM)
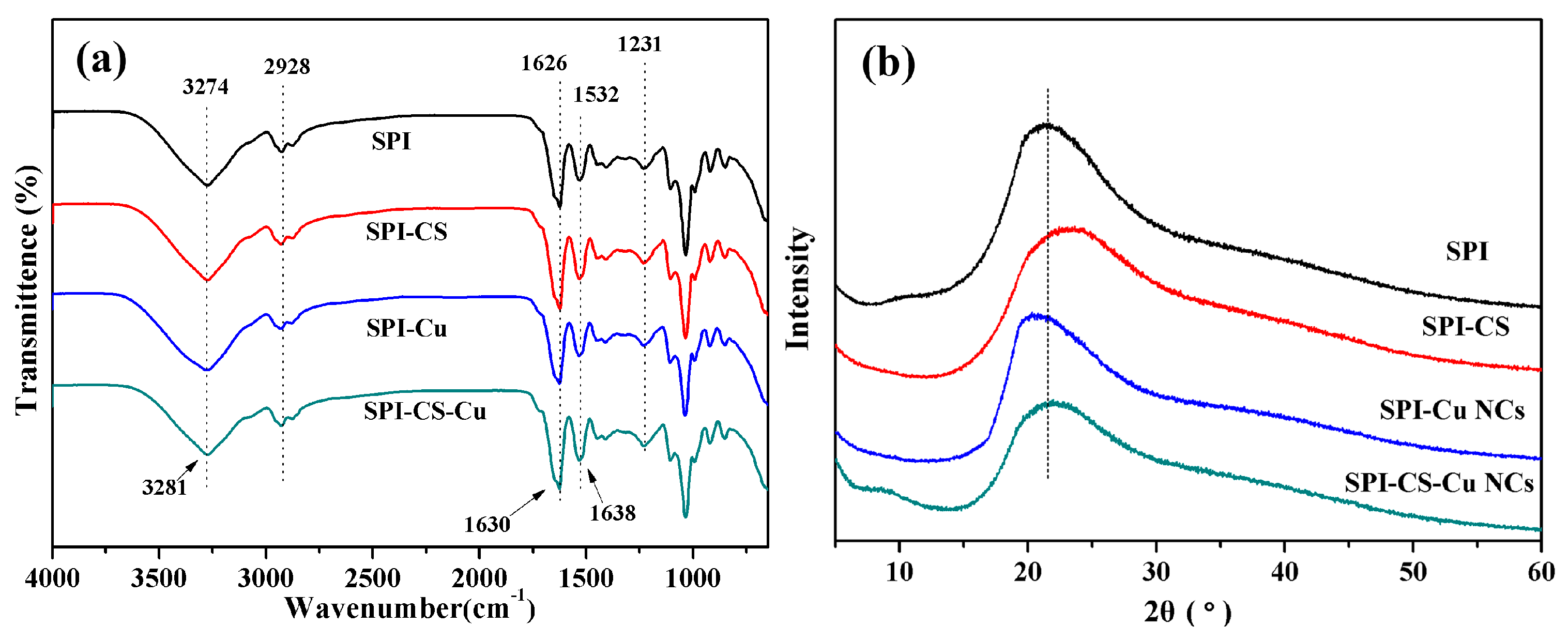
2.4.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
2.4.3. X-Ray Diffraction Analysis
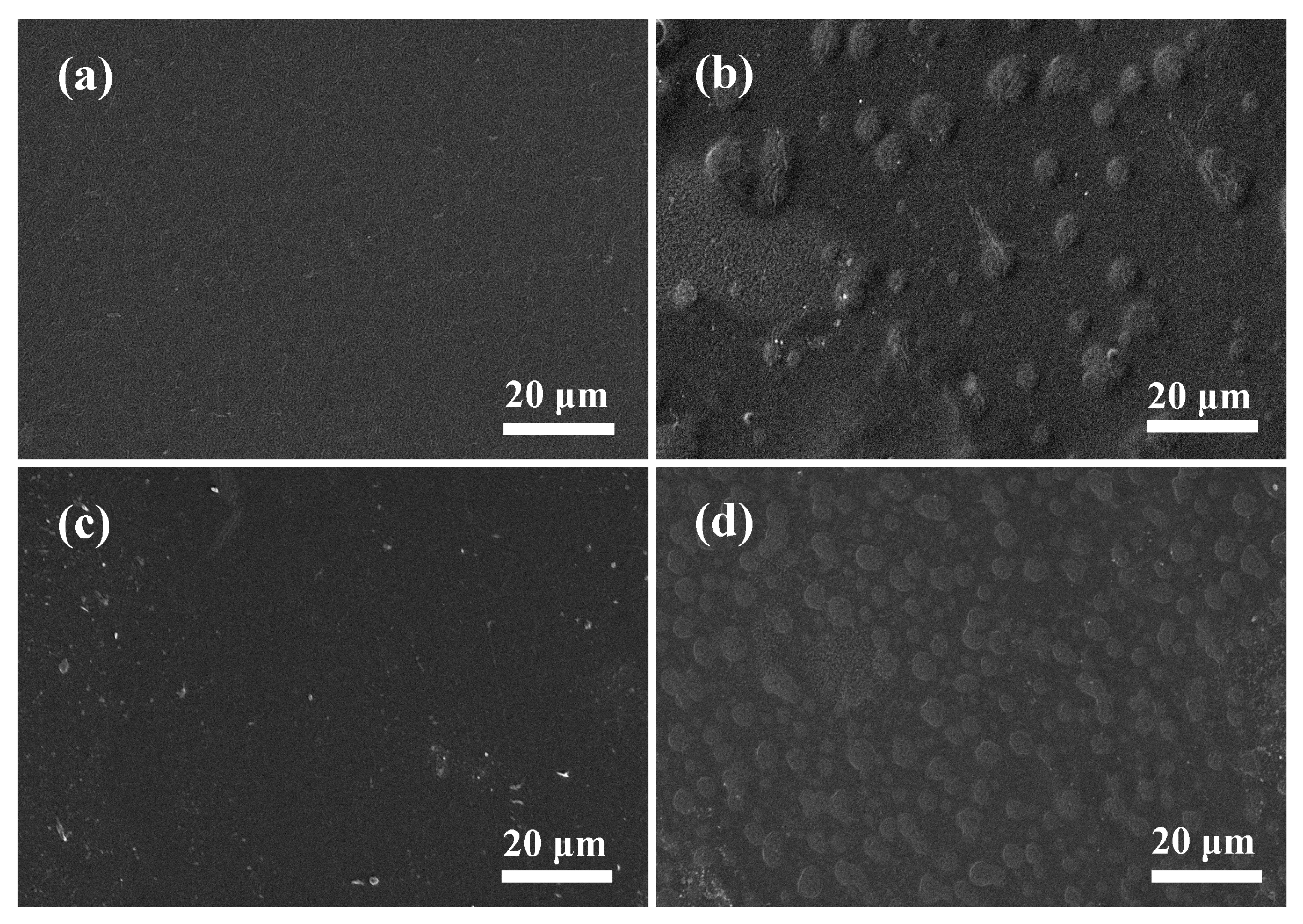
2.4.4. Scanning Electron Microscope
2.4.5. Mechanical Properties
2.4.6. Surface Contact Angles
2.4.7. Thermo-Gravimetric Analysis
2.4.8. Moisture Content
2.4.9. Water Vapor Permeability
2.4.10. Statistical Analysis
3. Results
3.1. Characterization of SPI–Cu NCs
3.2. Structural Analysis
3.3. Micromorphology of SPI-Based Film
3.4. Physical and Mechanical Properties
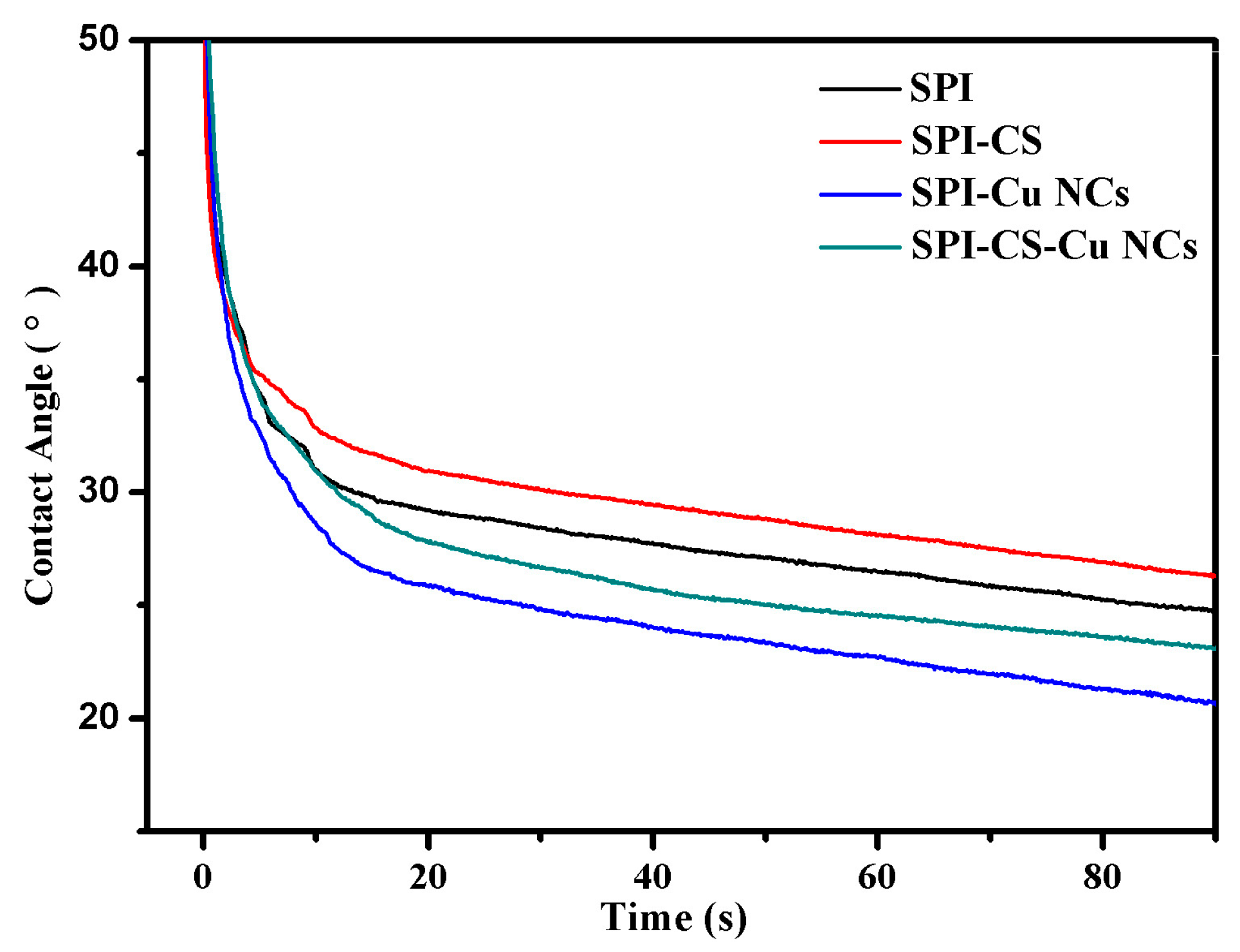
3.5. Contact Angles Analysis
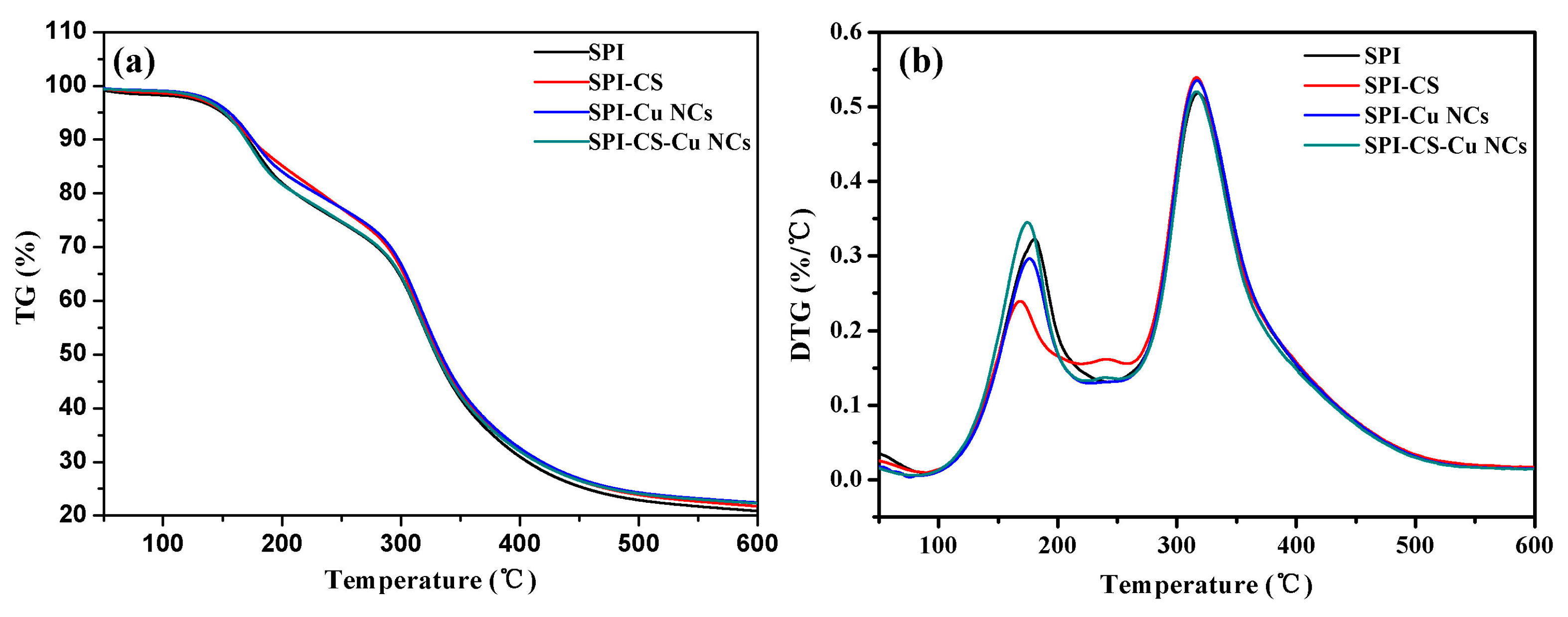
3.6. Thermo-Gravimetric Analysis
3.7. Water Resistance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Müller, K.; Jesdinszki, M.; Schmid, M. Modification of functional properties of whey protein isolate nanocomposite films and coatings with nanoclays. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Weizman, O.; Dotan, A.; Nir, Y.; Ophir, A. Modified whey protein coatings for improved gas barrier properties of biodegradable films. Polym. Adv. Technol. 2017, 28, 261–270. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Garrido, T.; Etxabide, A.; Peñalba, M.; de la Caba, K.; Guerrero, P. Preparation and characterization of soy protein thin films: Processing–properties correlation. Mater. Lett. 2013, 105, 110–112. [Google Scholar] [CrossRef]
- Dash, S.; Swain, S.K. Effect of nanoboron nitride on the physical and chemical properties of soy protein. Compos. Sci. Technol. 2013, 84, 39–43. [Google Scholar] [CrossRef]
- Morales, R.; Martinez, K.D.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochem. 2015, 26, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Anandjiwala, R.D.; Kumar, A. Thermal and mechanical properties of mandelic acid-incorporated soy protein films. J. Therm. Anal. Calorim. 2015, 123, 1273–1279. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by blown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Jiang, B.; Chen, J.; Jin, Z. Blend-modification of soy protein/lauric acid edible films using polysaccharides. Food Chem. 2014, 151, 1–6. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Strumia, M.C.; Alvarez Igarzabal, C.I. Cross-linked soy protein as material for biodegradable films: Synthesis, characterization and biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Nanocrystal-reinforced soy protein films and their application as active packaging. Food Hydrocoll. 2015, 43, 777–784. [Google Scholar] [CrossRef]
- Jensen, A.; Lim, L.T.; Barbut, S.; Marcone, M. Development and characterization of soy protein films incorporated with cellulose fibers using a hot surface casting technique. LWT Food Sci. Technol. 2015, 60, 162–170. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Wang, H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr. Polym. 2016, 138, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Li, C.; Gan, L.; He, M.; He, X.; Tian, W.; Li, M.; Xu, L.; Li, Y.; et al. Improvement in physical and biological properties of chitosan/soy protein films by surface grafted heparin. J. Biol. Macromol. 2016, 83, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein-chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886. [Google Scholar] [CrossRef]
- Boy, R.; Maness, C.; Kotek, R. Properties of chitosan/soy protein blended films with added plasticizing agent as a function of solvent type at acidic pH. Int. J. Polym. Mater. Polym. Biomater. 2015, 65, 11–17. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Kehoe, T.; Latorre, M.; Serrano, C.; Philippe, S.; Schmid, M. Recent prospects in the inline monitoring of nanocomposites and nanocoatings by optical technologies. Nanomaterials 2016, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bolz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Xu, W.; Xu, S. Luminescent composite polymer fibers: In situ synthesis of silver nanoclusters in electrospun polymer fibers and application. Mater. Sci. Eng. C 2014, 42, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, G.U. Metal nanoclusters: Protein corona formation and implications for biological applications. Int. J. Biochem. Cell Biol. 2016, 75, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Water-soluble fluorescent silver nanoclusters. Adv. Mater. 2010, 22, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Fang, Y.; Yao, K. Water vapor barrier and mechanical properties of konjac glucomannan–chitosan–soy protein isolate edible films. Food Bioprod. Process. 2009, 87, 7–10. [Google Scholar] [CrossRef]
- Schmid, M.; Reichert, K.; Hammann, F.; Stäbler, A. Storage time-dependent alteration of molecular interaction–property relationships of whey protein isolate-based films and coatings. J. Mater. Sci. 2015, 50, 4396–4404. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Dong, Y.; Li, K.; Li, L.; Li, J. Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Ind. Crops Prod. 2016, 82, 133–140. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M. Synthesis and characterization of ZnS nanoclusters via hydrothermal processing from [bis (salicylidene) zinc (II)]. J. Alloys Compd. 2009, 470, 502–506. [Google Scholar] [CrossRef]
- Li, K.; Chen, H.; Li, Y.; Li, J.; He, J. Endogenous Cu and Zn nanocluster-regulated soy protein isolate films: Excellent hydrophobicity and flexibility. RSC Adv. 2015, 5, 66543–66548. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Osako, K. Development and characterisation of blend films based on fish protein isolate and fish skin gelatin. Food Hydrocoll. 2014, 39, 58–67. [Google Scholar] [CrossRef]
- Guerrero, P.; Stefani, P.M.; Ruseckaite, R.A.; de la Caba, K. Functional properties of films based on soy protein isolate and gelatin processed by compression molding. J. Food Eng. 2011, 105, 65–72. [Google Scholar] [CrossRef]
- Guerrero, P.; Leceta, I.; Peñalba, M.; de la Caba, K. Optical and mechanical properties of thin films based on proteins. Mater. Lett. 2014, 124, 286–288. [Google Scholar] [CrossRef]
- Ferreira, C.O.; Nunes, C.A.; Delgadillo, I.; Lopes-da-Silva, J.A. Characterization of chitosan-whey protein films at acid pH. Food Res. Int. 2009, 42, 807–813. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Chen, H.; Lin, L.; Li, H.; Li, J.; Lin, J.-M. Aggregation-induced structuretransition of protein-stabilized zinc/copper nanoclusters for amplified chemiluminescence. ACS Nano 2015, 9, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A high-performance soy protein isolate-based nanocomposite film modified with microcrystalline cellulose and Cu and Zn nanoclusters. Polymers 2017, 9, 167–178. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Zhang, L.; Quan, C.; Zhang, X. Improved flexibility and water resistance of soy protein thermoplastics containing waterborne polyurethane. Ind. Crops Prod. 2010, 32, 13–20. [Google Scholar] [CrossRef]
- Schmidt, V.; Giacomelli, C.; Soldi, V. Thermal stability of films formed by soy protein isolate-sodium dodecyl sulfate. Polym. Degrad. Stab. 2005, 87, 25–31. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Hambleton, A.; Lenart, A.; Voilley, A. Protein and glycerol contents affect physic-chemical properties of soy protein isolate-based edible films. Food Sci. Emerg. 2010, 11, 503–510. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Song, F.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Roles of soft segment length in structure and property of soy protein isolate/waterborne polyurethane blend films. Ind. Eng. Chem. Res. 2016, 55, 1229–1235. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Olsen, C.W.; Bilbao-Sáinz, C.; McHugh, T.H. Mechanical and water barrier properties of isolated soy protein composite edible films as affected by carvacrol and cinnamaldehyde micro and nanoemulsions. Food Hydrocoll. 2016, 57, 72–79. [Google Scholar] [CrossRef]
| Samples | Thickness | TS | E | EB |
|---|---|---|---|---|
| (mm) | (MPa) | (MPa) | (%) | |
| SPI | 0.213 (0.019) b | 2.29 (0.16) c | 58.75 (2.52) c | 17.63 (0.09) c |
| SPI–CS | 0.190 (0.024) c | 3.02 (0.28) b | 67.89 (3.29) c | 62.86 (0.04) a |
| SPI–Cu NCs | 0.234 (0.015) a | 3.55 (0.21) b | 149.20 (3.40) b | 17.05 (0.17) c |
| SPI–CS–Cu NCs | 0.240 (0.026) a | 5.01 (0.34) a | 197.50 (4.05) a | 30.84 (0.13) b |
| Samples | Moisture content (%) | Water vapor permeation (g·mm·h−1·m−2·kPa−1) |
|---|---|---|
| SPI | 9.89 (1.3) c | 1.16 (0.15) b |
| SPI–CS | 15.40 (1.6) a | 1.27 (0.19) a |
| SPI–Cu NCs | 11.84 (1.1) b | 0.99 (0.12) c |
| SPI–CS–Cu NCs | 14.09 (1.8) a | 1.10 (0.16) b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Jin, S.; Liu, X.; Chen, H.; He, J.; Li, J. Preparation and Characterization of Chitosan/Soy Protein Isolate Nanocomposite Film Reinforced by Cu Nanoclusters. Polymers 2017, 9, 247. https://doi.org/10.3390/polym9070247
Li K, Jin S, Liu X, Chen H, He J, Li J. Preparation and Characterization of Chitosan/Soy Protein Isolate Nanocomposite Film Reinforced by Cu Nanoclusters. Polymers. 2017; 9(7):247. https://doi.org/10.3390/polym9070247
Chicago/Turabian StyleLi, Kuang, Shicun Jin, Xiaorong Liu, Hui Chen, Jing He, and Jianzhang Li. 2017. "Preparation and Characterization of Chitosan/Soy Protein Isolate Nanocomposite Film Reinforced by Cu Nanoclusters" Polymers 9, no. 7: 247. https://doi.org/10.3390/polym9070247