Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications
Abstract
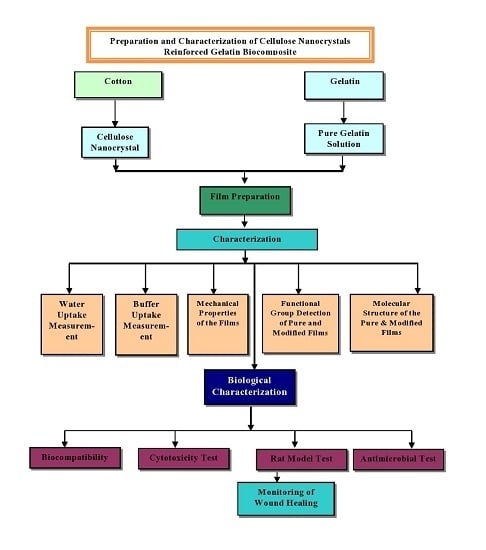
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Extraction of CCs
2.2.2. Preparation of Gelatin Solution
2.2.3. Preparation of Gelatin Film
2.2.4. Preparation of CC/Gelatin Biocomposite Film
2.3. Characterization
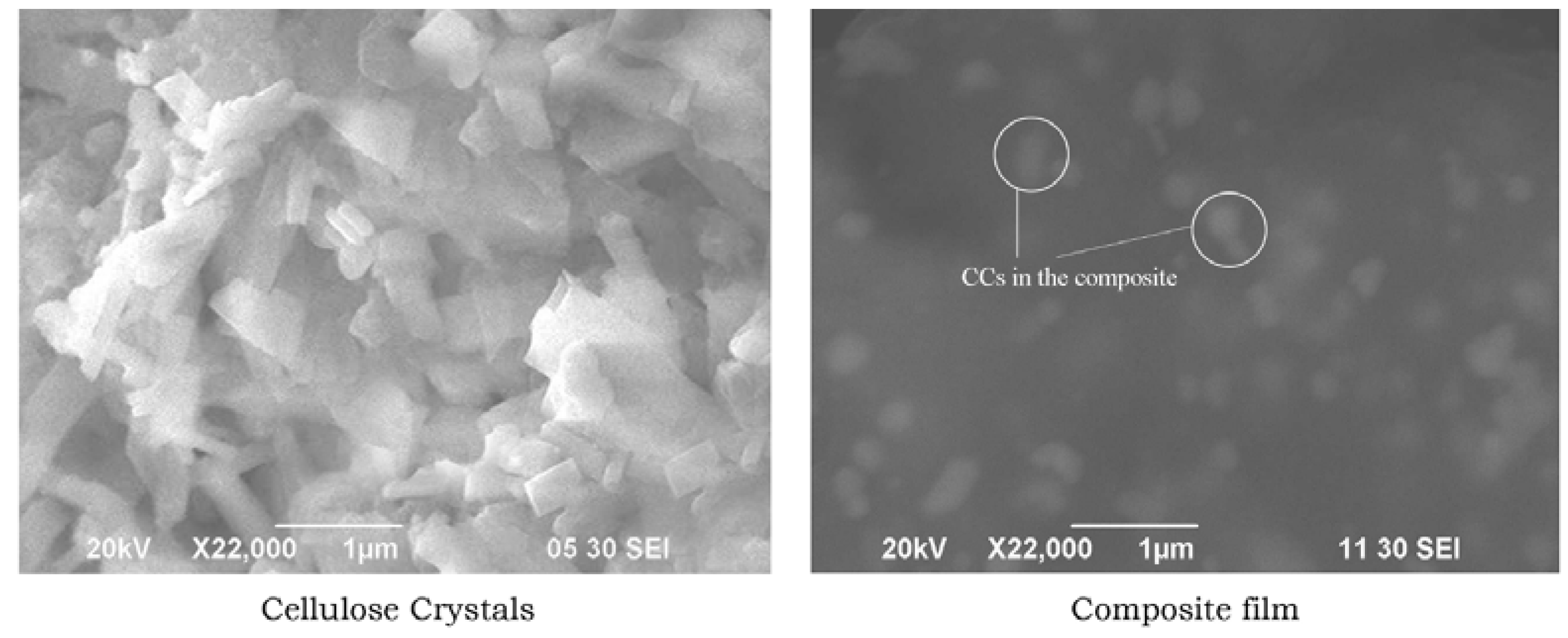
2.3.1. Scanning Electron Microscopy (SEM)
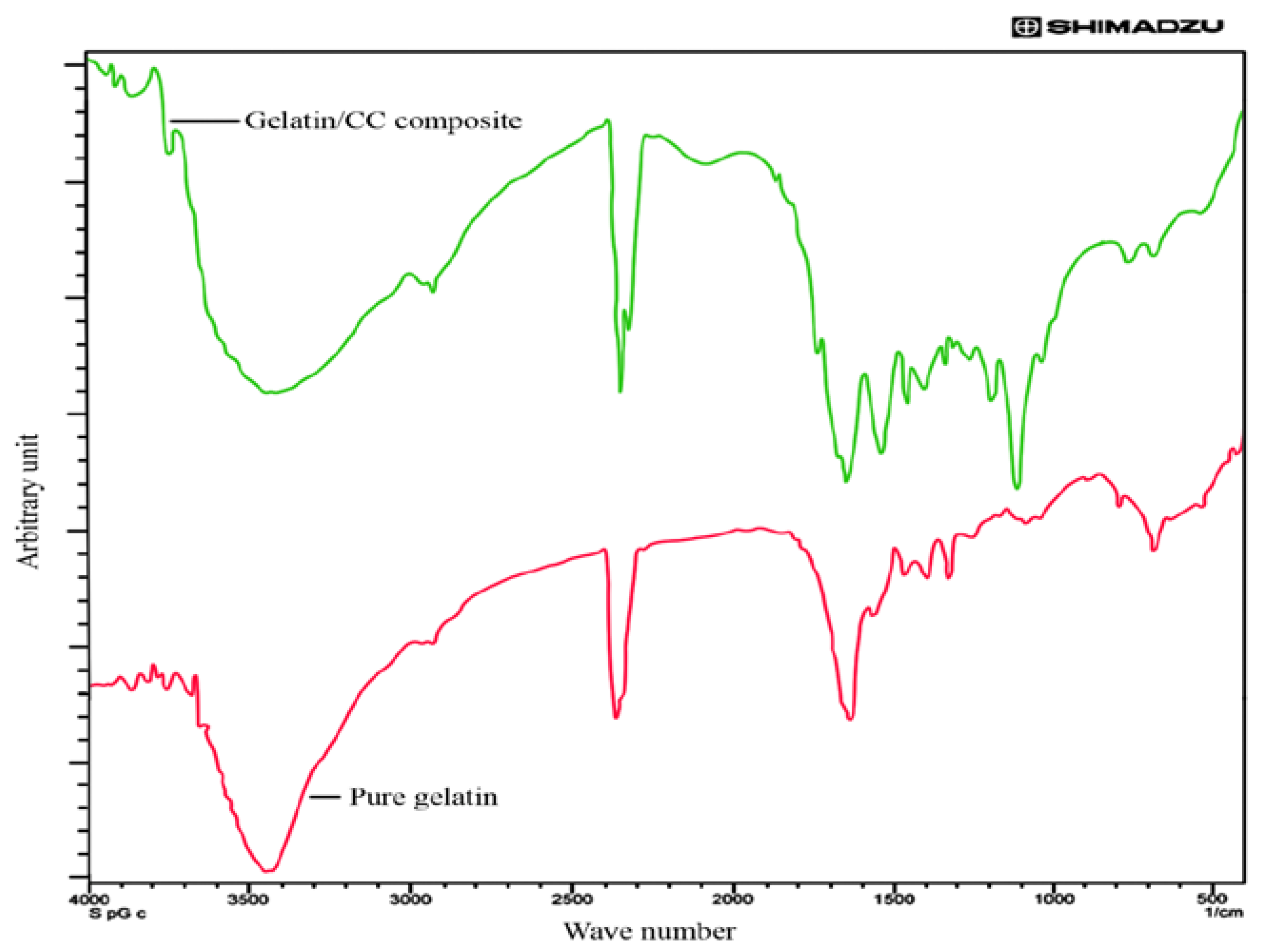
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
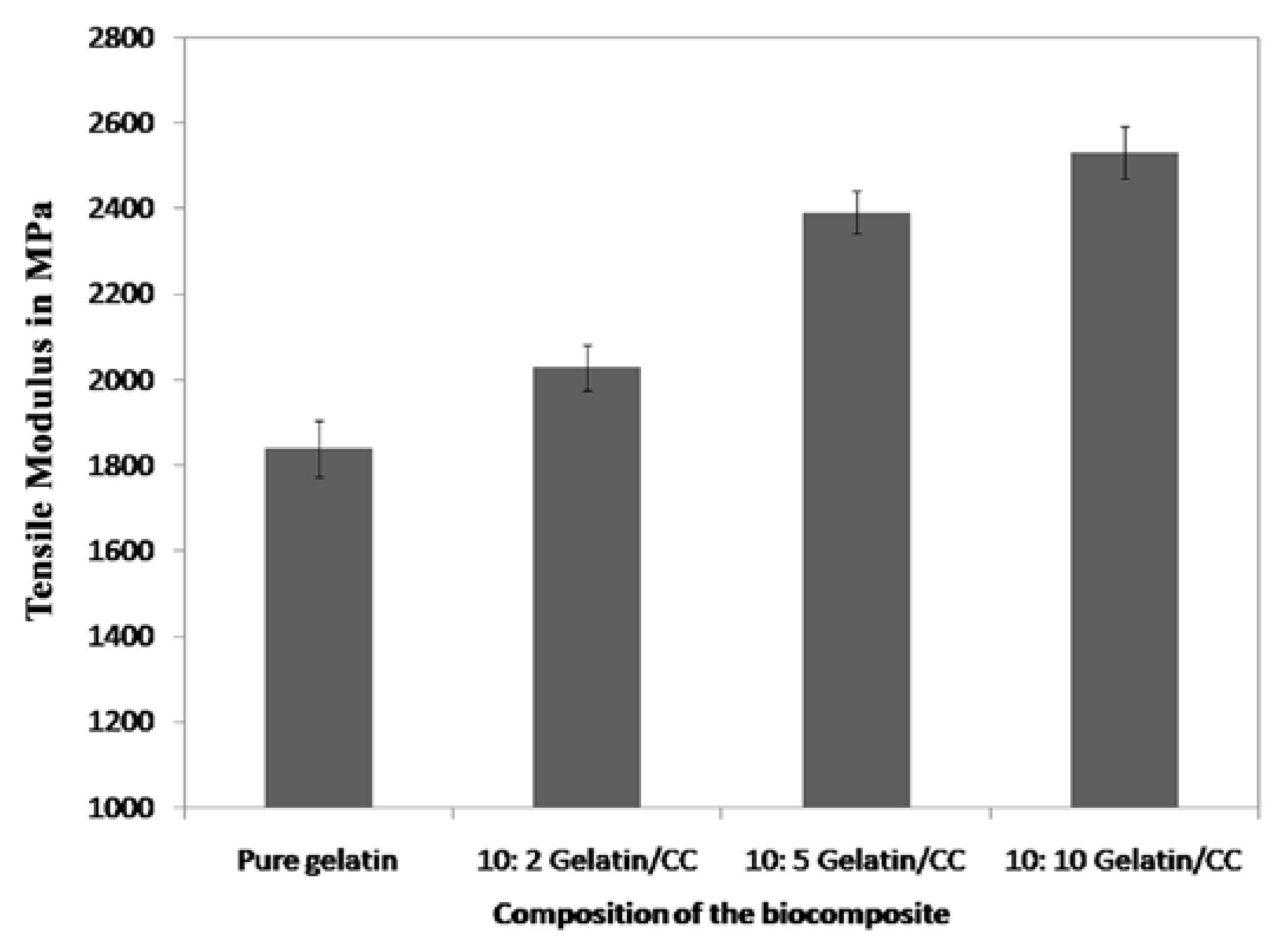
2.3.3. Mechanical Properties
2.3.4. Buffer Uptake Properties
2.3.5. In Vitro Biocompatibility Analysis
2.3.6. Antimicrobial Property
2.3.7. In Vitro Cytotoxicity Study
2.3.8. In Vivo Wound Healing
3. Result and Discussion
3.1. Morphology of CCs Derived from Cotton
3.2. FTIR Analysis of Pure and Blend Biocomposite Films
3.3. Mechanical Properties Analysis
3.4. Fluid Drainage Properties
3.4.1. Buffer Uptake
3.4.2. In Vitro Biocompatibility Analysis
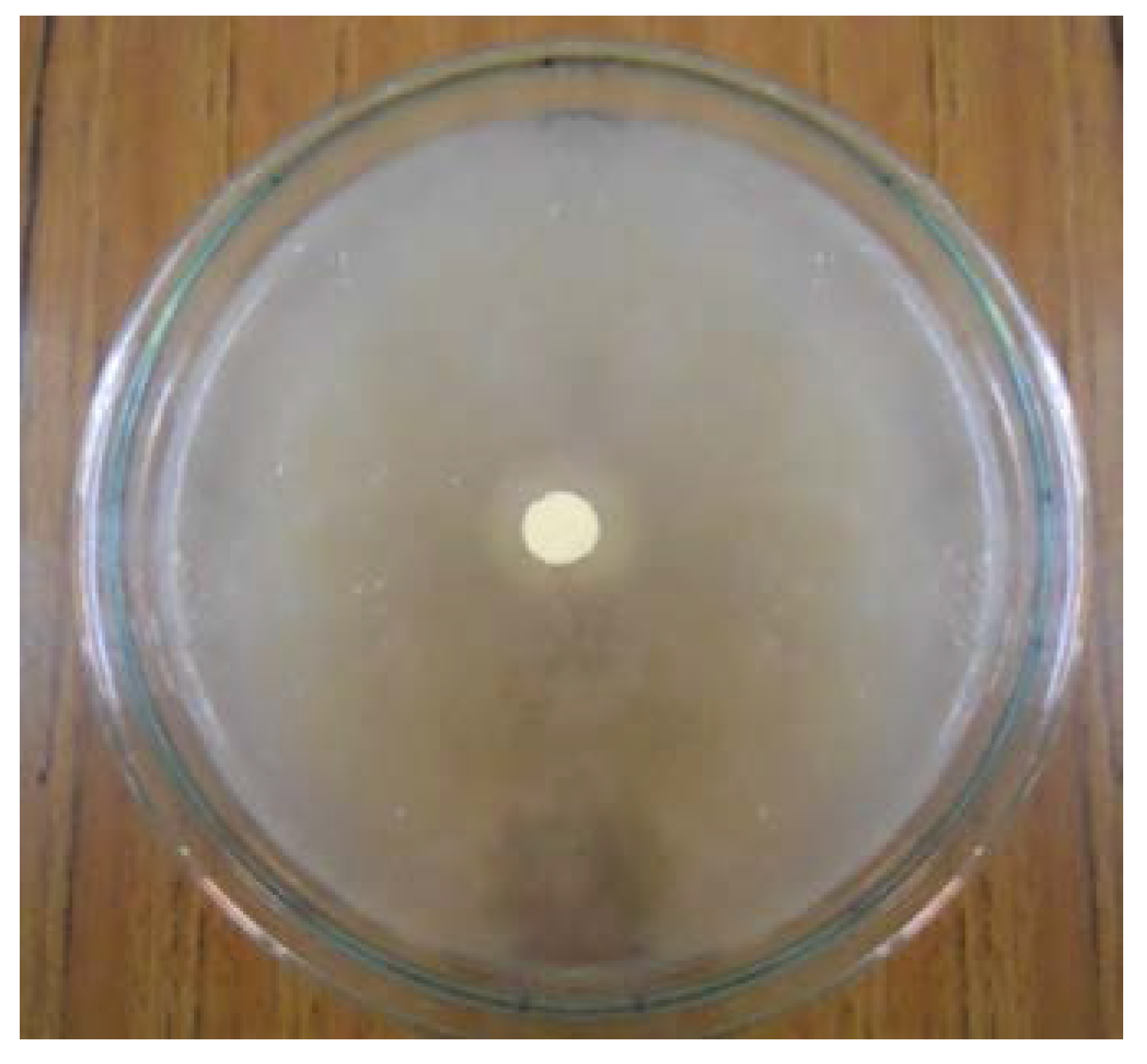
3.4.3. Microbial Sensitivity Analysis
3.4.4. In Vitro Cytotoxicity Test
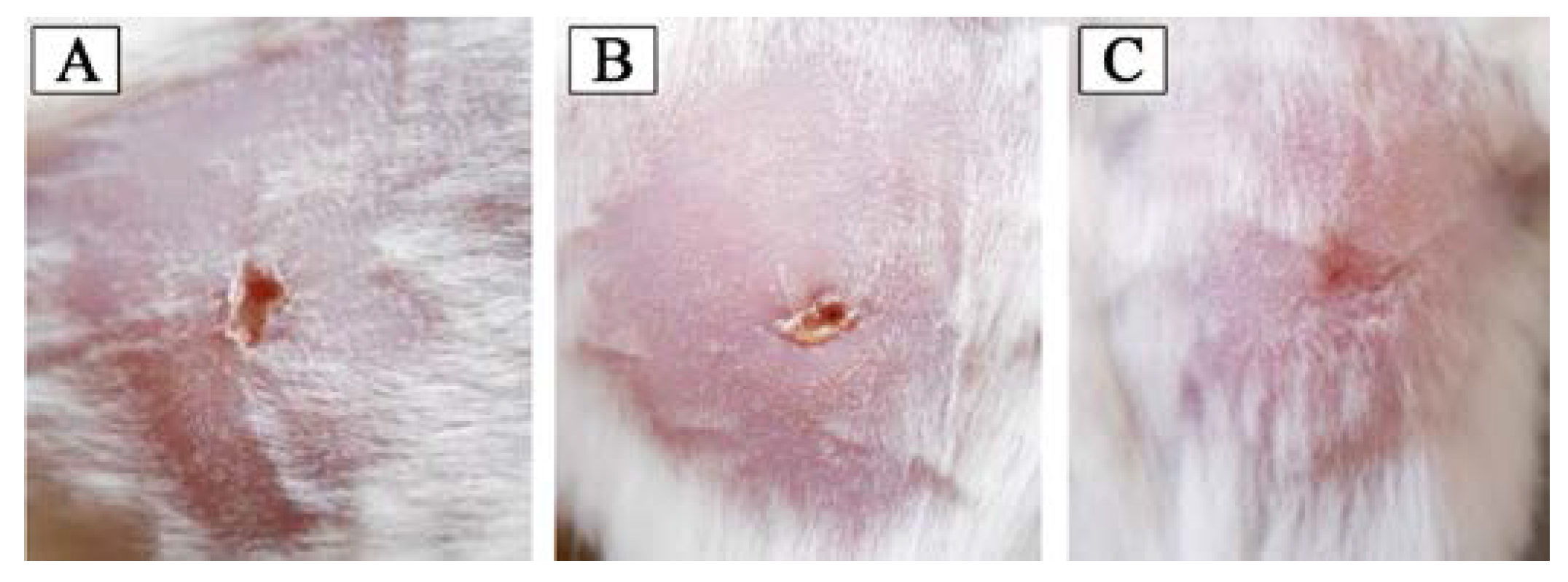
3.4.5. In Vivo Wound Healing
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Islam, J.M.; Tareq, H.; Rahman, M.F.; Habib, S.M.A.; Rahman, N.A.; Molla, E.; Khan, M.A. Preparation and characterization of low cost asymmetric thin film as accelerating wound healing material. Am. Acad. Sch. Res. J. 2013, 5, 16. [Google Scholar]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arabian J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Schaschke, C.; Audic, J.-L. Editorial: Biodegradable materials. Int. J. Mol. Sci. 2014, 15, 21468–21475. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Samir, M.A.S.A.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Wambua, P.; Ivens, J.; Verpoest, I. Natural fibres: Can they replace glass in fibre reinforced plastics? Compos. Sci. Technol. 2003, 63, 1259–1264. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 2008, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- De Morais Teixeira, E.; Corrêa, A.C.; Manzoli, A.; de Lima Leite, F.; de Oliveira, C.R.; Mattoso, L.H.C. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Trittschuh, S.; Seidel, S.; Rowan, S.J.; Weder, C. Polymer nanocomposites with nanowhiskers isolated from microcrystalline cellulose. Biomacromolecules 2009, 10, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind. Eng. Chem. Res. 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Pääkkö, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindström, T.; Berglund, L.A.; Ikkala, O. Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 4, 2492–2499. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Pandey, J.K.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Recent advances in the application of natural fiber based composites. Macromol. Mater. Eng. 2010, 295, 975–989. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Juntaro, J.; Sain, M.; Manuspiya, H. Development of transparent bacterial cellulose nanocomposite film as substrate for flexible organic light emitting diode (OLED) display. Ind. Crops Prod. 2012, 35, 92–97. [Google Scholar] [CrossRef]
- Lo, S.; Hsu, M.; Hu, W. Using wound bed preparation to heal a malignant fungating wound: A single case study. J. Wound Care 2007, 16, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Pan, H.; Chen, W. Self-crosslinkable hydrogels composed of partially oxidized hyaluronan and gelatin: In vitro and in vivo responses. J. Biomed. Mater. Res. Part A 2008, 85, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Ulubayram, K.; Nur Cakar, A.; Korkusuz, P.; Ertan, C.; Hasirci, N. EGF containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Ito, A.; Mase, A.; Takizawa, Y.; Shinkai, M.; Honda, H.; Hata, K.; Ueda, M.; Kobayashi, T. Transglutaminase-mediated gelatin matrices incorporating cell adhesion factors as a biomaterial for tissue engineering. J. Biosci. Bioeng. 2003, 95, 196–199. [Google Scholar] [CrossRef]
- Rocasalbas, G.; Francesko, A.; Touriño, S.; Fernández-Francos, X.; Guebitz, G.M.; Tzanov, T. Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 2013, 92, 989–996. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, R.; Grosso, C. Characterization of gelatin based films modified with transglutaminase, glyoxal and formaldehyde. Food Hydrocoll. 2004, 18, 717–726. [Google Scholar] [CrossRef]
- Dawlee, S.; Sugandhi, A.; Balakrishnan, B.; Labarre, D.; Jayakrishnan, A. Oxidized chondroitin sulfate-cross-linked gelatin matrixes: A new class of hydrogels. Biomacromolecules 2005, 6, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Fernandez, A.C.; Mohanan, P.V.; Jayakrishnan, A. Evaluation of the effect of incorporation of dibutyryl cyclic adenosine monophosphate in an in situ-forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2006, 27, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen cryogel cross-linked by dialdehyde starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Liu, Y.; Chen, M.; Hu, Y.; Yang, Z. Study on the grafting of chitosan-gelatin microcapsules onto cotton fabrics and its antibacterial effect. Colloids Surf. B Biointerfaces 2013, 109, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Khazraji, A.C.; Robert, S. Interaction effects between cellulose and water in nanocrystalline and amorphous regions: A novel approach using molecular modeling. J. Nanomater. 2013, 2013, 44. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Zhang, Q. Dynamic rheology studies of in situ polymerization process of polyacrylamide-cellulose nanocrystal composite hydrogels. Colloid Polym. Sci. 2011, 289, 247–255. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Vicentini, N.; Dupuy, N.; Leitzelman, M.; Cereda, M.; Sobral, P. Prediction of cassava starch edible film properties by chemometric analysis of infrared spectra. Spectrosc. Lett. 2005, 38, 749–767. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Islam, J.M.M.; Kabir, W.; Rahman, A.; Mizan, M.; Rahman, M.F.; Amin, J.; Khan, M.A. Development of hydrocolloid Bi-layer dressing with bio-adhesive and non-adhesive properties. Mater. Sci. Eng. C 2016, 69, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.M.; Hossan, M.A.; Alom, F.R.; Khan, M.A. Extraction and characterization of crystalline cellulose from jute fiber and application as reinforcement in biocomposite: Effect of gamma radiation. J. Compos. Mater. 2017, 51, 31–38. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Hettiarachchy, N.; Johnson, M. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella gaminara. J. Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Polymer nanocomposites from biological sources. In Encyclopdia of Nanosciences and Nanotechnology, 3rd ed.; Nalwa, H.S., Ed.; American Scientific Publisher: Valencia, CA, USA, 2010. [Google Scholar]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Zaman, H.U.; Beg, M. Improvement of physico-mechanical, thermomechanical, thermal and degradation properties of PCL/gelatin biocomposites: Effect of gamma radiation. Radiat. Phys. Chem. 2015, 109, 73–82. [Google Scholar] [CrossRef]
- Islam, M.M.; Khan, M.A.; Rahman, M.M. Preparation of gelatin based porous biocomposite for bone tissue engineering and evaluation of gamma irradiation effect on its properties. Mater. Sci. Eng. C 2015, 49, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.M.; de Graaf, L.A.; Reis, R.L.; Cunha, A.M. Effect of crosslinking, thermal treatment and UV irradiation on the mechanical properties and in vitro degradation behavior of several natural proteins aimed to be used in the biomedical field. J. Mater. Sci. Mater. Med. 2003, 14, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Effect of air exposure and occlusion on experimental human skin wounds. Nature 1963, 200, 378–379. [Google Scholar] [CrossRef] [PubMed]



| Sample No. | Sample Name | Dose (mg/mL) | No. of Nauplii Present after Incubation | Mortality (%) |
|---|---|---|---|---|
| 1. | Positive control (vincristine sulfate) | 0.5 | 0 | 100 |
| 2. | Negative control (artificial sea water) | - | 10 | 0 |
| 3. | Biocomposite film | 0.125 | 10 | 0 |
| 4. | Biocomposite film | 0.25 | 10 | 0 |
| 5. | Biocomposite film | 0.5 | 9 | 10 |
| 6. | Biocomposite film | 0.75 | 8 | 20 |
| 7. | Biocomposite film | 1.00 | 8 | 20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmik, S.; Islam, J.M.M.; Debnath, T.; Miah, M.Y.; Bhattacharjee, S.; Khan, M.A. Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications. Polymers 2017, 9, 222. https://doi.org/10.3390/polym9060222
Bhowmik S, Islam JMM, Debnath T, Miah MY, Bhattacharjee S, Khan MA. Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications. Polymers. 2017; 9(6):222. https://doi.org/10.3390/polym9060222
Chicago/Turabian StyleBhowmik, Shukanta, Jahid M. M. Islam, Tonmoy Debnath, Muhammed Yusuf Miah, Shovon Bhattacharjee, and Mubarak A. Khan. 2017. "Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications" Polymers 9, no. 6: 222. https://doi.org/10.3390/polym9060222