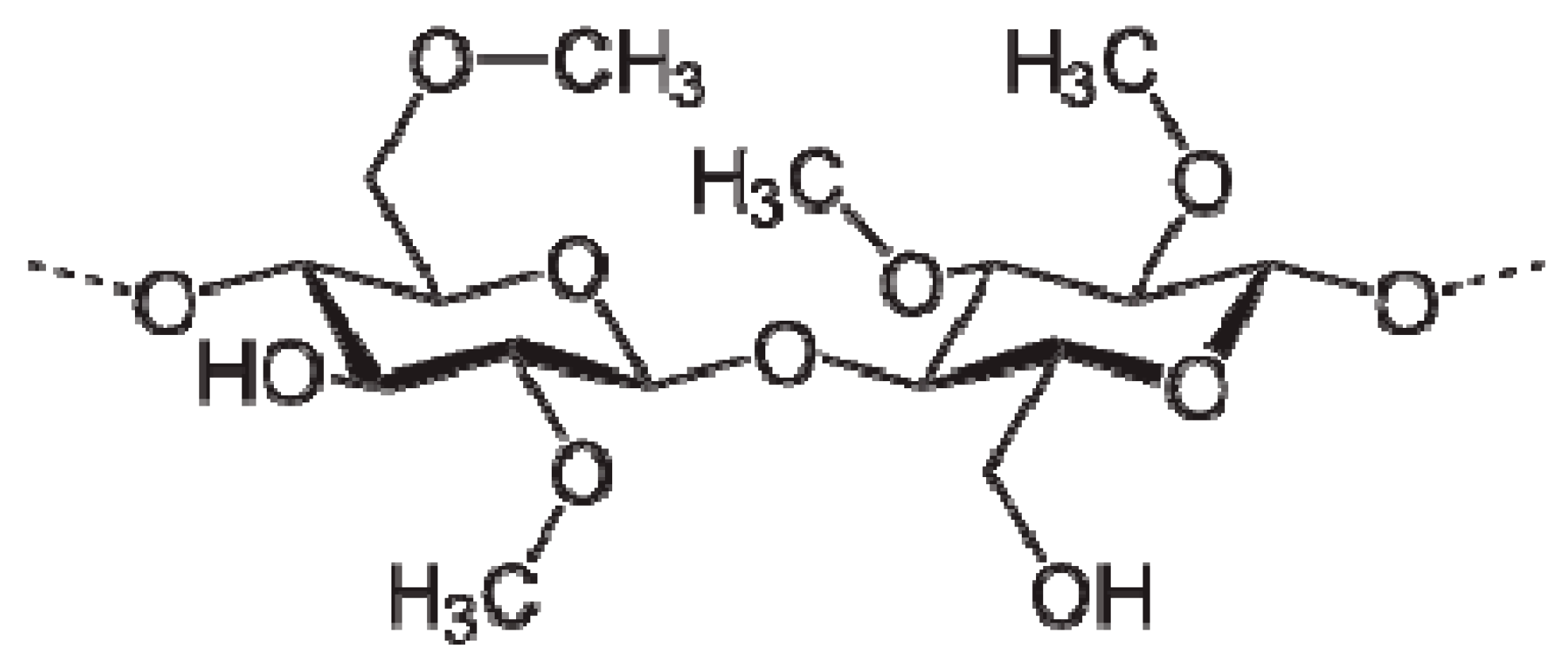
Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose](1−x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with Desired Optical Band Gaps
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Sample Preparation
2.2. Ultraviolet-Visible (UV-Vis) Spectroscopy
2.3. Fourier Transform Infrared (FTIR) and X-ray Diffraction (XRD) Spectroscopies
3. Results and Discussion
3.1. Optical Properties
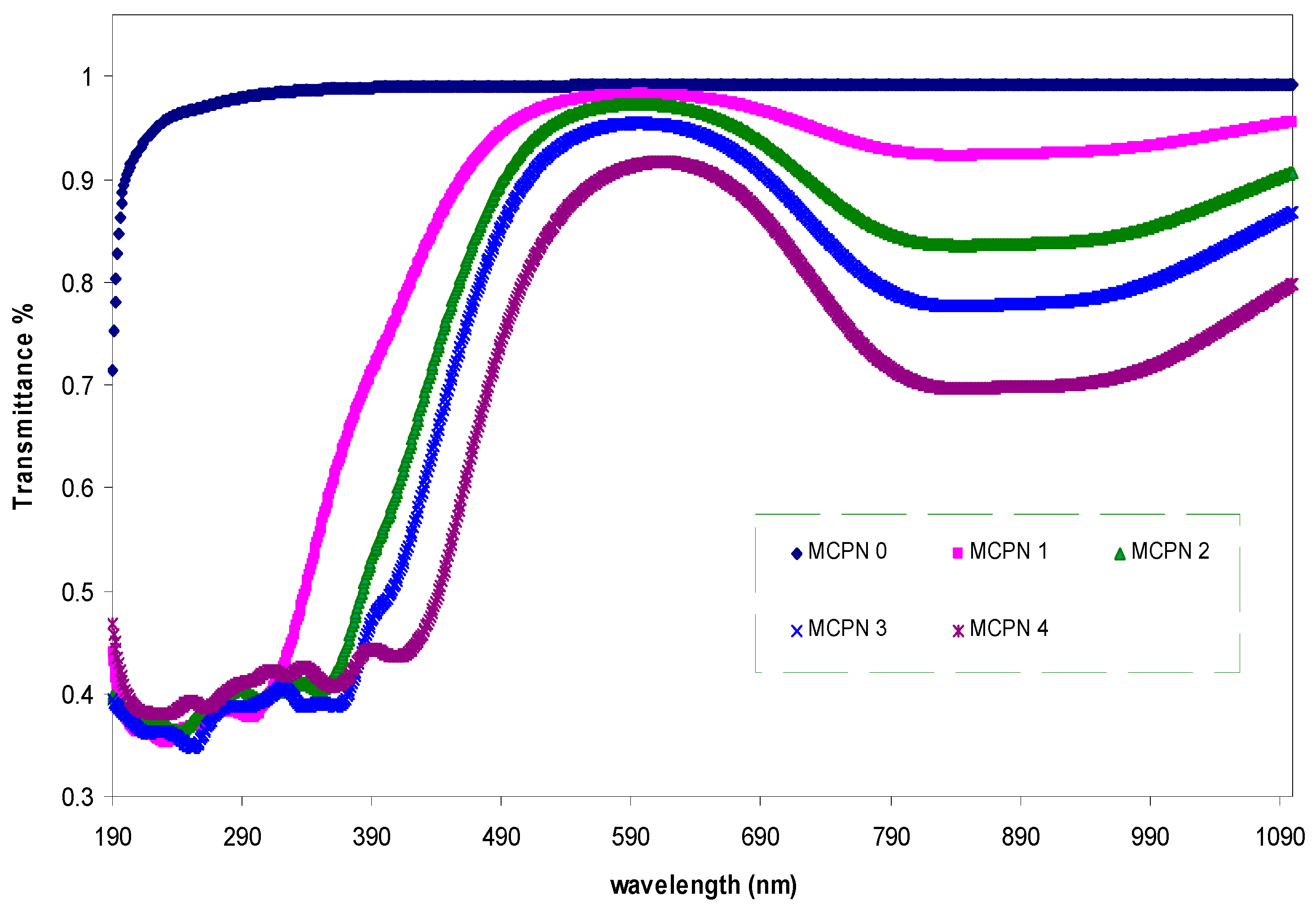
3.1.1. Transmittance and Absorption Study
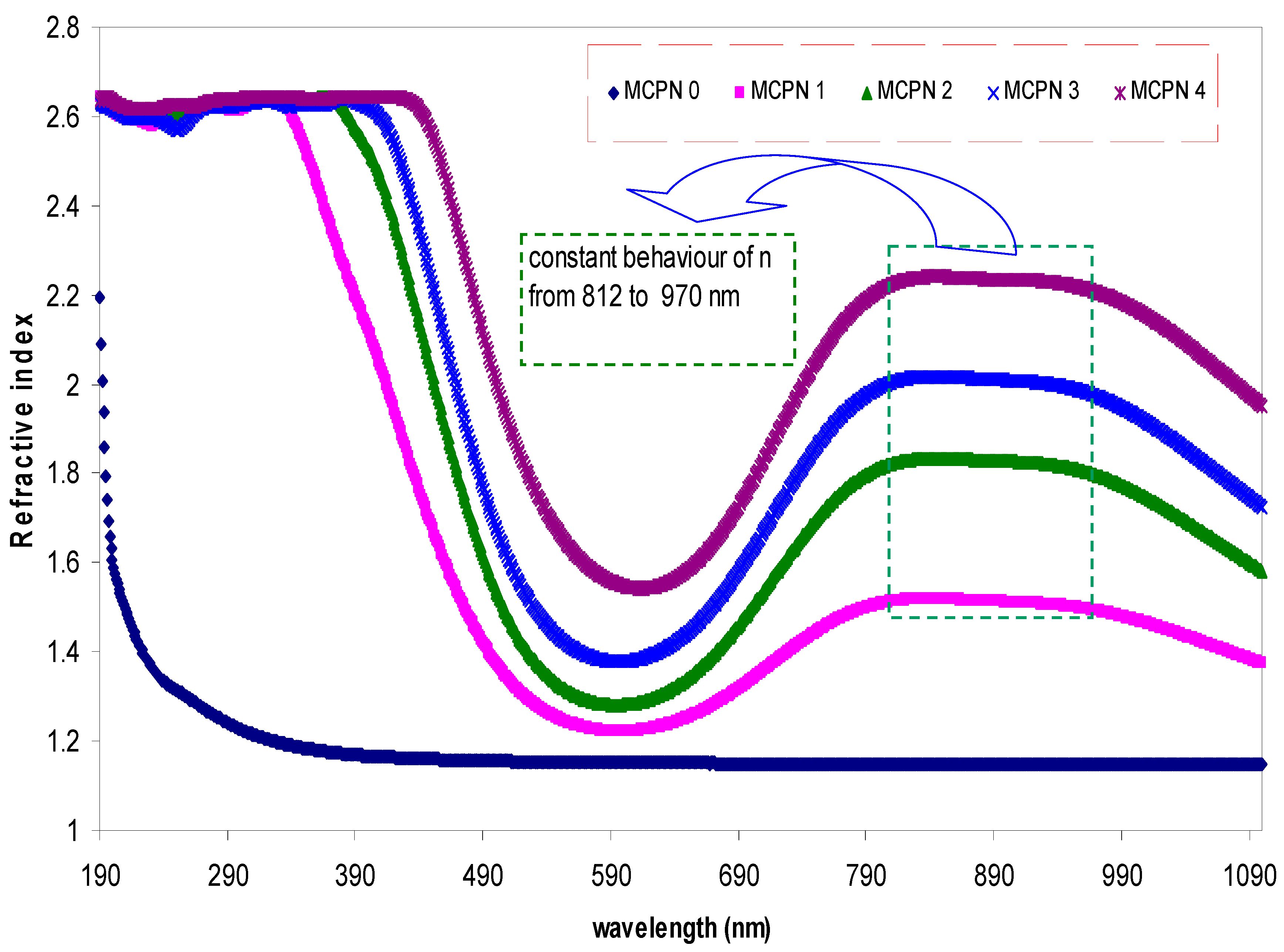
3.1.2. Refractive Index Study
3.1.3. Bandgap Study: Tauc and Optical Dielectric Methods
3.1.4. Tail Study
3.2. FTIR Study
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Wu, Y.; Wadia, C.; Ma, W.; Sadtler, B.; Alivisatos, A.P. Synthesis and Photovoltaic Application of Copper (I) Sulfide Nanocrystals. Nano Lett. 2008, 8, 2551–2555. [Google Scholar] [CrossRef] [PubMed]
- Brelle, M.C.; Torres-Martinez, C.L.; McNulty, J.C.; Mehra, R.K.; Zhang, J.Z. Synthesis and characterization of CuxS nanoparticles. Nature of the infrared band and charge-carrier dynamics. Pure Appl. Chem. 2000, 72, 101–117. [Google Scholar] [CrossRef]
- Serrano, T.; Gomez, I. One pot synthesis of PbS/Cu2S core-shell nanoparticles and their optical properties. Rev. Mex. Fis. 2014, 60, 14–21. [Google Scholar]
- Chai, M.N.; Isa, M.I.N. The Oleic Acid Composition Effect on the Carboxymethyl Cellulose Based Biopolymer Electrolyte. J. Cryst. Proc. Technol. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Rengui, W.; Lihui, C.; Shan, L.; Hui, Z.; Hui, W.; Kai, L.; Shilin, C.; Liulian, H. Preparation and Characterization of Antibacterial Cellulose/Chitosan Nanofiltration Membranes. Polymers 2017, 9, 116. [Google Scholar] [CrossRef]
- Mantravadi, R.; Chinnam, P.R.; Dikin, D.A.; Wunder, S.L. High Conductivity, High Strength Solid Electrolytes Formed by in situ Encapsulation of Ionic Liquids in Nanofibrillar Methyl Cellulose Networks. ACS Appl. Mater. Interfaces 2016, 21, 13426–13436. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, A.; Garci, M.A.; Martino, M.N.; Zaritzky, N.E. Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocoll. 2007, 21, 66–72. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Majid, S.R.; Arof, A.K. Transport studies of NH4NO3 doped methyl cellulose electrolyte. Synth. Met. 2010, 160, 1040–1044. [Google Scholar] [CrossRef]
- Li, S.; Lin, M.M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 5214. [Google Scholar] [CrossRef] [PubMed]
- Yashar, A.; Uumut, A.; Emsettin, T. Dielectric and Optical Properties of CdS–Polymer Nanocomposites Prepared by the Successive Ionic Layer Adsorption and Reaction (SILAR). J. Electron. Mater. 2014, 43, 1226–1231. [Google Scholar]
- Tiwari, A.; Khan, S.A.; Kher, R.S.; Dhobled, S.J.; Chandel, A.L.S. Synthesis, characterization and optical properties of polymer-based ZnSnanocomposites. Luminescence 2016, 31, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Bockstaller, M.R.; Thomas, E.L. Optical Properties of Polymer-Based Photonic Nanocomposite Materials. J. Phys. Chem. B 2003, 107, 10017–10024. [Google Scholar] [CrossRef]
- Shunichiro, I.; Amane, H.; Madoka, Y.; Kazuo, T.; Yoshiki, C. Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element. Polymers 2017, 9, 68. [Google Scholar] [CrossRef]
- Tanwistha, G.; Jayanthy, S.P.; Vijayakumar, C.N. Self-Assembled Organic Materials for Photovoltaic Application. Polymers 2017, 9, 112. [Google Scholar] [CrossRef]
- Mozer, A.J.; Sariciftci, N.S. Conjugated polymer photovoltaic devices and materials. C.R. Chimie 2006, 9, 568–577. [Google Scholar] [CrossRef]
- Mallajosyula, A.T.; Srivastava, N.; Kumar, S.S.; Mazhari, B. Characterization of matrix and isolated organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1319–1323. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]
- Rao, Y.Q.; Chen, S. Molecular Composites Comprising TiO2 and Their Optical Properties. Macromolecules 2008, 41, 4838–4844. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Huang, Q.; Huang, M.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Longo, A.; Hison, C.L. Tuned linear optical properties of gold-polymer nanocomposites. J. Mater. Chem. 2009, 19, 5744–5750. [Google Scholar] [CrossRef]
- Srivastana, S.; Haridas, M.; Basu, J.K. Optical properties of polymer nanocomposites. Bull. Mater. Sci. 2008, 31, 213–217. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Kadir, M.F.Z. Innovative method to avoid the reduction of silver ions to silver nanoparticles (Ag+→Ago) in silver ion conducting based polymer electrolytes. Phys. Scr. 2015, 90, 035808. [Google Scholar] [CrossRef]
- Hong, L.V.; Cat, D.T.; Chi, L.H.; Thuy, N.T.; Hung, T.V.; Tai, L.N.; Long, P.D. Plasmonic Effect in Au-Added TiO2-Based Solar Cell. J. Electron. Mater. 2016, 45, 4833–4837. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosan-silver triflate electrolyte membrane. eXPRESS Polym. Lett. 2010, 4, 300–310. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Effect of silver nanoparticles on the DC conductivity in chitosan-silver triflate polymer electrolyte. Phys. B 2010, 405, 4429–4433. [Google Scholar] [CrossRef]
- Abdulwahid, R.T.; Abdullah, O.G.; Aziz, S.B.; Hussein, S.A.; Muhammad, F.F.; Yahya, M.Y. The study of structural and optical properties of PVA: PbO2 based solid polymer nanocomposites. J. Mater. Sci. 2016, 27, 12112–12118. [Google Scholar]
- El-Menyawy, E.M.; Mahmoud, G.M.; Ibrahim, R.S.; Terra, F.S.; El-Zahed, H.; El Zawawi, I.K. Structural, optical and electrical properties of PbS and PbSe quantum dot thin films. J. Mater. Sci. 2016, 27, 10070–10077. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuningthe absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci. 2015, 26, 8022–8028. [Google Scholar]
- Nakabayashi, K.; Takeuchi, Y.; Mori, H. Perylene Bisimide-Based DonorAcceptor Materials Incorporating Oligothiophenes: Synthesis, Characterization, Thin-Film Properties, and Nanomorphology. Bull. Chem. Soc. Jpn. 2016, 89, 1063–1068. [Google Scholar] [CrossRef]
- Ghanipour, M.; Dorranian, D. Effect of Ag-nanoparticles doped in Polyvinyl Alcohol on the structural and optical properties of PVA films. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. J. Mater. Sci. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Rao, Y.Q.; Chen, S. Molecular composites comprising TiO2 and their optical properties. Macromolecules 2008, 41, 4838–4844. [Google Scholar] [CrossRef]
- Mohan, V.M.; Raja, V.; Bhargav, P.B.; Sharma, A.K.; Rao, V.V.R.N. Structural, electrical and optical properties of pure and NaLaF4 doped PEO polymer electrolyte films. J. Polym. Res. 2007, 14, 283–290. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, R.; Johan, M.R. Conductivity and optical studies of plasticized solid polymer electrolytes doped with carbon nanotube. J. Lumin. 2012, 132, 147–152. [Google Scholar] [CrossRef]
- Kumar, K.K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Rao, V.V.R.N. Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys. B 2011, 406, 1706–1712. [Google Scholar] [CrossRef]
- Mohan, V.M.; Bhargav, P.B.; Raja, V.; Sharma, A.K.; Rao, V.V.R.N. Optical and Electrical Properties of Pure and Doped PEO Polymer Electrolyte Films. Soft Mater. 2007, 5, 33–46. [Google Scholar] [CrossRef]
- Devi, C.U.; Sharma, A.K.; Rao, V.V.R.N. Electrical and optical properties of pure and silver nitrate-doped polyvinyl alcohol films. Mater. Lett. 2002, 56, 167–174. [Google Scholar] [CrossRef]
- Ahmad, F.; Sheha, E. Preparation and physical properties of (PVA)0.7 (NaBr)0.3 (H3PO4)xM solid acid membrane for phosphoric acid—Fuel cells. J. Adv. Res. 2013, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.J.; Shahabuddin, M.; Karikomi, M.; Salim, M.; Nasuno, E.; Kato, N.; Iimura, K. Structural and Photoelectrical Characterization of Thin Films of a Novel Amphiphilic Oxa[9]helicene Derivative. Bull. Chem. Soc. Jpn. 2016, 89, 203–211. [Google Scholar] [CrossRef]
- Weiwei, L.; Koen, H.H.; Martijn, M.W.; René, A.J.J. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar]
- Dong, M.; Dan, S.; Chengmei, Z.; Tao, L.; Bingbing, F.; Lijun, H.; Yan, L.; Wei, J.; Hyosung, C.; Taehyo, K.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar]
- Wakamiya, A.; Yamaguchi, S. Designs of Functional 468 π-Electron Materials based on the Characteristic Features of Boron. Bull. Chem. Soc. Jpn. 2015, 88, 1357–1377. [Google Scholar] [CrossRef]
- Biskri, Z.E.; Rached, H.; Bouchear, M.; Rached, D.; Aida, M.S. A Comparative Study of Structural Stability and Mechanical and Optical Properties of Fluorapatite (Ca5(PO4)3F) and Lithium Disilicate (Li2Si2O5) Components Forming Dental Glass–Ceramics: First Principles Study. J. Electron. Mater. 2016, 45, 5082–5095. [Google Scholar] [CrossRef]
- Ravindra, N.M.; Ganapathy, P.; Choi, J. Energy gap–refractive index relations in semiconductors—An overview. Infrared Phys. Technol. 2007, 50, 21–29. [Google Scholar] [CrossRef]
- Cardona, M. Optical Properties and Electronic Density of States. J. Res. Nat. Bur. Stand. 1970, 74A, 253–265. [Google Scholar] [CrossRef]
- Yu, L.; Li, D.; Zhao, S.; Li, G.; Yang, K. First principles study on electronic structure and optical properties of ternary GaAs:Bi alloy. Materials 2012, 5, 2486–2497. [Google Scholar] [CrossRef]
- Sheha, E.; Khoder, H.; Shanap, T.S.; El-Shaarawy, M.G.; El Mansy, M.K. Structure, dielectric and optical properties of p-type (PVA/CuI) nanocomposite polymer electrolyte for photovoltaic cells. Optik 2012, 123, 1161–1166. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying Poly(Vinyl Alcohol) (PVA) from Insulator to SmallBandgap Polymer: A Novel Approach for Organic Solar Cells and Optoelectronic Devices. J. Electron. Mater. 2016, 45, 736–745. [Google Scholar] [CrossRef]
- Abdel-Baset, T.; Elzayat, M.; Mahrous, S. Characterization and Optical and Dielectric Properties of Polyvinyl Chloride/Silica Nanocomposites Films. Int. J. Polym. Sci. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Prasher, S.; Kumar, M.; Singh, S. Electrical and Optical Properties of O6+ Ion Beam–Irradiated Polymers. Int. J. Polym. Anal. Charact. 2014, 19, 204–211. [Google Scholar] [CrossRef]
- Saq’an, S.A.; Ayesh, A.S.; Zihlif, A.M.; Martuscelli, E.; Ragosta, G. Physical properties of polystyrene/alum composites. Polym. Test. 2004, 23, 739–745. [Google Scholar] [CrossRef]
- Liu, P.; Xiangmei, W.; Zhong, L. Miscibility Study of Chitosan and Methylcellulose Blends. Adv. Mater. Res. 2013, 750–752, 802–805. [Google Scholar] [CrossRef]
- Salleh, N.S.; Aziz, S.B.; Aspanut, Z.; Kadir, M.F.Z. Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics 2016, 22, 2157–2167. [Google Scholar] [CrossRef]
- Turhan, K.N.; Ahbaz, F.S.; Guner, A. A Spectrophotometric Study of Hydrogen Bonding in Methylcellulose-based Edible Films Plasticized by Polyethylene Glycol. J. Food Sci. 2001, 66, 59–62. [Google Scholar] [CrossRef]
- Aziz, N.A.N.; Idris, N.K.; Isa, M.I.N. Solid Polymer Electrolytes Based on Methylcellulose: FT-IR and Ionic Conductivity Studies. Int. J. Polym. Anal. Charact. 2010, 15, 319–327. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Xiao, S.Y.; Li, M.X.; Chang, Z.; Wang, F.X.; Gao, J.; Wu, Y.P. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. J. Power Sources 2015, 288, 368–375. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Polat, T.G. Effects of montmorillonite on properties of methyl cellulose/carvacrol based active antimicrobial nanocomposites. Carbohydr. Polym. 2016, 150, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Buslov, D.K.; Sushko, N.I.; Tretinnikov, O.N. Study of thermal gelation of methylcellulose in water using FTIR-ATR spectroscopy. J. Appl. Spectrosc. 2008, 75, 514–518. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]

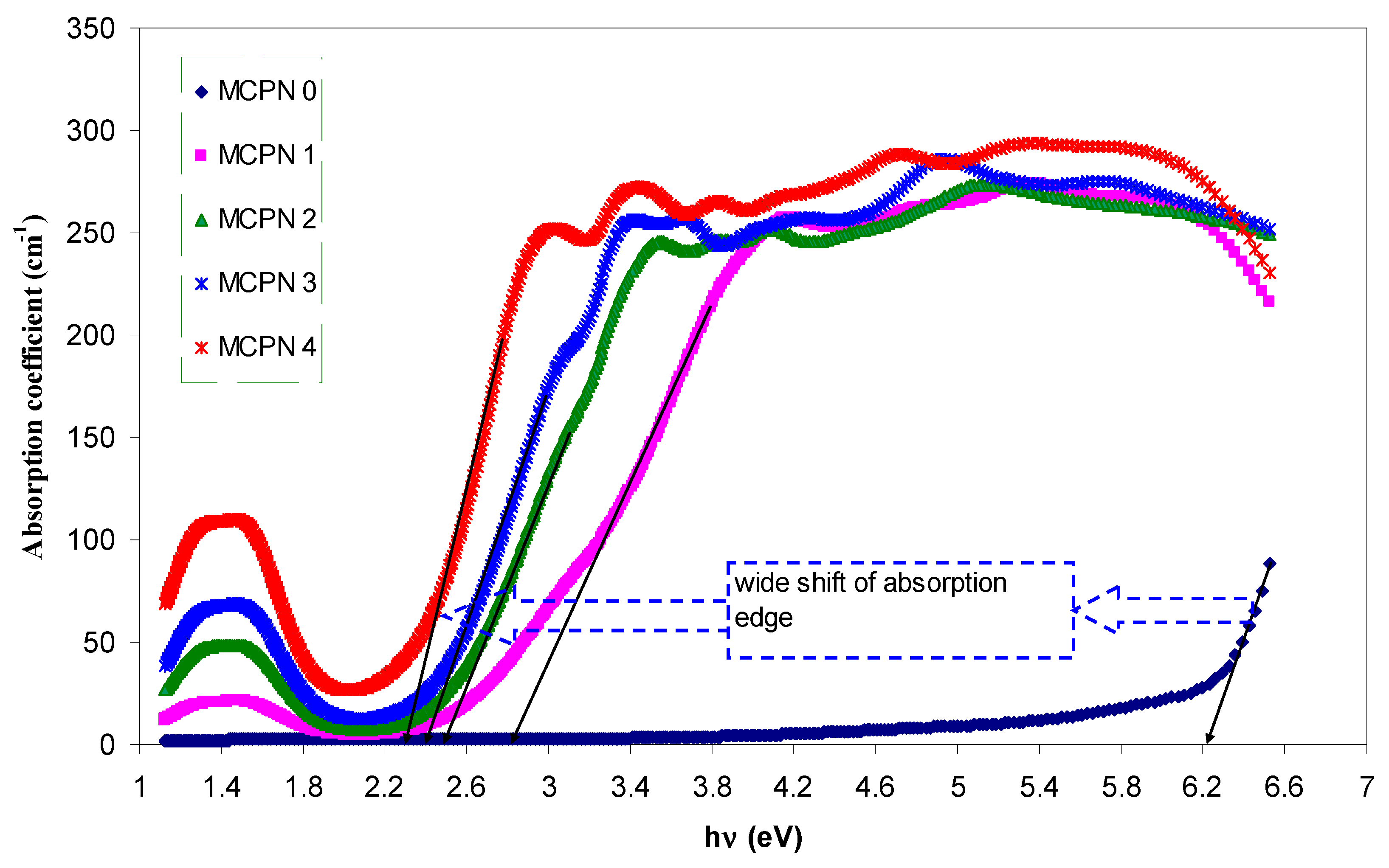

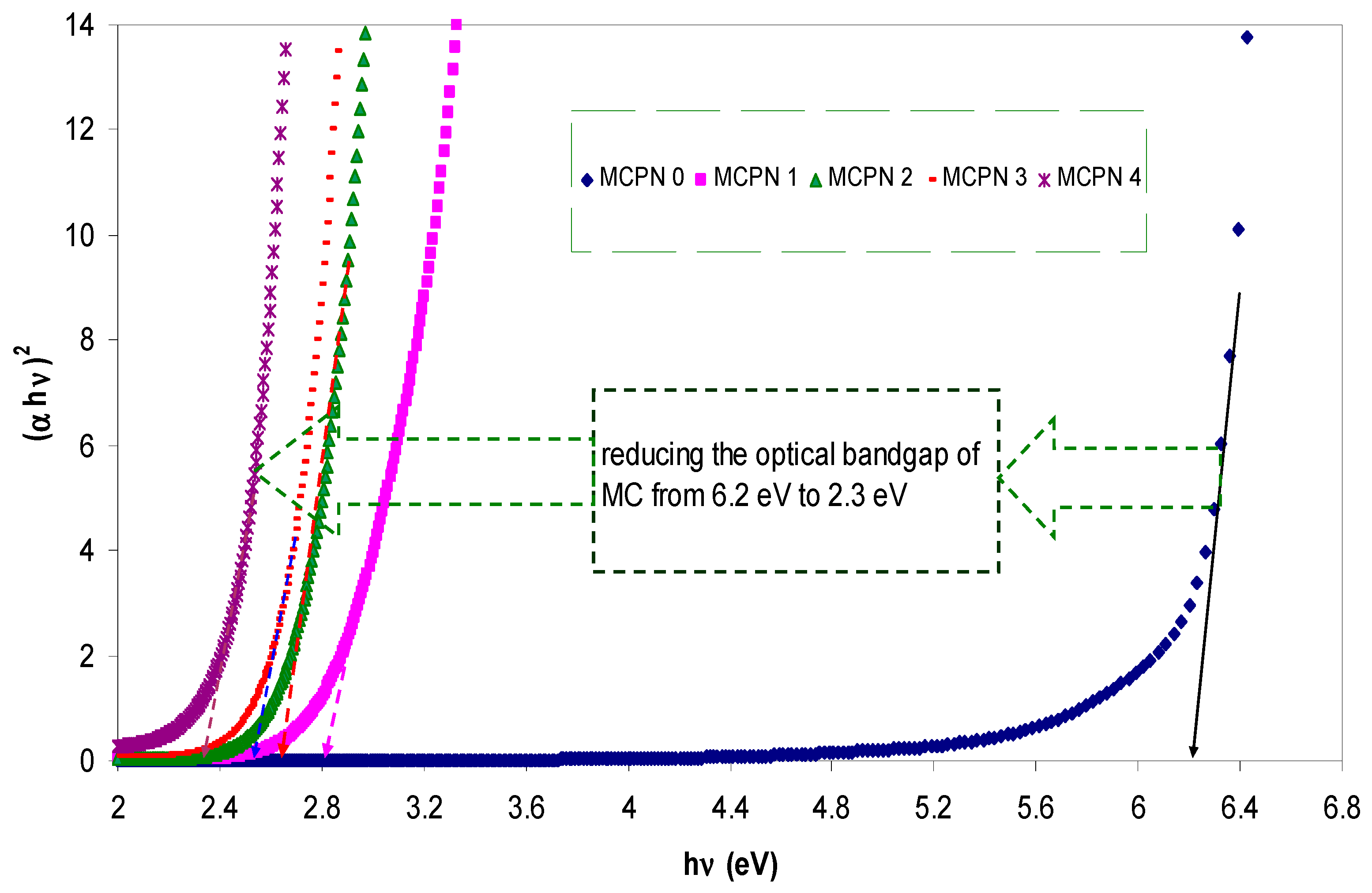
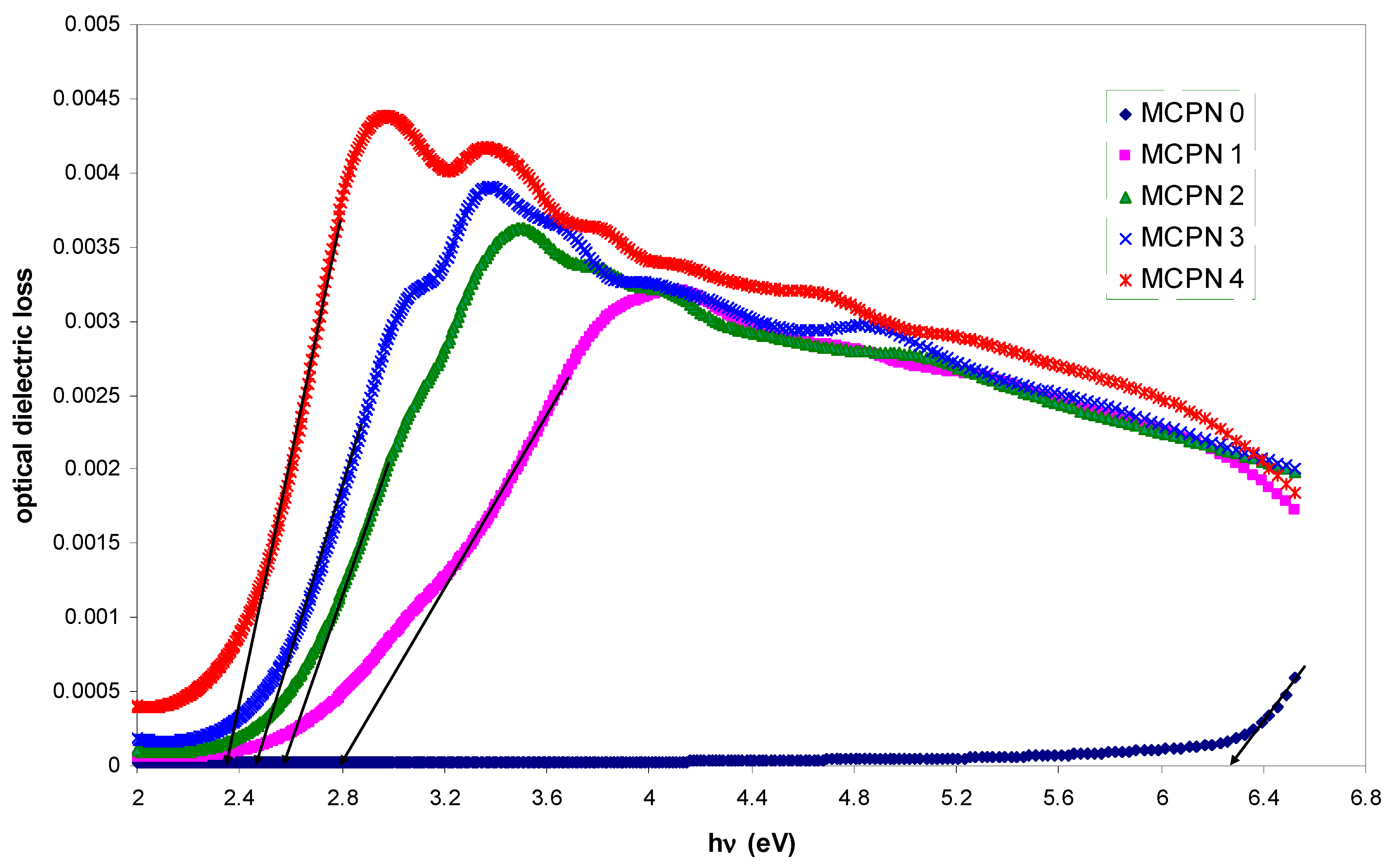
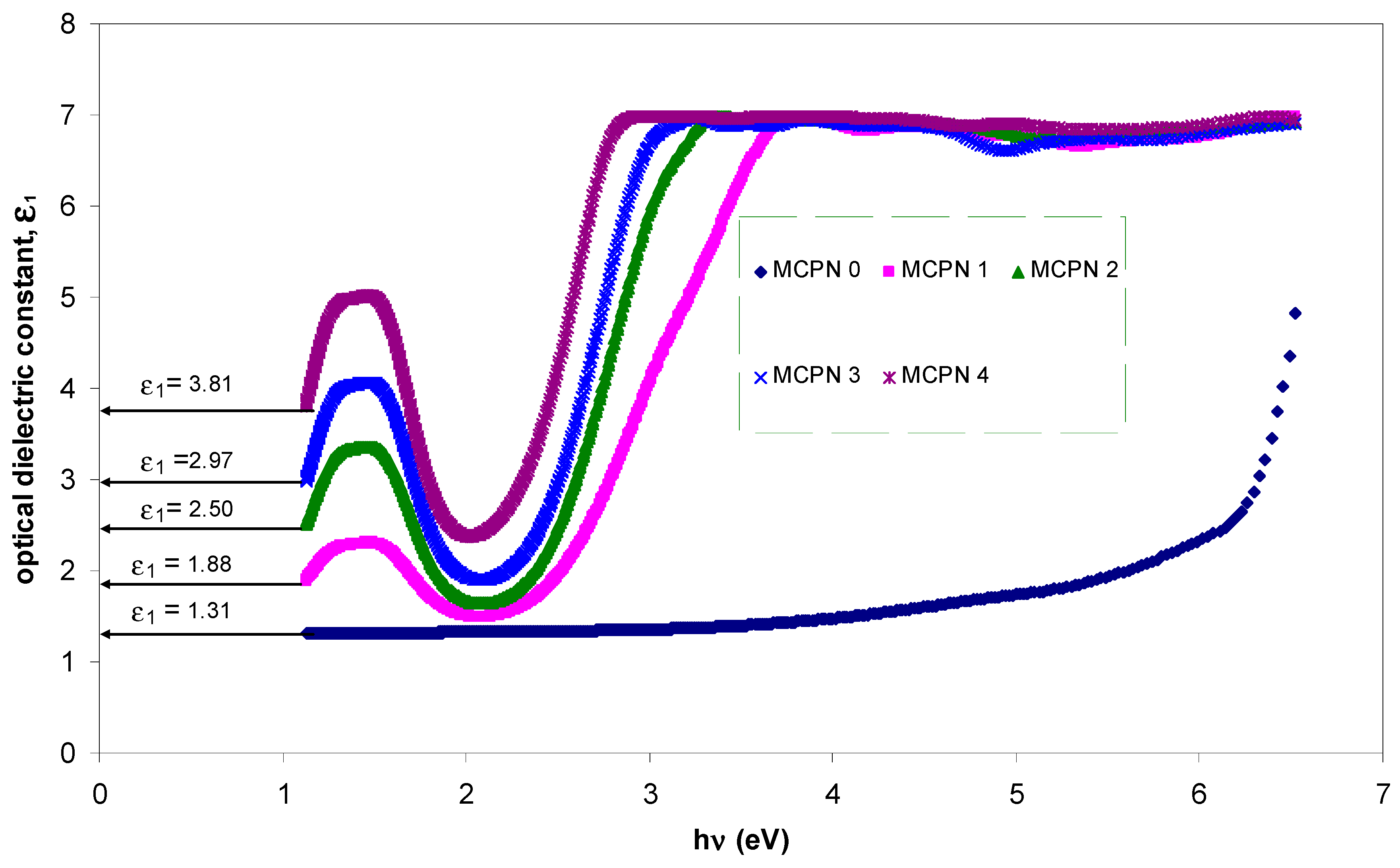
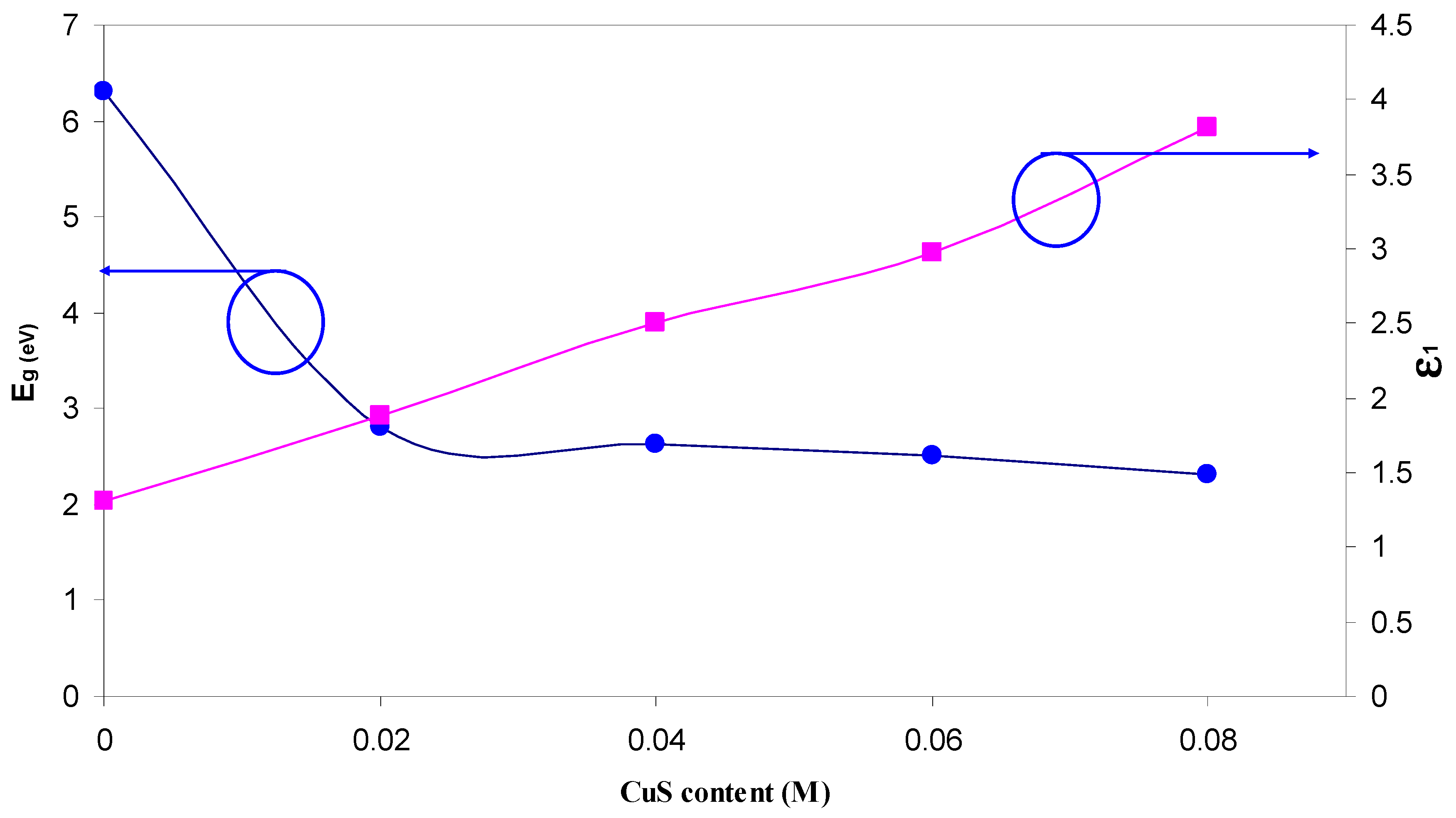
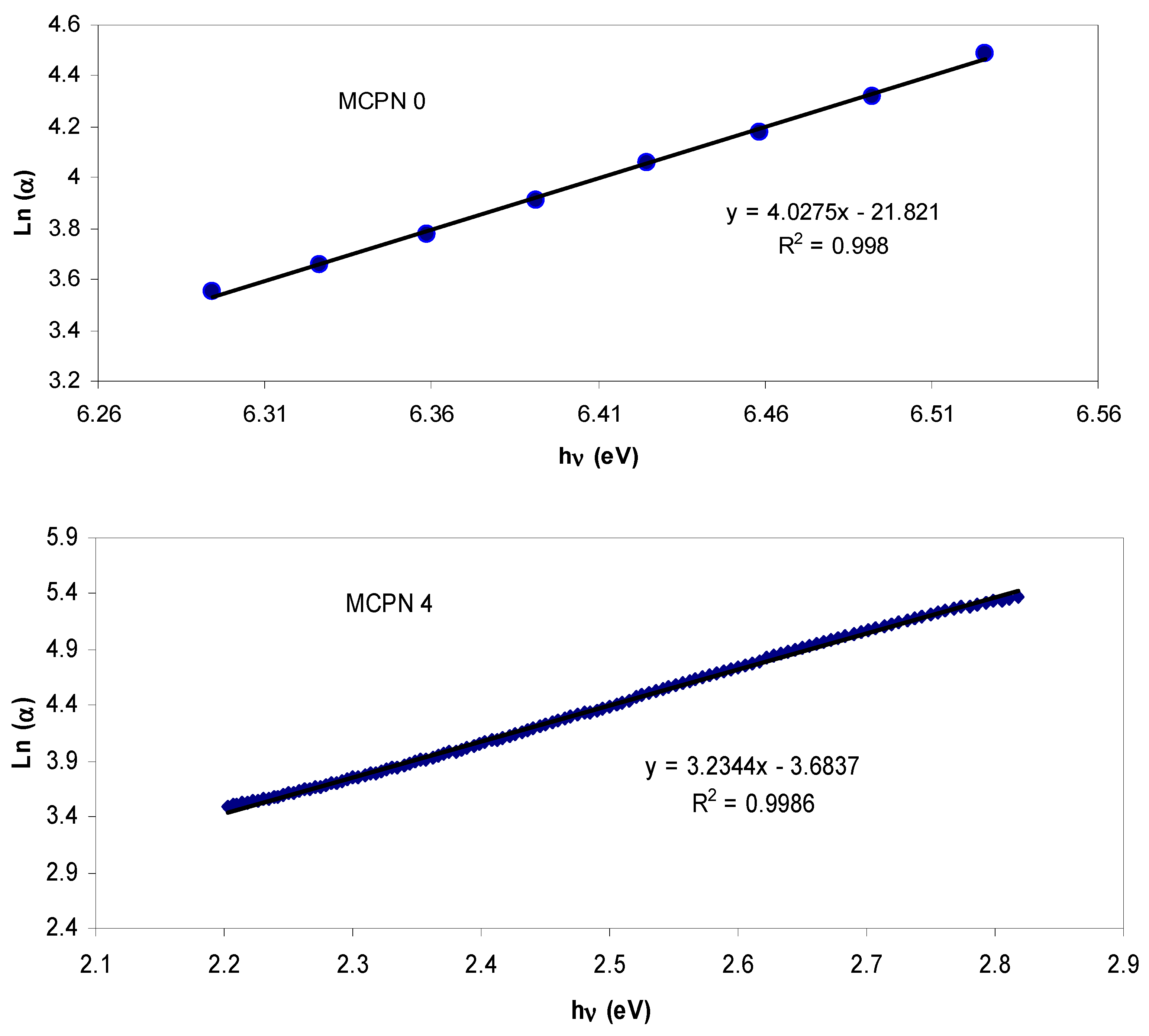
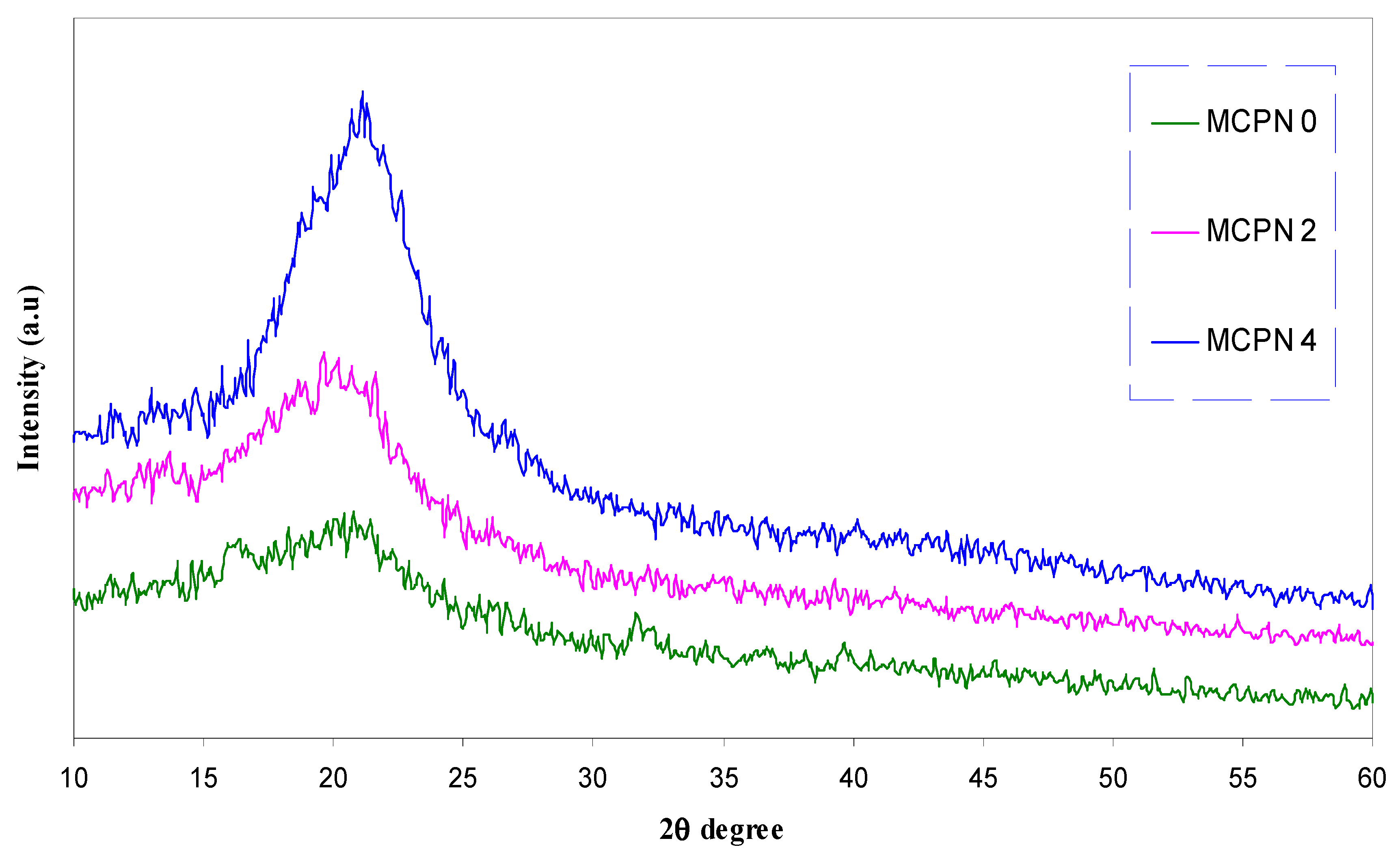
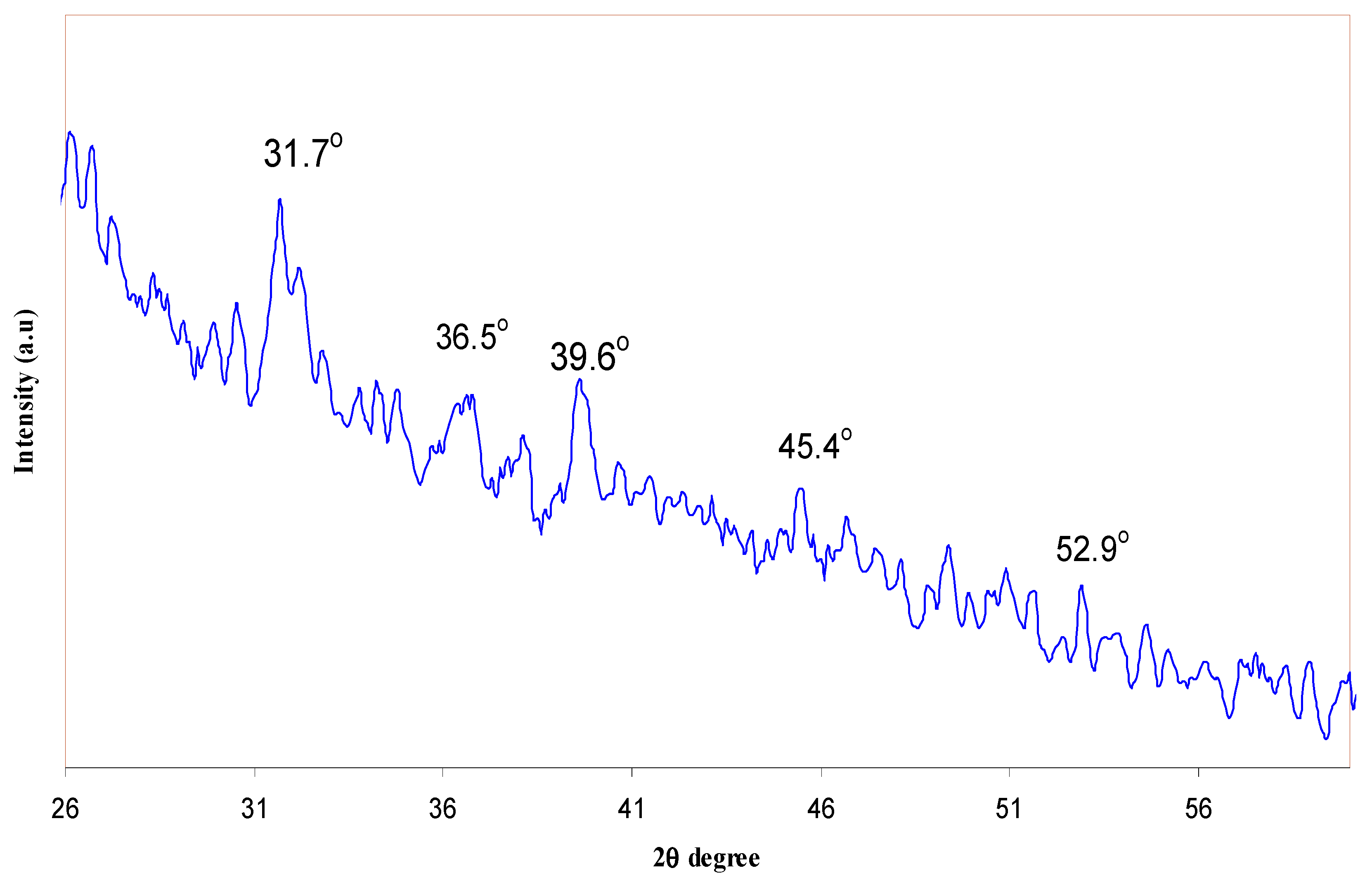
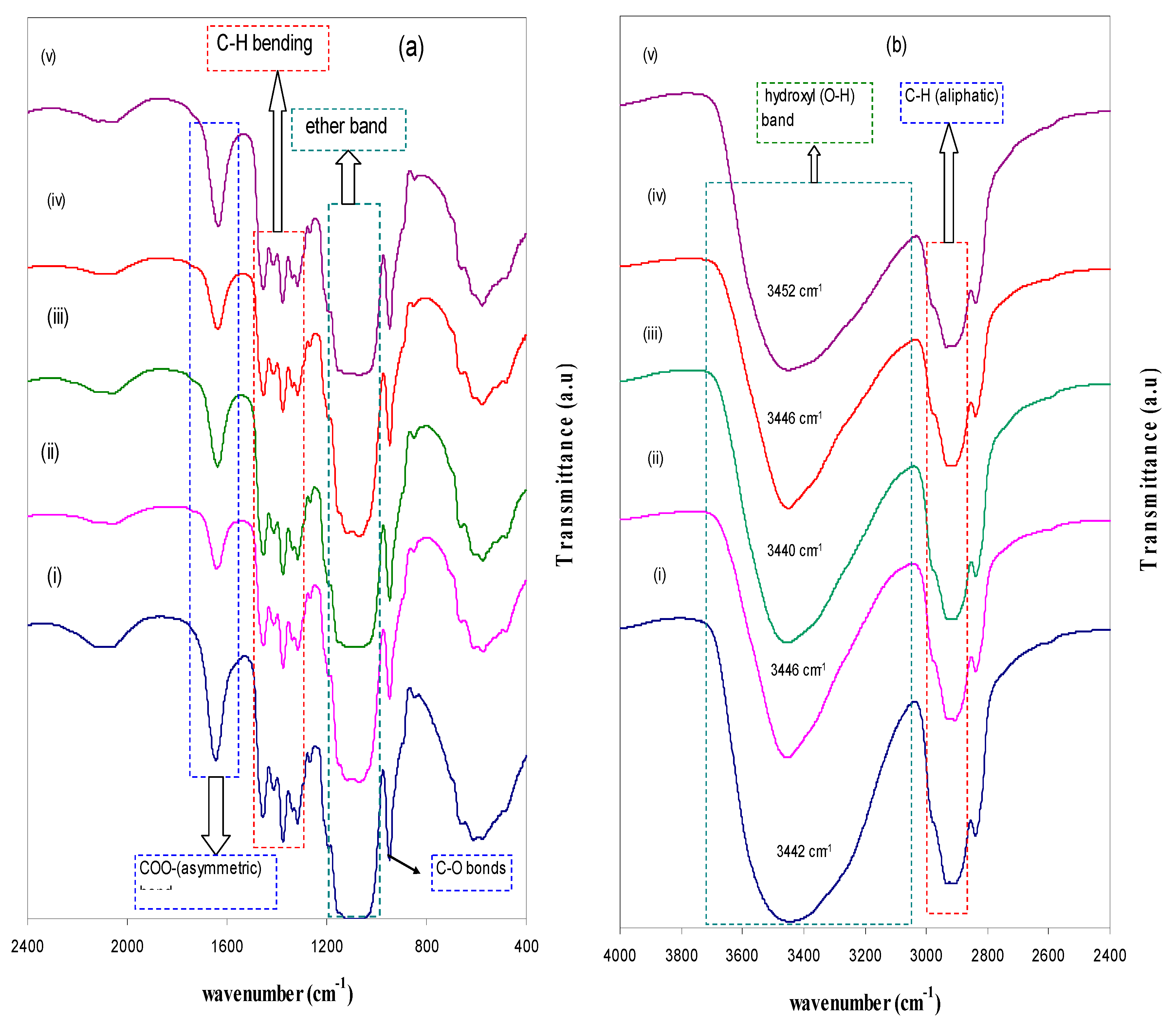
| Nano-Composite Designation | Absorption Edge (eV) | Indirect Bandgap (eV) | Estimated Bandgap from ɛ"-hυ Plot |
|---|---|---|---|
| MCPN0 | 6.2 | 6.19 | 6.24 |
| MCPN1 | 2.8 | 2.78 | 2.8 |
| MCPN2 | 2.5 | 2.6 | 2.6 |
| MCPN3 | 2.4 | 2.47 | 2.48 |
| MCPN4 | 2.28 | 2.33 | 2.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose](1−x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with Desired Optical Band Gaps. Polymers 2017, 9, 194. https://doi.org/10.3390/polym9060194
Aziz SB, Rasheed MA, Ahmed HM. Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose](1−x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with Desired Optical Band Gaps. Polymers. 2017; 9(6):194. https://doi.org/10.3390/polym9060194
Chicago/Turabian StyleAziz, Shujahadeen B., Mariwan A. Rasheed, and Hameed M. Ahmed. 2017. "Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose](1−x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with Desired Optical Band Gaps" Polymers 9, no. 6: 194. https://doi.org/10.3390/polym9060194






