Coumarin- and Carboxyl-Functionalized Supramolecular Polybenzoxazines Form Miscible Blends with Polyvinylpyrrolidone
Abstract
:1. Introduction
2. Experimental
2.1. Materials
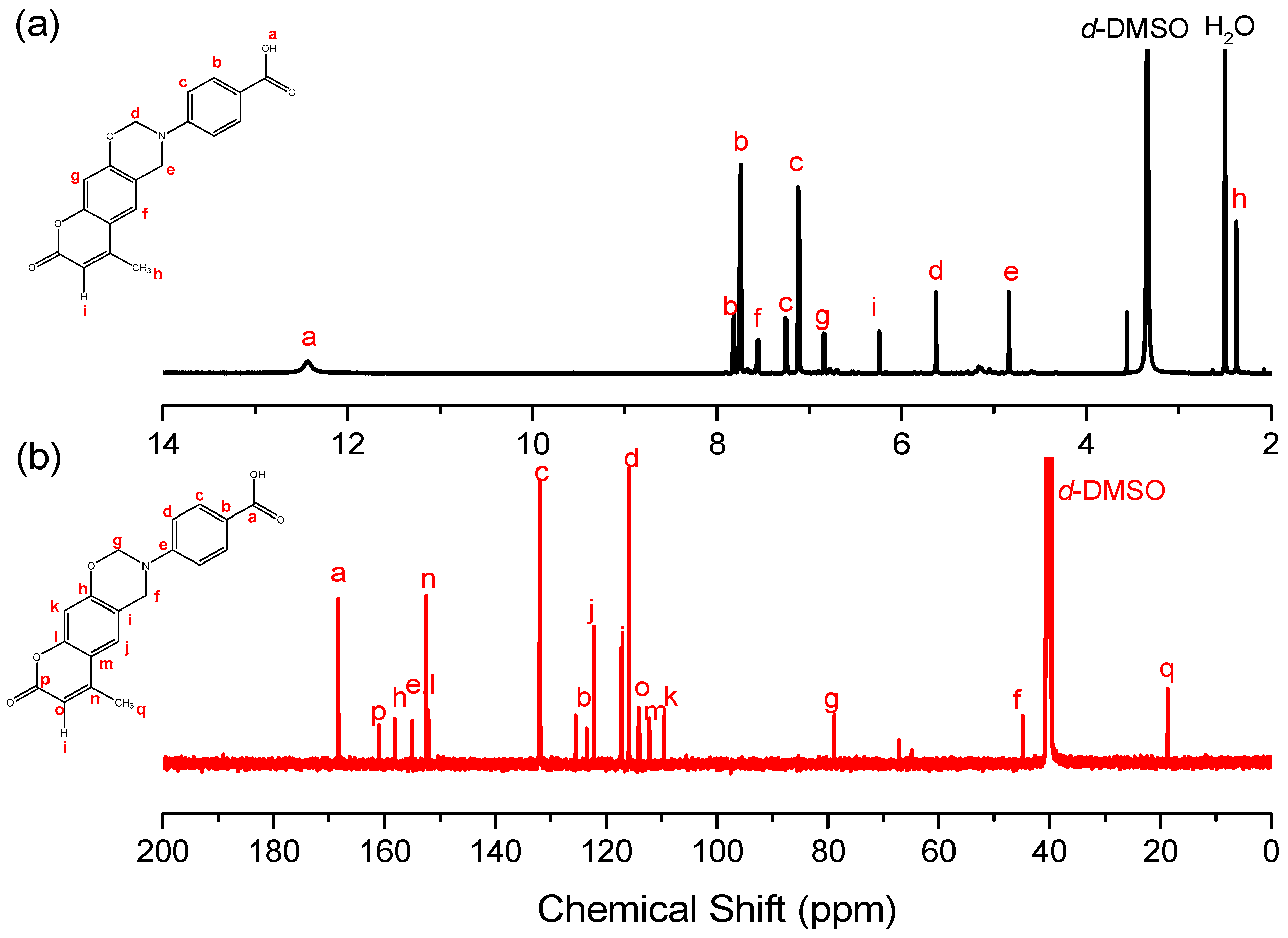
2.1.1. 4-(6-Methyl-8-oxochromeno[6,7-e][1,3]oxazin-3(2H,4H,8H)-yl)benzoic Acid (Coumarin-COOH BZ)
2.1.2. Coumarin-COOH BZ/PVP Blends
2.1.3. Photodimerization of Coumarin-COOH BZ
2.1.4. Poly(Coumarin-COOH BZ)/PVP Blends through Thermal Curing
2.2. Characterization
3. Results and Discussion
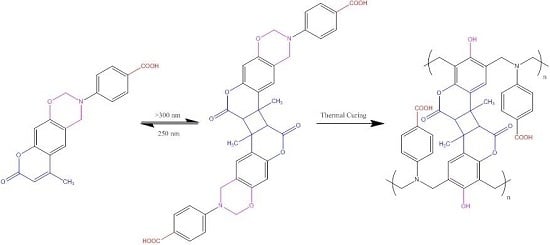
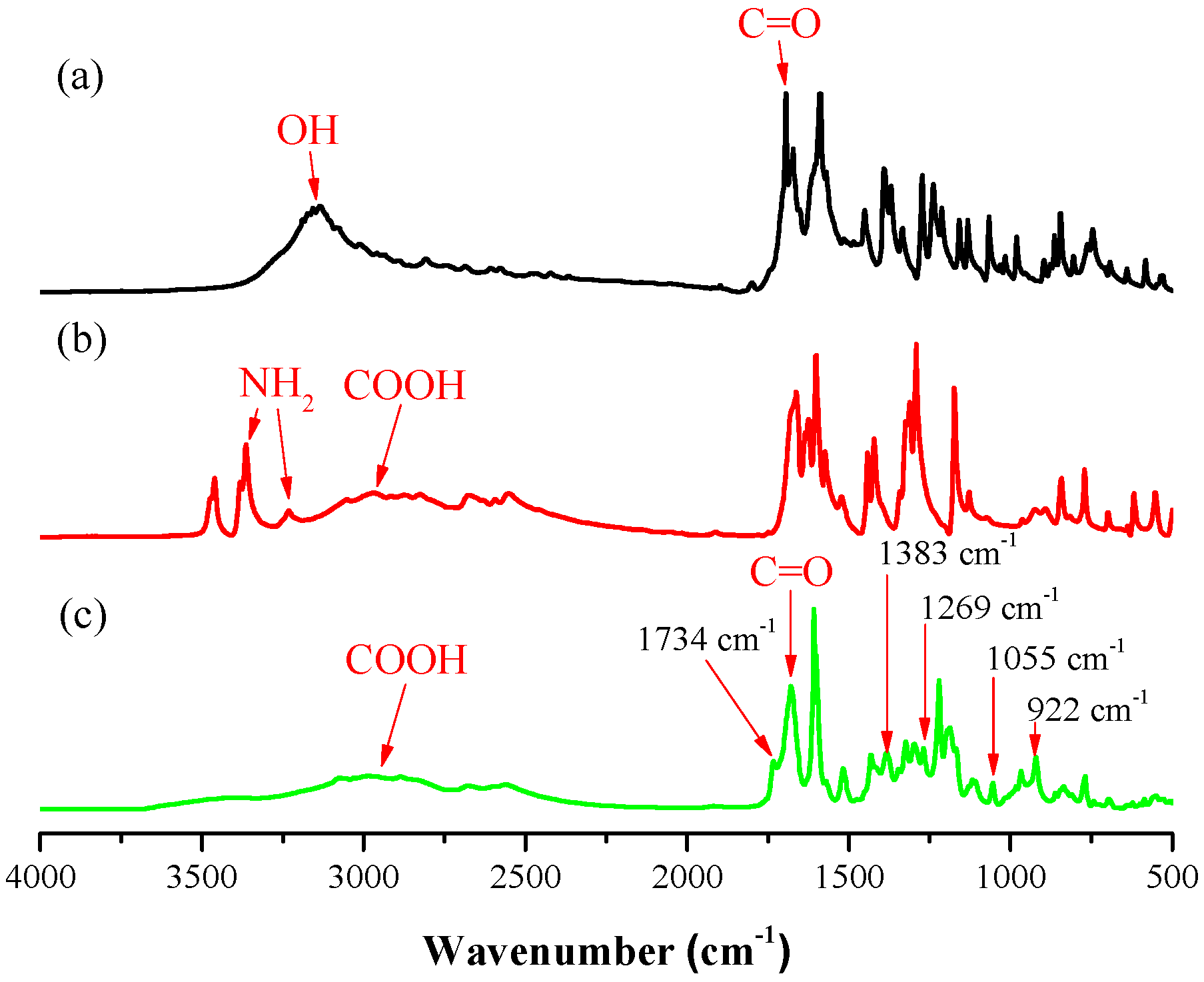
3.1. Synthesis of Coumarin-COOH BZ through Mannich Reaction of Coumarin-OH with 4-Aminobenzoic Acid and Paraformaldehyde
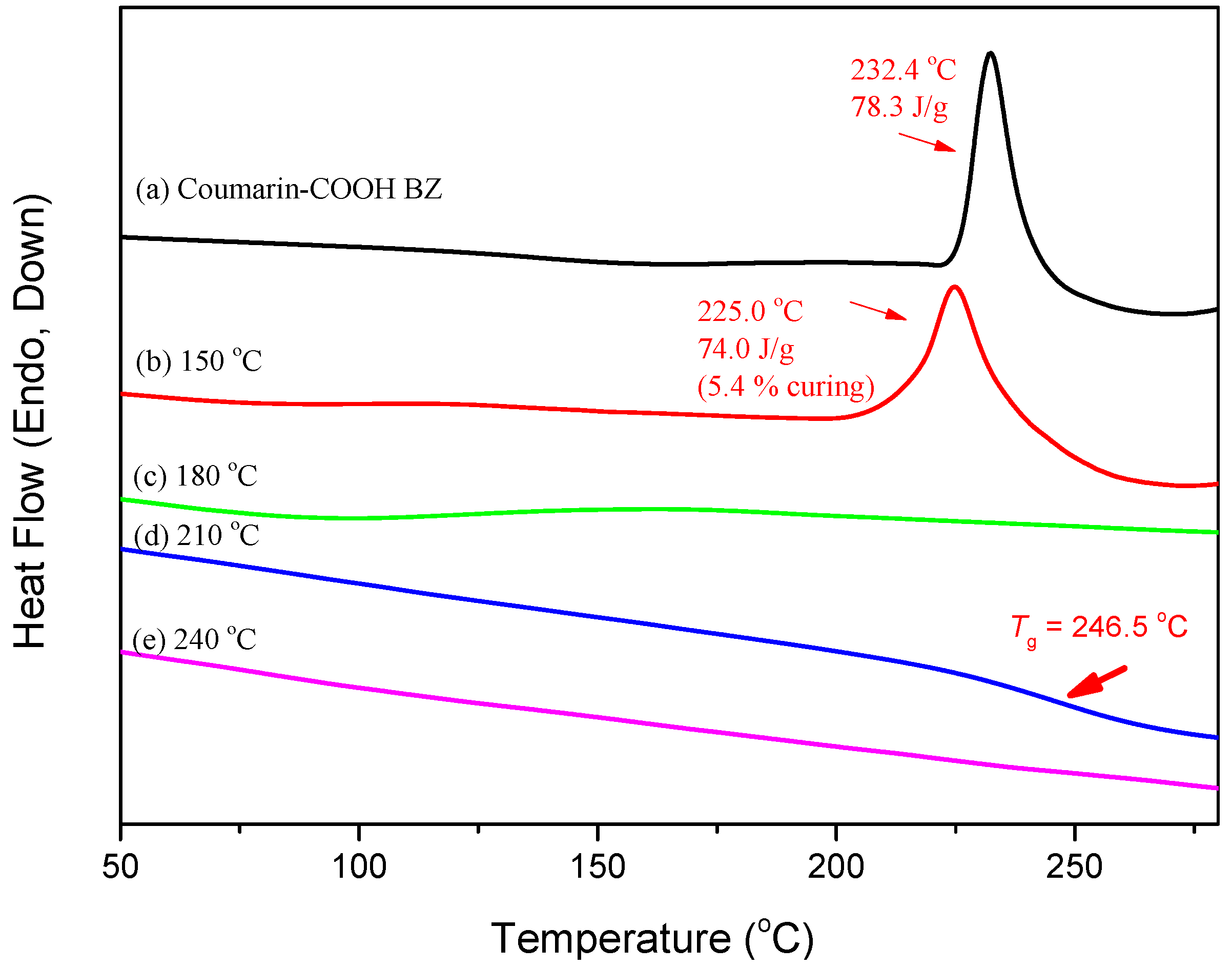
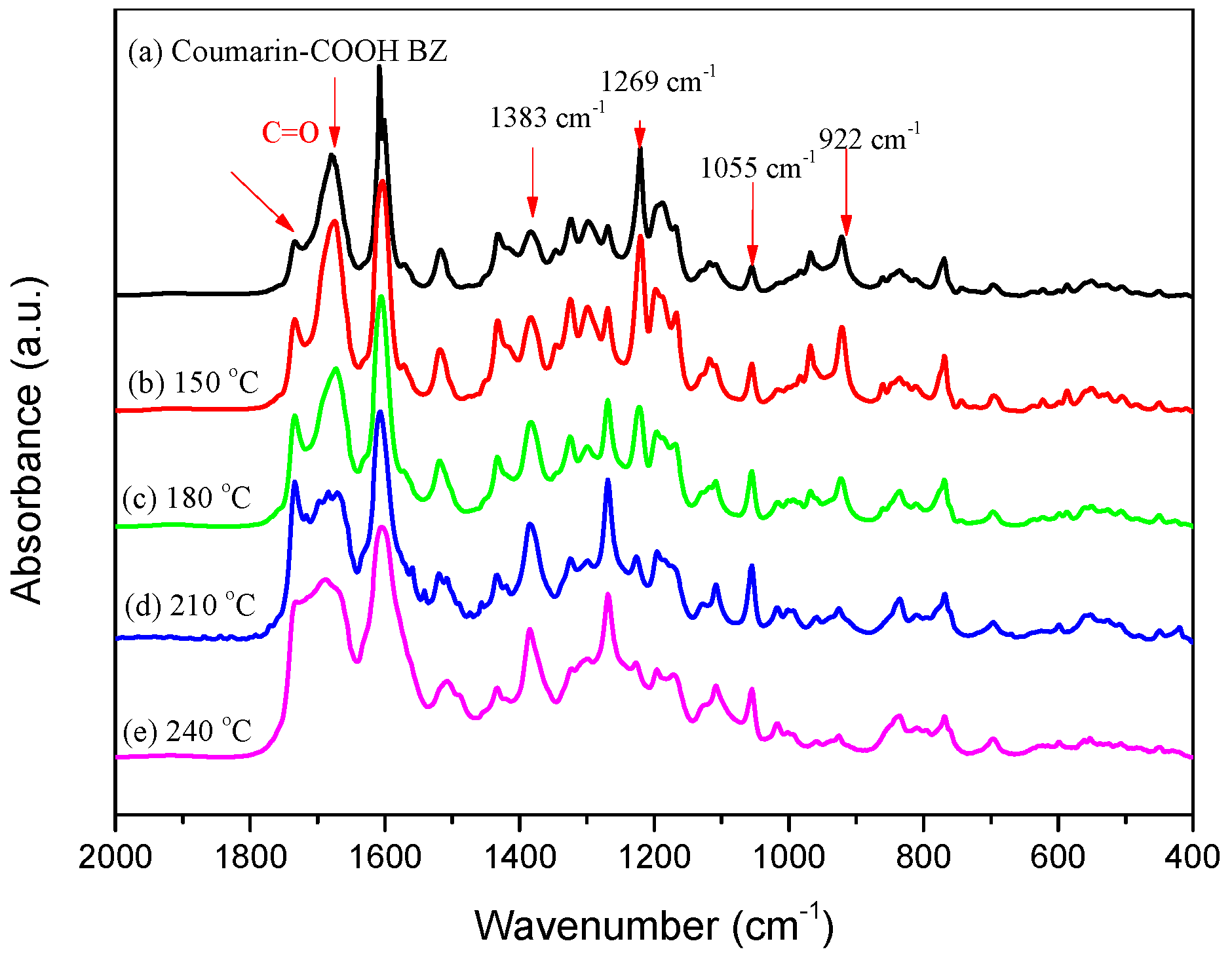
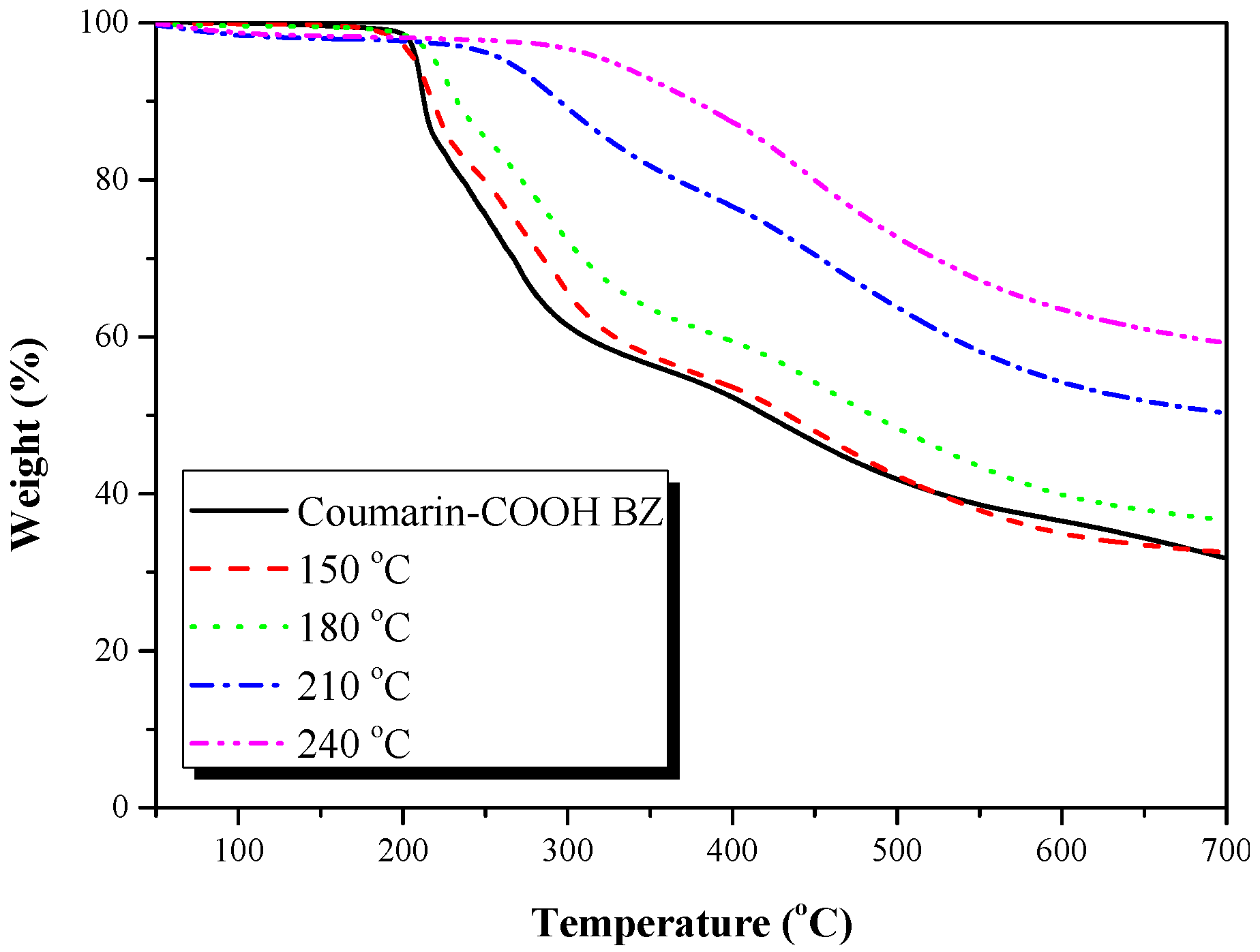
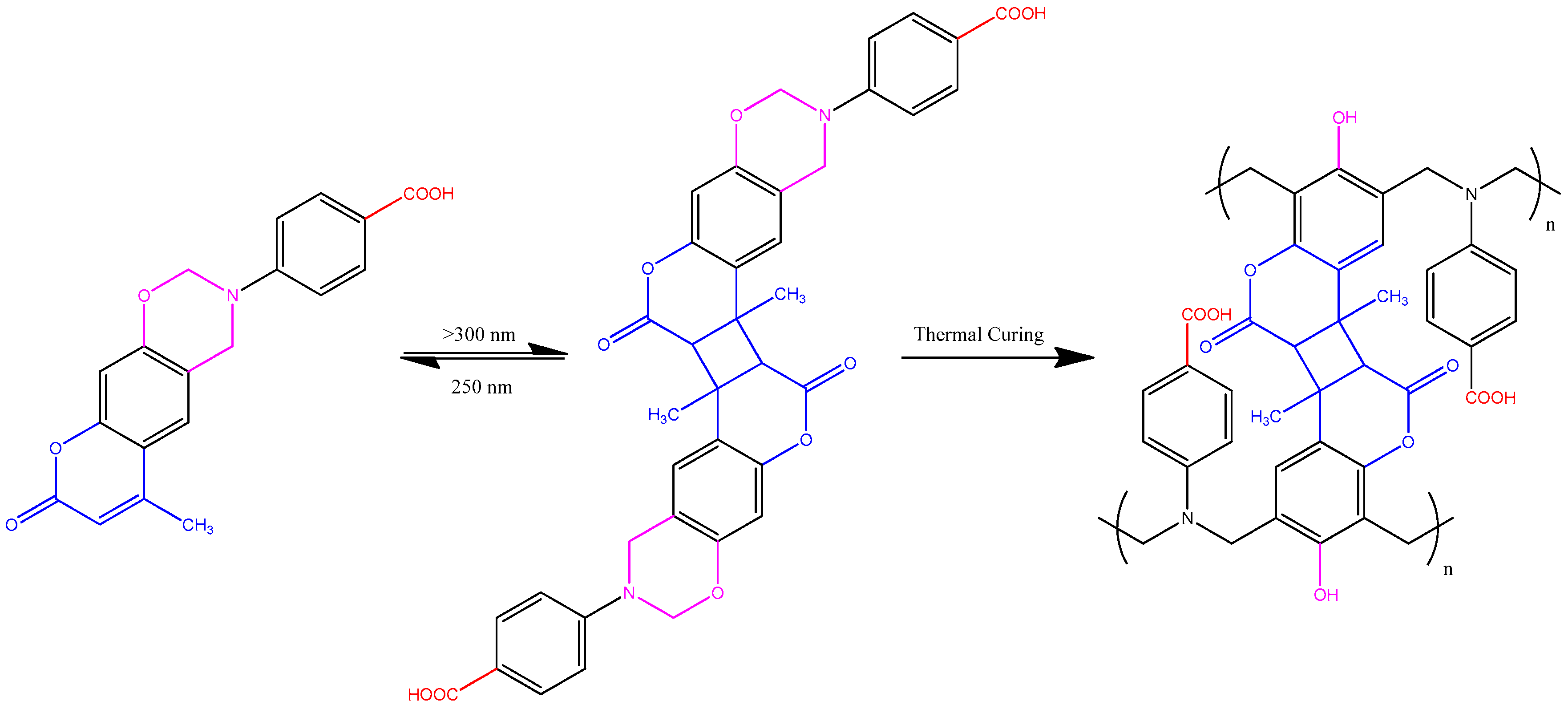
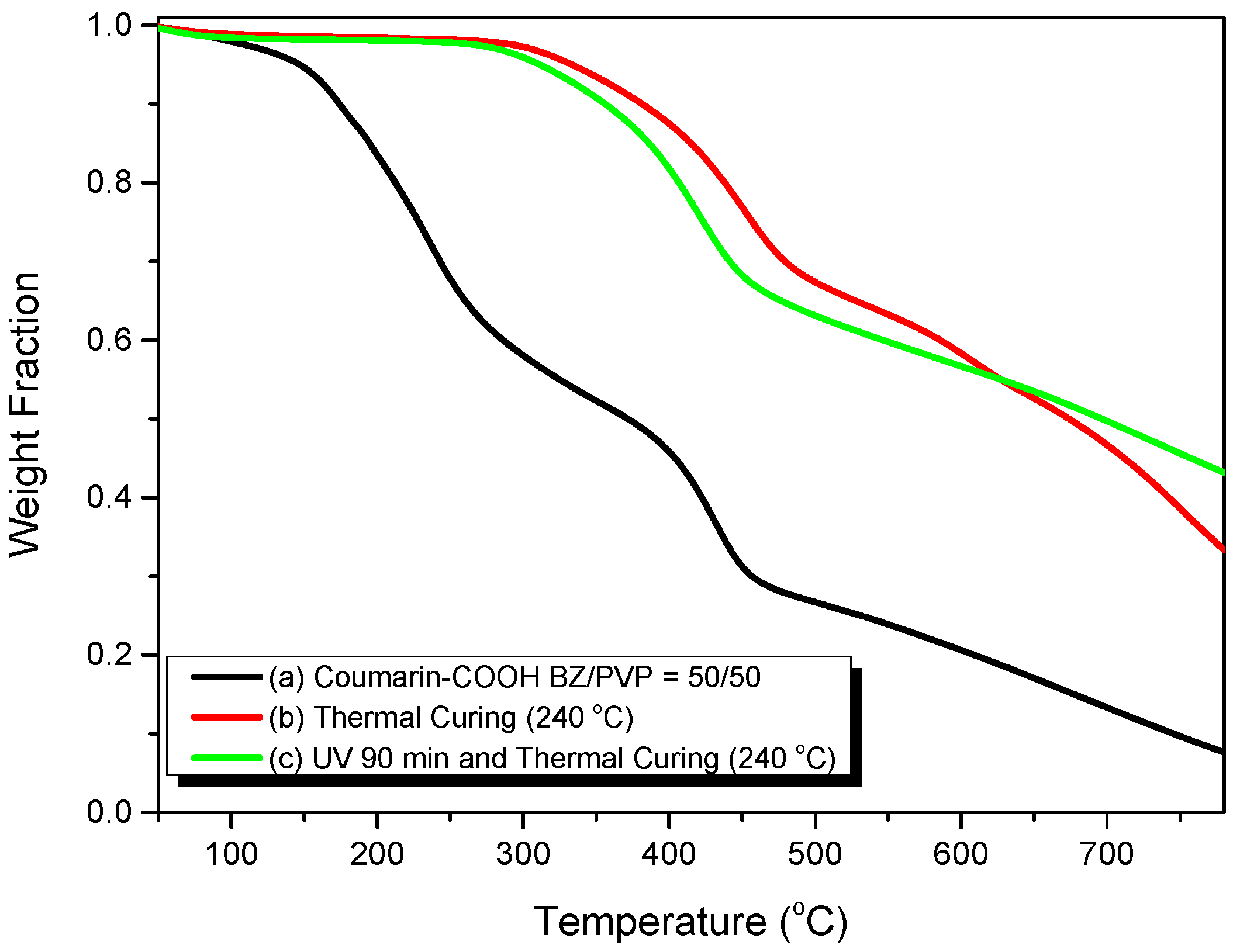
3.2. Thermal Polymerization of Coumarin-COOH BZ
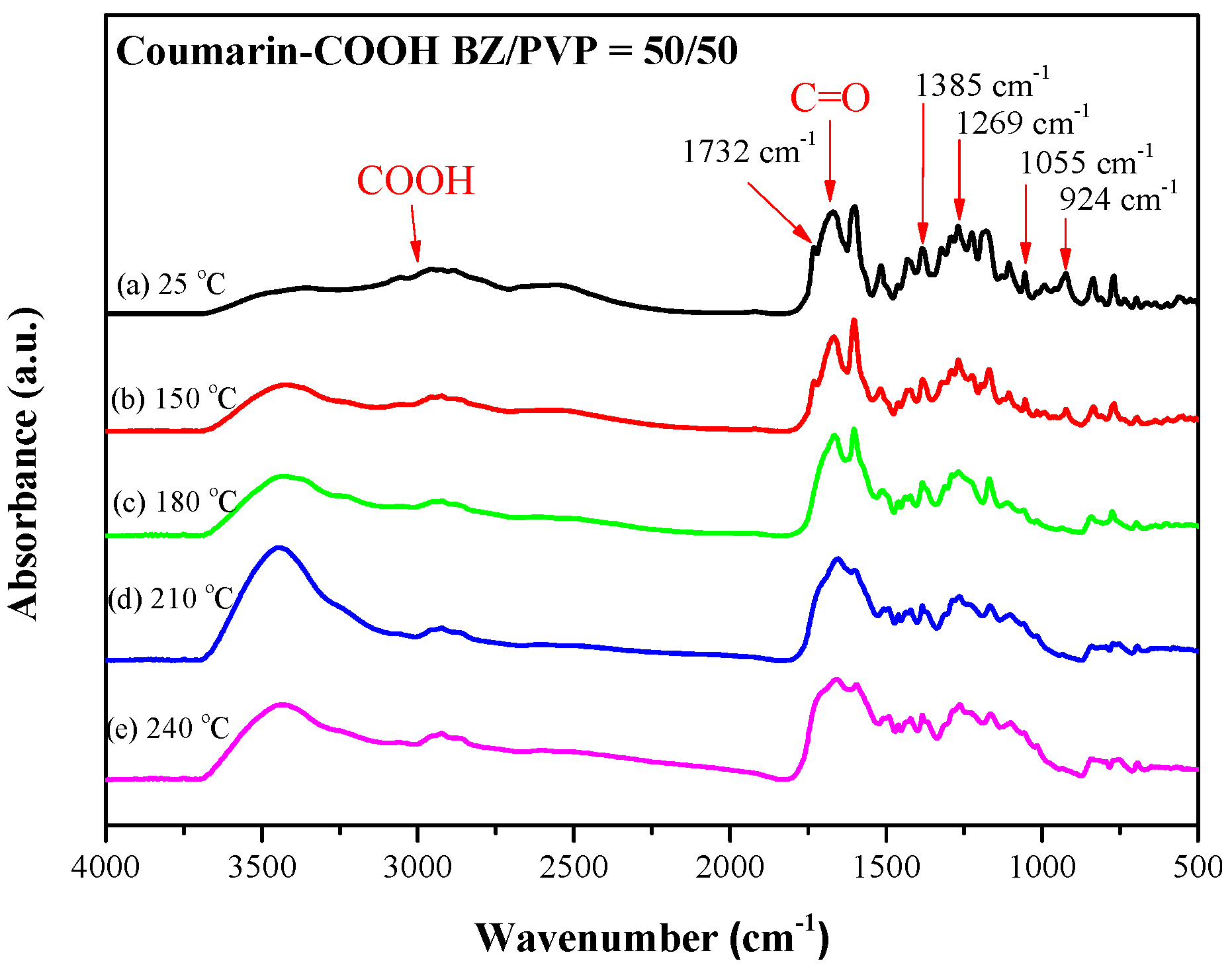
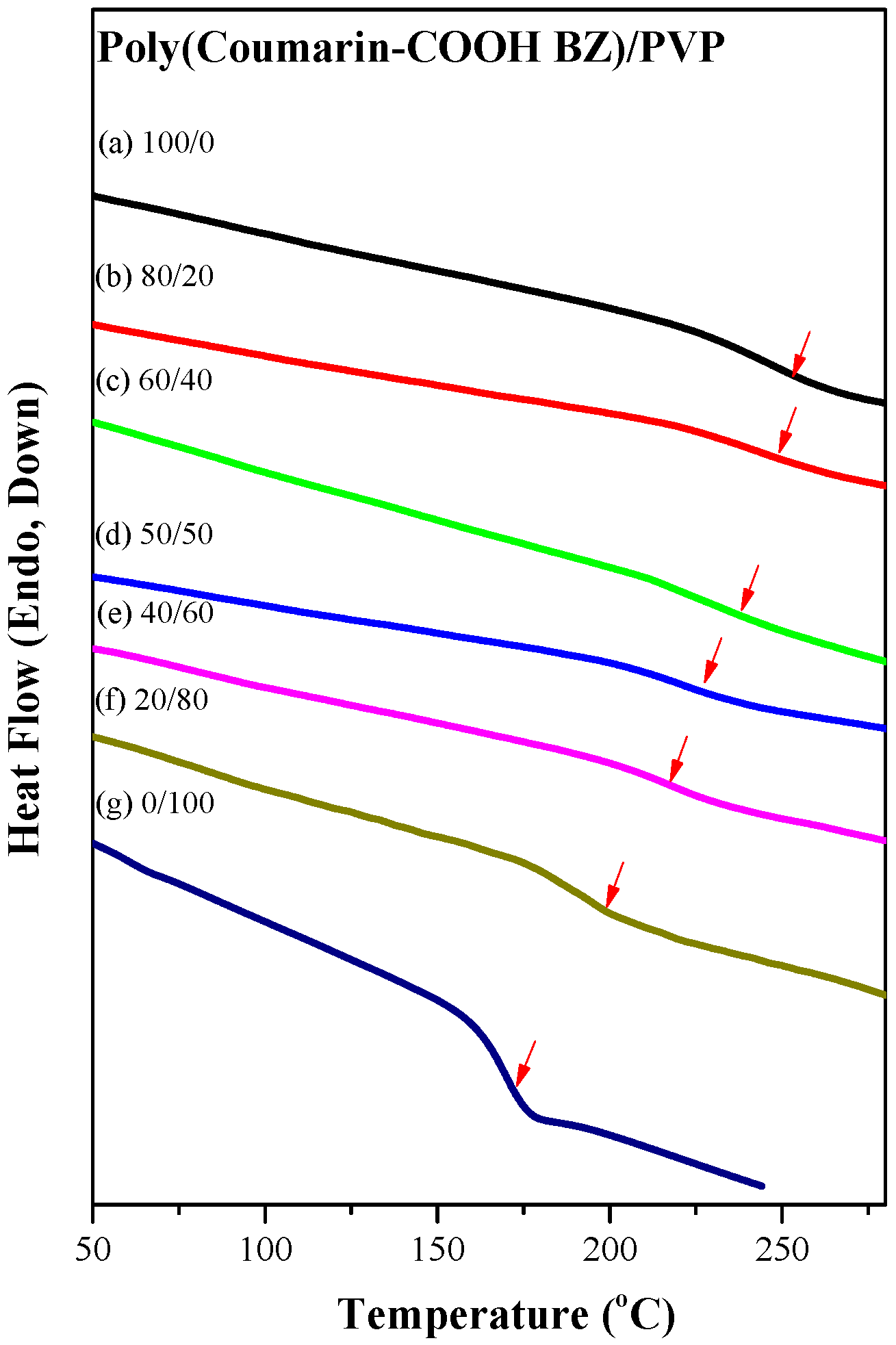
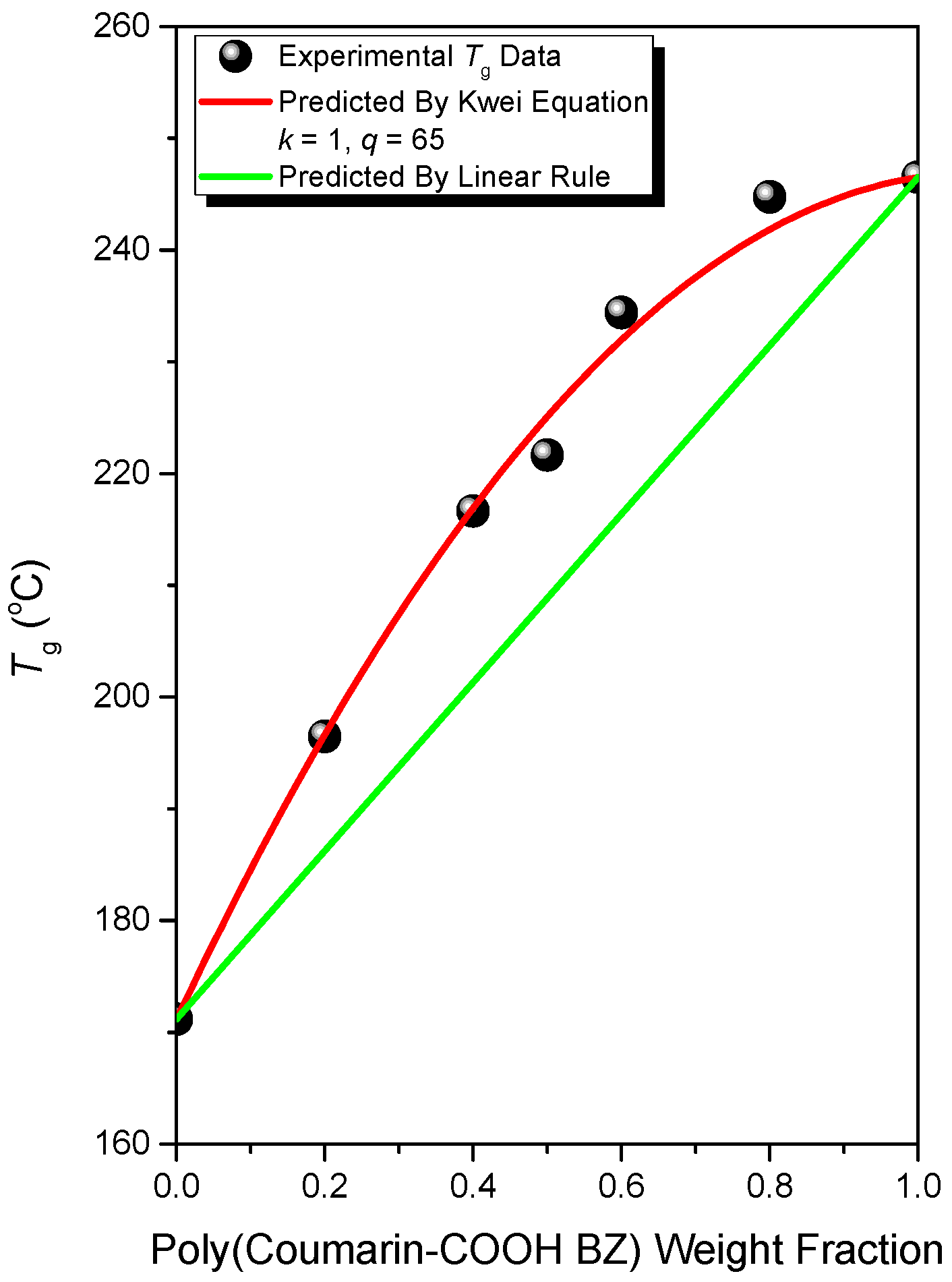
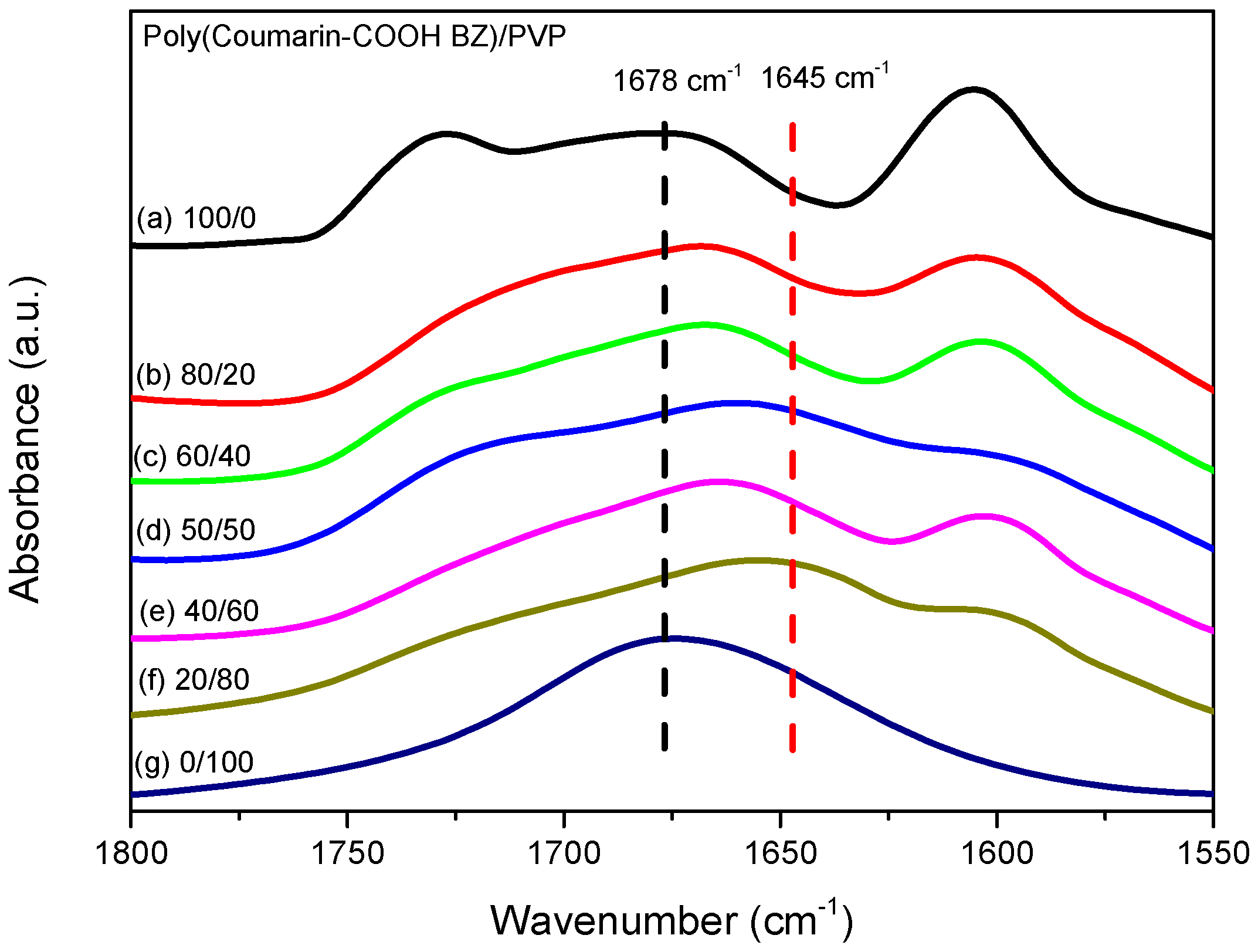
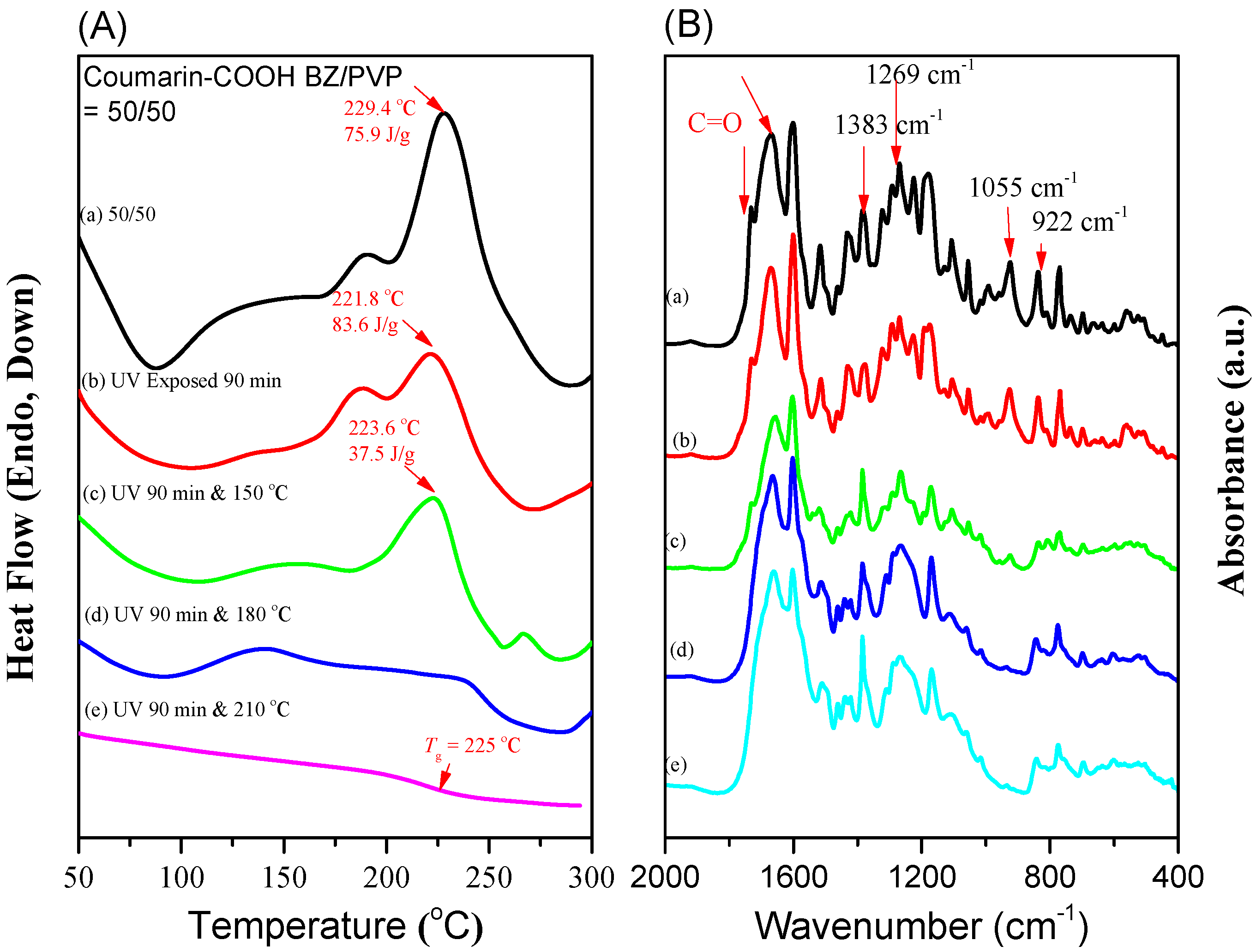
3.3. Thermal Polymerization of Coumarin-COOH BZ/PVP Blends
3.4. Photodimerization of Coumarin Moieties Through [2π + 2π] Cycloaddition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laobutee, A.; Chirachanchai, S.; Ishida, H. Asymmetric mono-Oxazine: An inevitable product from Mannich reaction of benzoxazine dimers. J. Am. Chem. Soc. 2001, 123, 9947–9955. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Tsutomu, T.; Takehiro, K.; Tarek, A. High performance polybenzoxazines as a novel type of phenolic resin. Polym. J. 2008, 40, 1121–1131. [Google Scholar]
- Wang, C.F.; Su, Y.C.; Kuo, S.W.; Huang, C.F.; Sheen, C.Y.; Chang, F.C. Low-surface-free-energy materials based on polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Synthesis and thermal characterization of polybenzoxazines based on acetylene-functional monomers. Polymer 1999, 40, 6565–6573. [Google Scholar] [CrossRef]
- Fu, H.K.; Huang, C.F.; Kuo, S.W.; Lin, H.C.; Yei, D.R.; Chang, F.C. Effect of an organically modified nanoclay on low-surface-energy materials of polybenzoxazine. Macromol. Rapid Commun. 2008, 29, 1216–1220. [Google Scholar] [CrossRef]
- Mohamed, G.M.; Kuo, S.W. Polybenzoxazine/polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Polymers 2016, 8, 225. [Google Scholar] [CrossRef]
- Huang, K.W.; Kuo, S.W. High performance polybenzoxazine nanocomposites containing multifunctional poss cores presenting vinyl-terminated benzoxazine groups. Macromol. Chem. Phys. 2010, 211, 2301–2311. [Google Scholar] [CrossRef]
- Wang, C.F.; Chen, H.Y.; Kuo, S.W.; Lai, Y.S.; Yang, P.F. Rapid, low temperature microwave synthesis of durable, superhydrophobic carbon nanotube-polybenzoxazine nanocomposites. RSC Adv. 2013, 3, 9764–9769. [Google Scholar] [CrossRef]
- Wang, C.F.; Kuo, S.W.; Lin, C.H.; Chen, H.G.; Liao, C.S.; Hung, P.R. Benzoxazine as a reactive noncovalent dispersant for carbon nanotubes. RSC Adv. 2014, 4, 36012–36016. [Google Scholar] [CrossRef]
- Mohamed, G.M.; Hsiao, C.H.; Luo, F.; Dai, L.Z.; Kuo, S.W. Multifunctional polybenzoxazine nanocomposites containing photoresponsive azobenzene units, catalytic carboxylic acid groups, and pyrene units capable of dispersing carbon nanotubes. RSC Adv. 2015, 5, 45201–45212. [Google Scholar] [CrossRef]
- Mohamed, G.M.; Hsu, K.C.; Kuo, S.W. Bifunctional polybenzoxazine nanocomposites containing photo-crosslinkable coumarin units and pyrene units capable of dispersing single-Walled carbon nanotubes. Polym. Chem. 2015, 6, 2423–2433. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polymer 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Lin, C.R.; Mohamed, G.M.; Jia, Y.W.; Yu, R.J.; Kuo, S.W. Multivalent photo-crosslinkable coumarin-containing polybenzoxazines exhibiting enhanced thermal and hydrophobic surface properties. RSC Adv. 2016, 6, 10683–10696. [Google Scholar] [CrossRef]
- Shih, H.K.; Chu, Y.L.; Chang, F.C.; Zhu, C.Y.; Kuo, S.W. A cross-Linkable triphenylamine derivative as a hole injection/transporting material in organic light-Emitting diodes. Polym. Chem. 2015, 6, 6227–6237. [Google Scholar] [CrossRef]
- Kudoh, R.; Sudo, A.; Endo, T. A highly reactive benzoxazine monomer, 1-(2-hydroxyethyl)-1,3-benzoxazine: Activation of benzoxazine by neighboring group participation of hydroxyl group. Macromolecules 2010, 43, 1185–1187. [Google Scholar] [CrossRef]
- Ohashi, S.; Kilbane, J.; Heyl, T.; Ishida, H. Synthesis and characterization of cyanate ester functional benzoxazine and its polymer. Macromolecules 2015, 48, 8412–8417. [Google Scholar] [CrossRef]
- Kan, Z.; Ishida, H. An anomalous trade-off effect on the properties of smart ortho-functional benzoxazines. Polym. Chem. 2015, 6, 2541–2550. [Google Scholar]
- Yang, C.C.; Lin, Y.C.; Wang, P.I.; Liaw, D.J.; Kuo, S.W. Polybenzoxazine/single-walled carbon nanotube nanocomposites stabilized through noncovalent bonding interactions. Polymer 2014, 55, 2044–2050. [Google Scholar] [CrossRef]
- Hu, H.W.; Huang, K.W.; Kuo, S.W. Heteronucleobase-Functionalized benzoxazine: Synthesis, thermal properties, and self-assembled structure formed through multiple hydrogen bonding interactions. Polym. Chem. 2012, 3, 1546–1554. [Google Scholar] [CrossRef]
- Su, Y.C.; Kuo, S.W.; Yei, D.R.; Xu, H.Y.; Chang, F.C. Thermal property and hydrogen bonding in polymer blend of polybenzoxazine/poly(N-vinyl-2-pyrrolidone). Polymer 2003, 44, 2187–2191. [Google Scholar] [CrossRef]
- Li, X.; Xia, Y.; Xu, W.; Ran, Q.; Gu, Y. The curing procedure for a benzoxazine-cyanate-epoxy system and the properties of the terpolymer. Polym. Chem. 2012, 3, 1629–1633. [Google Scholar] [CrossRef]
- Ohashi, S.; Pandey, V.; Arza, C.; Frimowicz, P.; Ishida, H. Simple and low energy consuming synthesis of cyanate ester functional naphthoxazines and their properties. Polym. Chem. 2016, 7, 2245–2252. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, R.; Zhu, X.; Yan, D. Photo-responsive polymeric micelles. Soft Matter 2014, 10, 6121–6138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tong, X.; Zhao, Y. A new design for light-breakable polymer micelles. J. Am. Chem. Soc. 2005, 127, 8290–8291. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhu, B.; Zhou, Y.; Yan, D.; Zhu, X. Reversible photoisomerization of azobenzene-containing polymeric systems driven by visible light. Polym. Chem. 2013, 4, 912–915. [Google Scholar] [CrossRef]
- Kehrlosser, D.; Trager, J.; Kim, H.C.; Hampp, N. Synthesis and photochemistry of coumarin-based self-assembled monolayers on silicon oxide surfaces. Langmuir 2010, 26, 3878–3882. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Kiskan, B. Thermally curable benzoxazine monomer with a photodimerizable coumarin group. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1670–1676. [Google Scholar]
- Froimowicz, P.; Arza, C.R.; Ohashi, S.; Ishida, H. Tailor-made and chemically designed synthesis of coumarin-Containing benzoxazines and their reactivity study toward their thermosets. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1428–1435. [Google Scholar] [CrossRef]
- Kwei, T. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, R.-C.; Mohamed, M.G.; Chen, T.; Kuo, S.-W. Coumarin- and Carboxyl-Functionalized Supramolecular Polybenzoxazines Form Miscible Blends with Polyvinylpyrrolidone. Polymers 2017, 9, 146. https://doi.org/10.3390/polym9040146
Lin R-C, Mohamed MG, Chen T, Kuo S-W. Coumarin- and Carboxyl-Functionalized Supramolecular Polybenzoxazines Form Miscible Blends with Polyvinylpyrrolidone. Polymers. 2017; 9(4):146. https://doi.org/10.3390/polym9040146
Chicago/Turabian StyleLin, Ruey-Chorng, Mohamed Gamal Mohamed, Tao Chen, and Shiao-Wei Kuo. 2017. "Coumarin- and Carboxyl-Functionalized Supramolecular Polybenzoxazines Form Miscible Blends with Polyvinylpyrrolidone" Polymers 9, no. 4: 146. https://doi.org/10.3390/polym9040146