Hydrophilic Polyelectrolyte Multilayers Improve the ELISA System: Antibody Enrichment and Blocking Free
Abstract
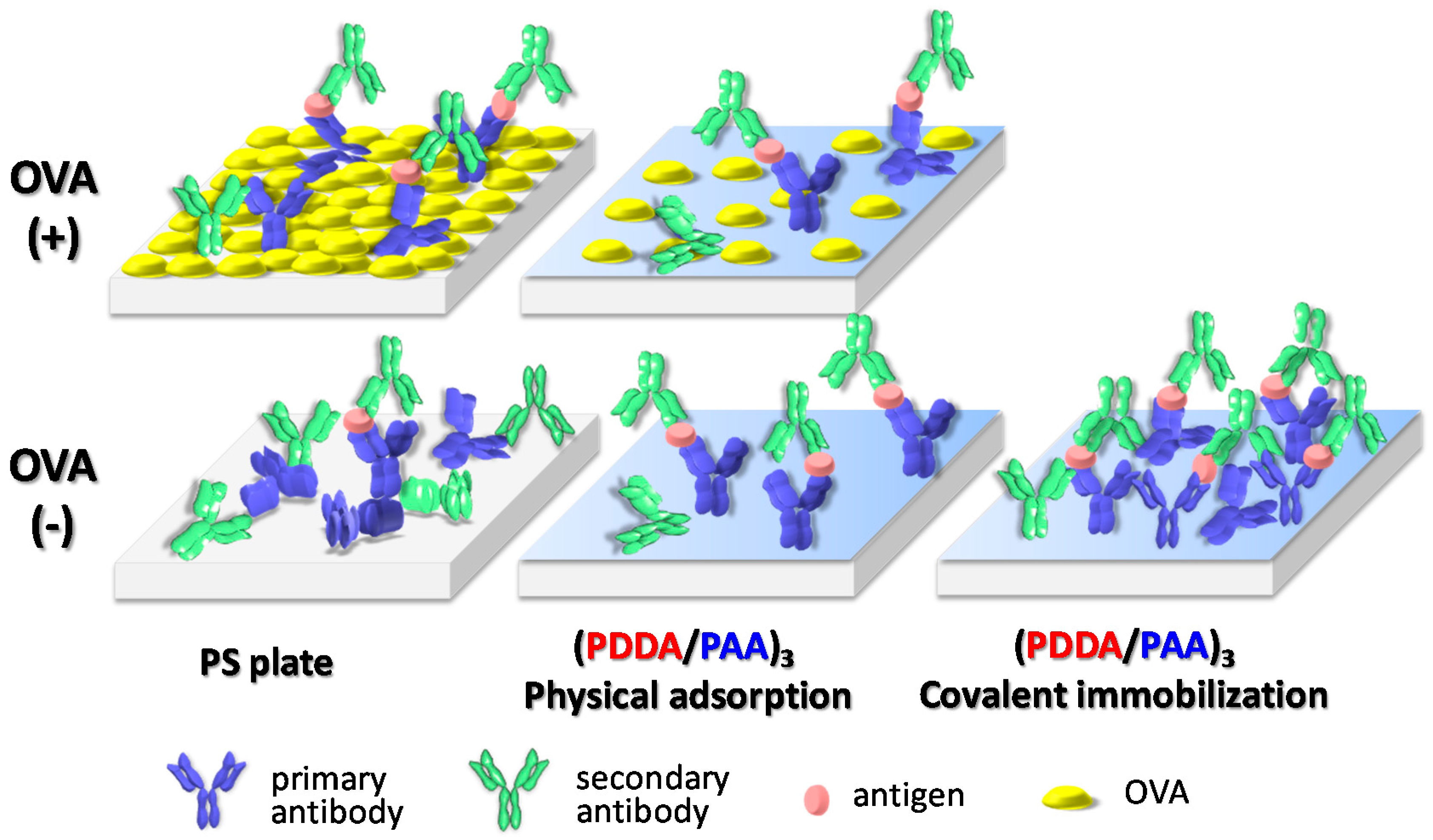
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PEMs in PS Microplate Wells
2.3. Determination of Physical Adsorption and Covalent Immobilization of Protein
2.4. ELISA Protocol on the PS and PEMs Substrate
3. Results and Discussion
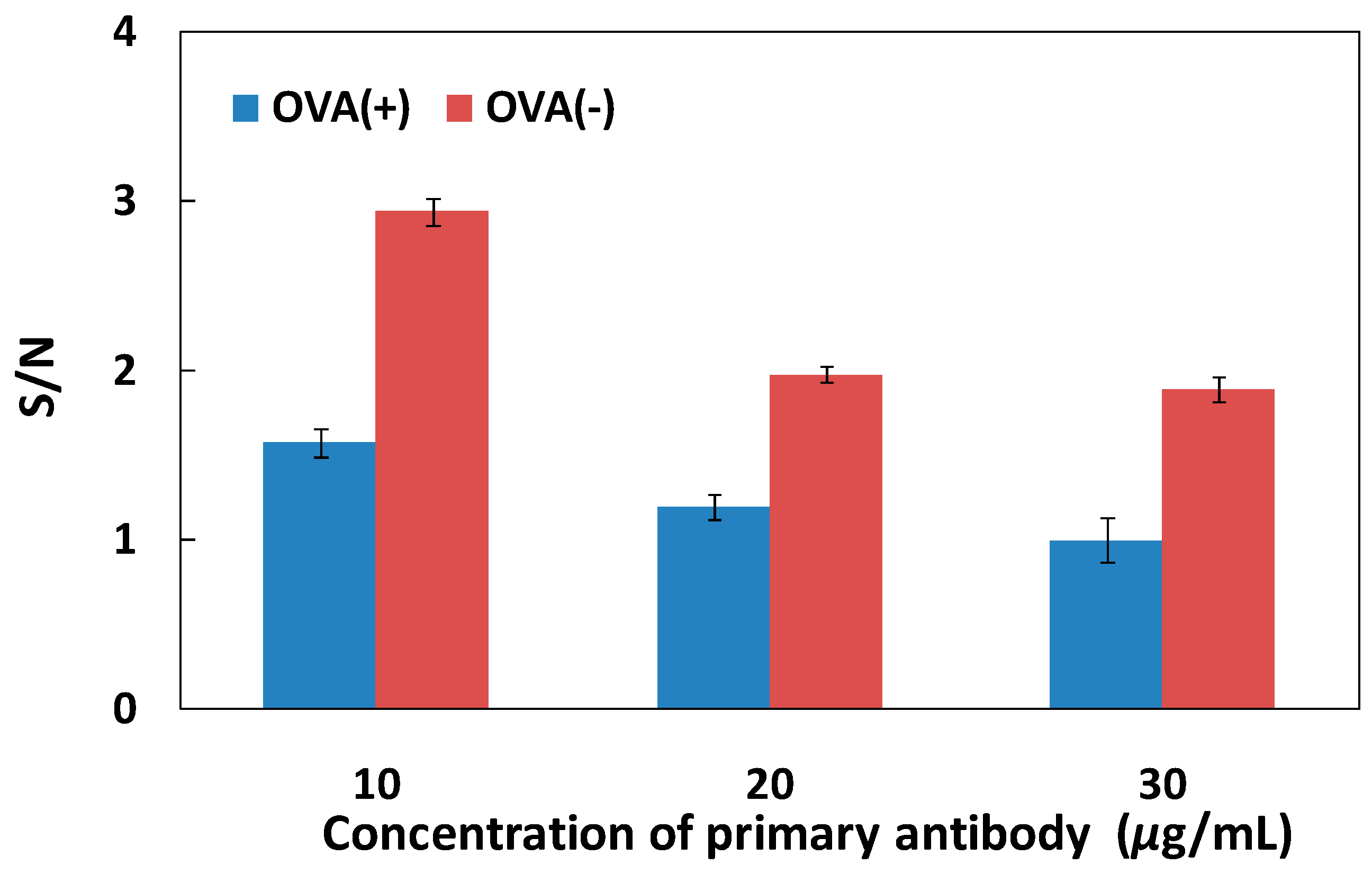
3.1. Determination of Primary Antibody and Blocking Adsorption
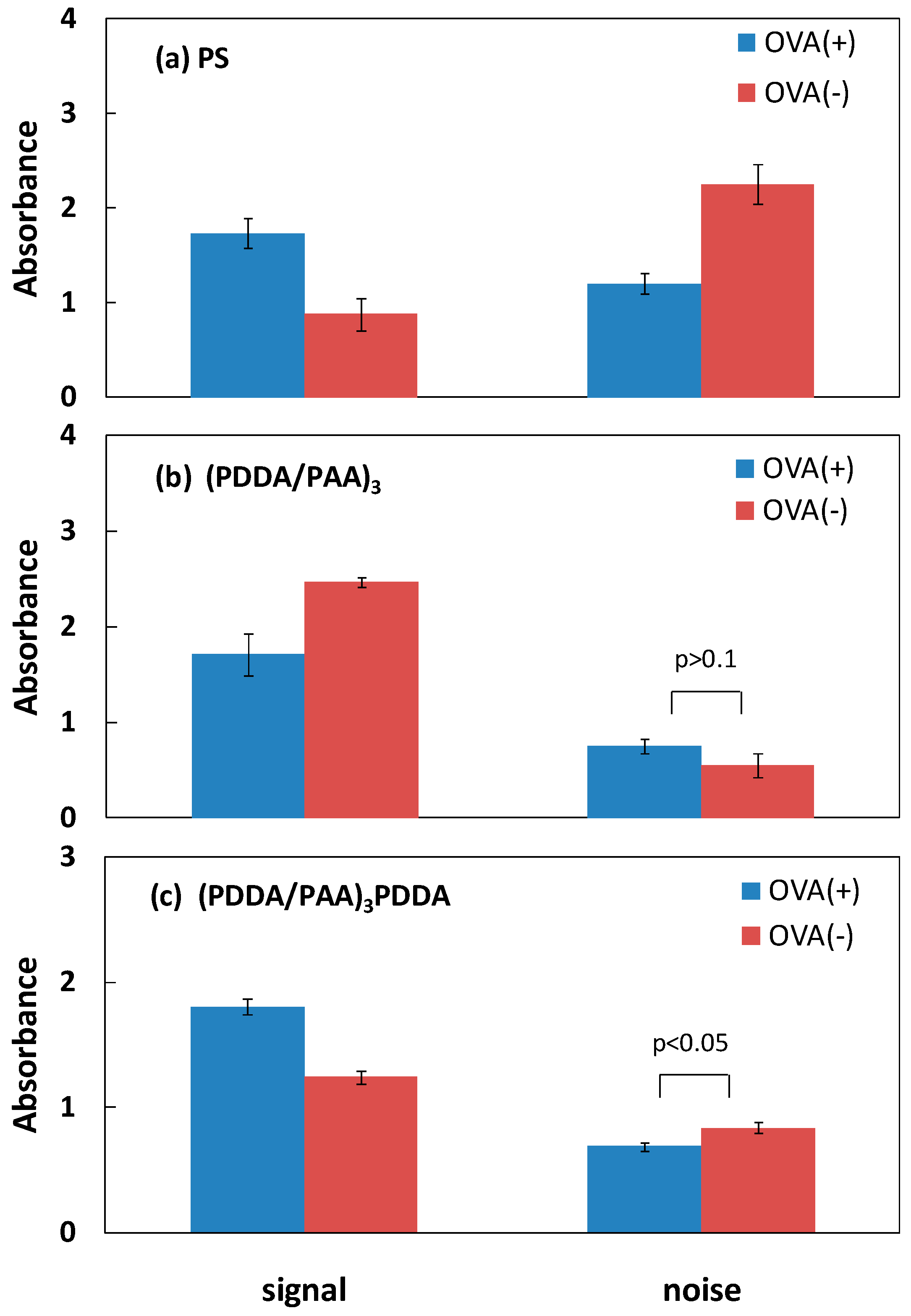
3.2. Blocking Ability of PAA PEMs
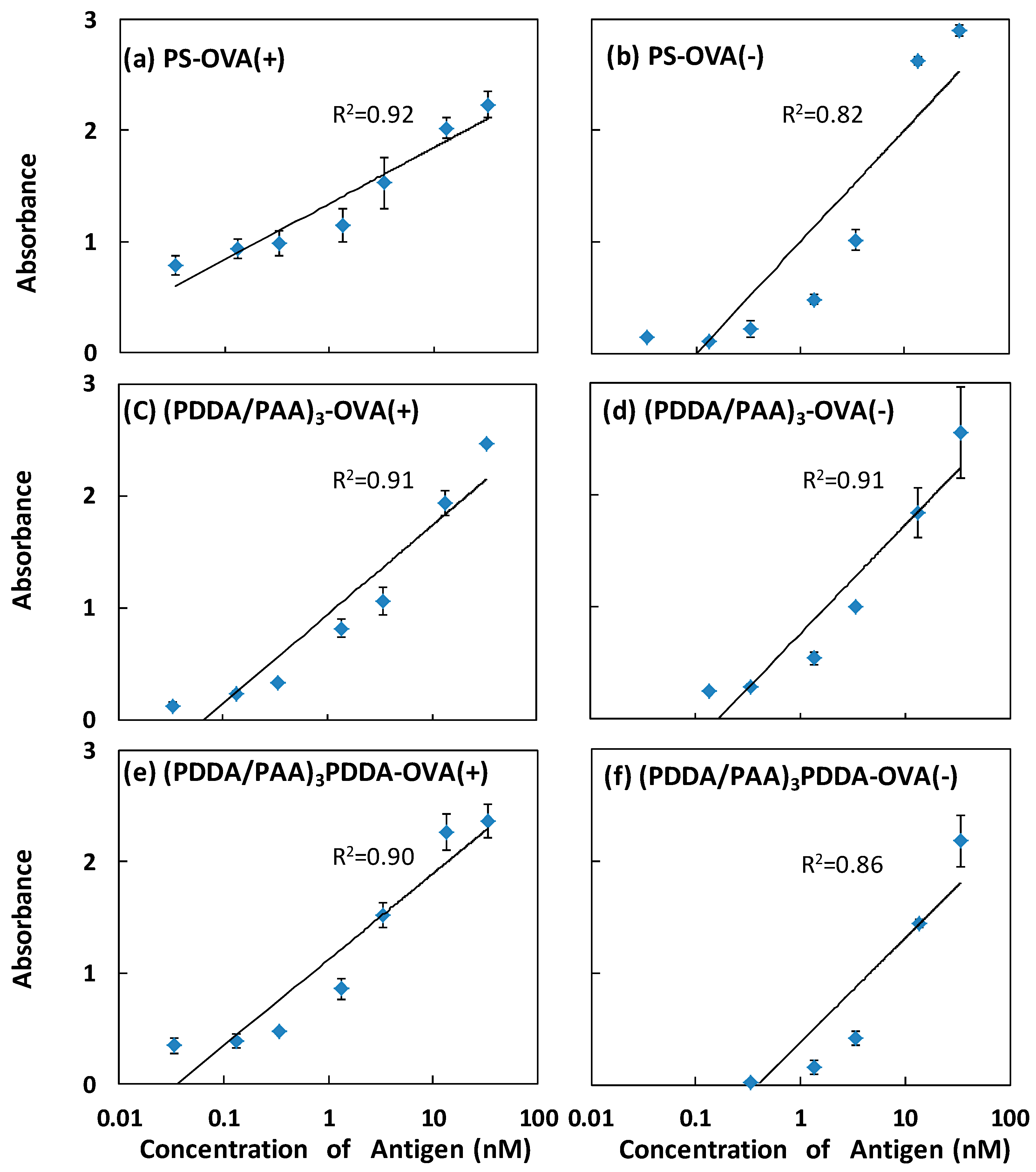
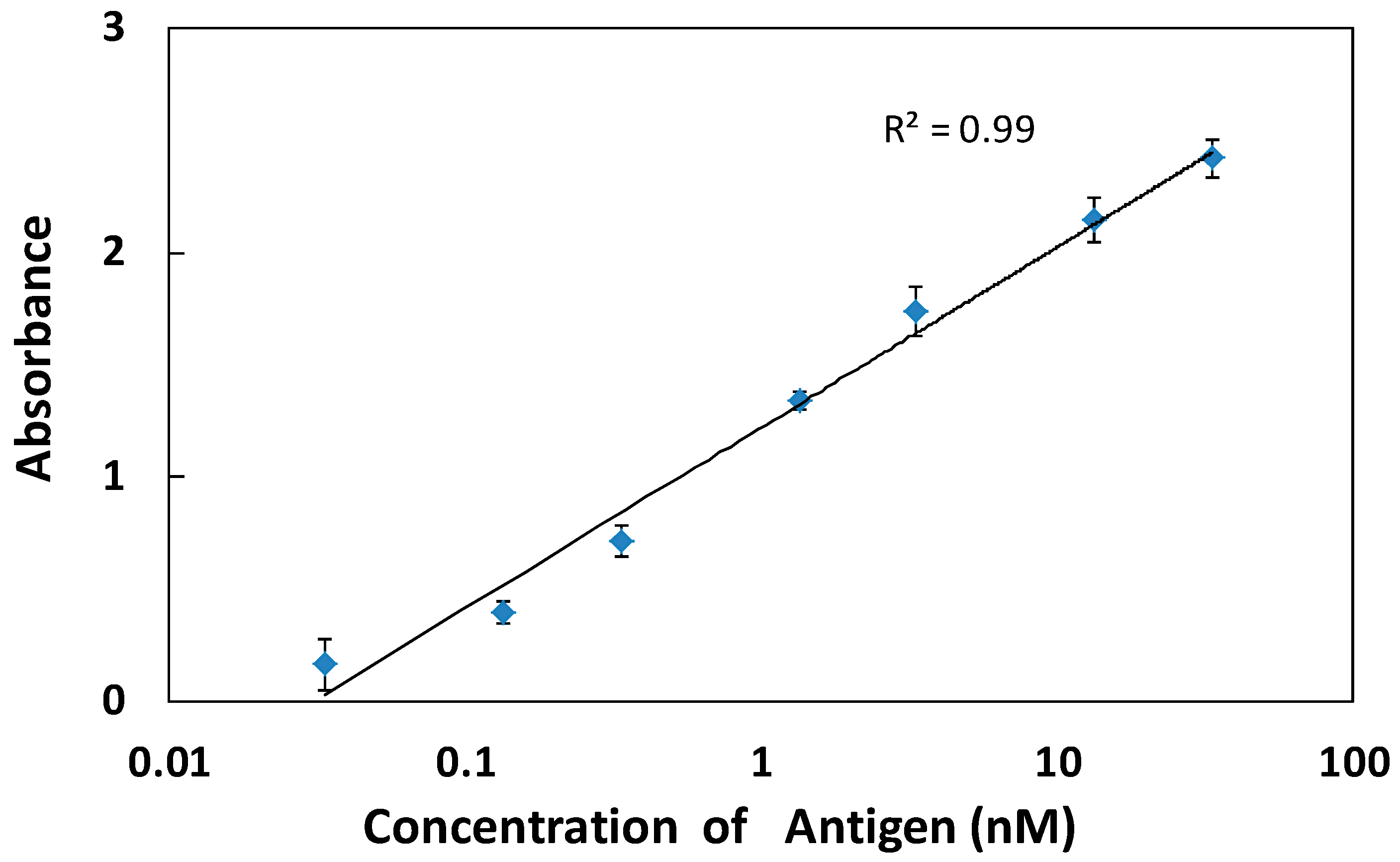
3.3. Higher Sensitivity Induced by Antibody Enrichment on (PDDA/PAA)3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lazcka, O.; Del Campo, F.J.; Muñoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Gutschow, P.; Schmidt, P.J.; Han, H.; Ostland, V.; Bartnikas, T.B.; Pettiglio, M.A.; Herrera, C.; Butler, J.S.; Nemeth, E.; Ganz, T. A competitive enzyme-linked immunosorbent assay specific for murine hepcidin-1: Correlation with hepatic mrna expression in established and novel models of dysregulated iron homeostasis. Haematologica 2015, 100, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Galarini, R.; Diana, F.; Moretti, S.; Puppini, B.; Saluti, G.; Persic, L. Development and validation of a new qualitative elisa screening for multiresidue detection of sulfonamides in food and feed. Food Control 2014, 35, 300–310. [Google Scholar] [CrossRef]
- Steiner, J.M.; Xenoulis, P.G.; Schwierk, V.M.; Suchodolski, J.S. Development and analytical validation of an enzyme-linked immunosorbent assay for the measurement of feline tumor necrosis factor α in serum. Vet. Clin. Pathol. 2014, 43, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Watanabe, J.; Akashi, M. Polyelectrolyte multilayers-modified membrane filter for rapid immunoassay: Protein condensation by centrifugal permeation. Polym. J. 2010, 43, 35–40. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, M.K.; Lee, E.J.; Ahn, K.Y.; Lee, K.E.; Jung, J.H.; Cho, Y.; Han, S.S.; Kim, Y.K.; Lee, J. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nat. Nanotechnol. 2009, 4, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Cheow, L.F.; Ko, S.H.; Kim, S.J.; Kang, K.H.; Han, J. Increasing the sensitivity of enzyme-linked immunosorbent assay using multiplexed electrokinetic concentrator. Anal. Chem. 2010, 82, 3383–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yuan, L.; Wang, H.; Li, D.; Chen, H. Gold nanoparticle layer: A promising platform for ultra-sensitive cancer detection. Langmuir 2011, 27, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Du, D.; Zhu, M.J.; Lin, Y.; Dai, Z. An improved ultrasensitive enzyme-linked immunosorbent assay using hydrangea-like antibody–enzyme–inorganic three-in-one nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 6329–6335. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Xu, H.; Xu, P.; Chen, K.; Mu, R.; Fu, J.; Gu, H. Ultrasensitive elisa using enzyme-loaded nanospherical brushes as labels. Anal. Chem. 2014, 86, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, Y.; Kobayashi, H.; Katsuyama, Y.; Jomura, T.; Sakura, T. Enhanced immunoresponse of antibody/mixed-peg co-immobilized surface construction of high-performance immunomagnetic elisa system. J. Colloid. Interface Sci. 2007, 309, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y.; Miyachi, S.; Nagasaki, Y. High-performance surface acoustic wave immunosensing system on a peg/aptamer hybridized surface. Langmuir 2013, 29, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Ishihara, K. Instantaneous determination via bimolecular recognition: Usefulness of fret in phosphorylcholine group enriched nanoparticles. Bioconj. Chem. 2007, 18, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Nagasaka, Y.; Matsuno, H.; Shimoyama, M.; Kurita, K. A stereocomplex platform efficiently detecting antigen−antibody interactions. Bioconj. Chem. 2007, 18, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.P.; Zhong, X.Q.; Hua, B.; Liu, M.Y.; Jing, F.X.; Lou, X.H.; Yao, S.H.; Xiang, J.Q.; Jin, Q.H.; Zhao, J.L. Nano-elisa for highly sensitive protein detection. Biosens. Bioelectron. 2009, 24, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Berichte der Bunsengesellschaft für Physikalische Chemieolloid 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G. Toward layered polymeric multicomposites. Science 1997, 227, 1232–1237. [Google Scholar] [CrossRef]
- Volodkin, D.; Klitzing, R.V.; Moehwald, H. Polyelectrolyte multilayers: Towards single cell studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- O’Driscoll, R.L.; Mcclatchie, S. Human mesenchymal stem cell osteoblast differentiation, ecm deposition, and biomineralization on pah/paa polyelectrolyte multilayers. J. Biomed. Mater. Res. Part A 2015, 103, 1818–1827. [Google Scholar]
- Wu, H.; Guo, L.; Zhang, J.; Miao, S.; He, C.; Wang, B.; Wu, Y.; Chen, Z. Polyelectrolyte-free layer by layer self-assembled multilayer films of cationic phthalocyanine cobalt(ii) and carbon nanotube for the efficient detection of 4-nitrophenol. Sens. Actuators B Chem. 2016, 230, 359–366. [Google Scholar] [CrossRef]
- Matsusaki, M.; Ajiro, H.; Kida, T.; Serizawa, T.; Akashi, M. Layer-by-Layer assembly through weak interactions and their biomedical applications. Adv. Mater. 2012, 24, 454–474. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological applications of lbl multilayer capsules: From drug delivery to sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Ulasevich, S.A.; Brezesinski, G.; Möhwald, H.; Fratzl, P.; Schacher, F.H.; Poznyak, S.K.; Andreeva, D.V.; Skorb, E.V. Light-induced water splitting causes high-amplitude oscillation of ph-sensitive Layer-by-Layer assemblies on TiO2. Angew. Chem. Int. Ed. 2016, 55, 13001. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Pattekari, P.; Jesus, S.; Veiga, F.; Lvov, Y.; Ribeiro, A.J. Sonication-assisted Layer-by-Layer assembly for low solubility drug nanoformulation. ACS Appl. Mater. Interfaces 2015, 7, 11972. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Frueh, J.; Wu, Z.; He, Q. Leucocyte membrane-coated janus microcapsules for enhanced photothermal cancer treatment. Langmuir 2016, 32, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Watanabe, J.; Akashi, M. Heterofunctional interfaces achieve dual protein adsorption on polyelectrolyte multilayers. Polym. J. 2009, 41, 486–491. [Google Scholar] [CrossRef]
- Shen, H.; Watanabe, J.; Akashi, M. Polyelectrolyte multilayers-modified polystyrene plate improves conventional immunoassay: Full covering of the blocking reagent. Anal. Chem. 2009, 81, 6923–6928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, M.; Yuan, L.; Cheng, Z.; Wu, Z.; Chen, H. Sensitive sandwich elisa based on a gold nanoparticle layer for cancer detection. Analyst 2012, 137, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Hemmerlé, J.; Voegel, J.C.; Gonthier, E.; Decher, G.; Benkiranejessel, N.; Chassepot, A.; Mertz, D.; Lavalle, P.; Mésini, P. Polyelectrolyte multilayer coatings that resist protein adsorption at rest and under stretching. J. Mater. Chem. 2008, 36, 4242–4245. [Google Scholar] [CrossRef]
- Dai, J.; Baker, G.L.; Bruening, M.L. Use of porous membranes modified with polyelectrolyte multilayers as substrates for protein arrays with low nonspecific adsorption. Anal. Chem. 2006, 78, 135–140. [Google Scholar] [CrossRef] [PubMed]
- David, S.S.; Schlenoff, J.B. Protein adsorption modalities on polyelectrolyte multilayers. Biomacromolecules 2004, 5, 1089–1096. [Google Scholar]
- Sakaguchi, H.; Serizawa, T.; Akashi, M. Layer-by-Layer assembly on hydrogel surfaces and control of human whole blood coagulation. Chem. Lett. 2003, 32, 174–175. [Google Scholar] [CrossRef]
- Silverton, E.W.; Navia, M.A.; Davies, D.R. Three-dimensional structure of an intact human immunoglobulin. Proc. Nat. Acad. Sci. USA 1977, 74, 5140–5144. [Google Scholar] [CrossRef] [PubMed]
- Kamilya, T.; Prabir Pal, A.; Talapatra, G.B. Interaction of ovalbumin with phospholipids langmuir-blodgett film. J. Phys. Chem. B 2007, 111, 1199–1205. [Google Scholar] [CrossRef] [PubMed]



| Surface IgG a | Primary (μg/cm2) | IgG Coverage b (%) | OVA a (μg/cm2) | OVA Coverage c (%) | Ratio of Molecules d (OVA/IgG) | ||
|---|---|---|---|---|---|---|---|
| End-on | Side-on | End-on | Side-on | ||||
| PS | 0.17 ± 0.01 | 9 | 63 | 0.72 ± 0.01 | 171 | 266 | 14.1 |
| (PDDA/PAA)3 | 0.10 ± 0.01 | 5 | 36 | 0.21 ± 0.01 | 50 | 77 | 7.0 |
| (PDDA/PAA)3PDDA | 0.40 ± 0.01 | 21 | 146 | 1.91 ± 0.1 | 455 | 707 | 15.9 |
| Concentration of antibody (μg/mL) | Primary IgG a (μg/cm2) | IgG coverage b (%) | |
|---|---|---|---|
| End-on | Side-on | ||
| 10 | 0.28 ± 0.01 | 15 | 104 |
| 20 | 0.42 ± 0.01 | 23 | 156 |
| 30 | 0.56 ± 0.03 | 30 | 207 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Gao, G.; Watanabe, J.; Liu, H.; Shen, H. Hydrophilic Polyelectrolyte Multilayers Improve the ELISA System: Antibody Enrichment and Blocking Free. Polymers 2017, 9, 51. https://doi.org/10.3390/polym9020051
Lai X, Gao G, Watanabe J, Liu H, Shen H. Hydrophilic Polyelectrolyte Multilayers Improve the ELISA System: Antibody Enrichment and Blocking Free. Polymers. 2017; 9(2):51. https://doi.org/10.3390/polym9020051
Chicago/Turabian StyleLai, Xing, Gan Gao, Junji Watanabe, Huiyu Liu, and Heyun Shen. 2017. "Hydrophilic Polyelectrolyte Multilayers Improve the ELISA System: Antibody Enrichment and Blocking Free" Polymers 9, no. 2: 51. https://doi.org/10.3390/polym9020051






