Preparation and Material Application of Amylose-Polymer Inclusion Complexes by Enzymatic Polymerization Approach
Abstract
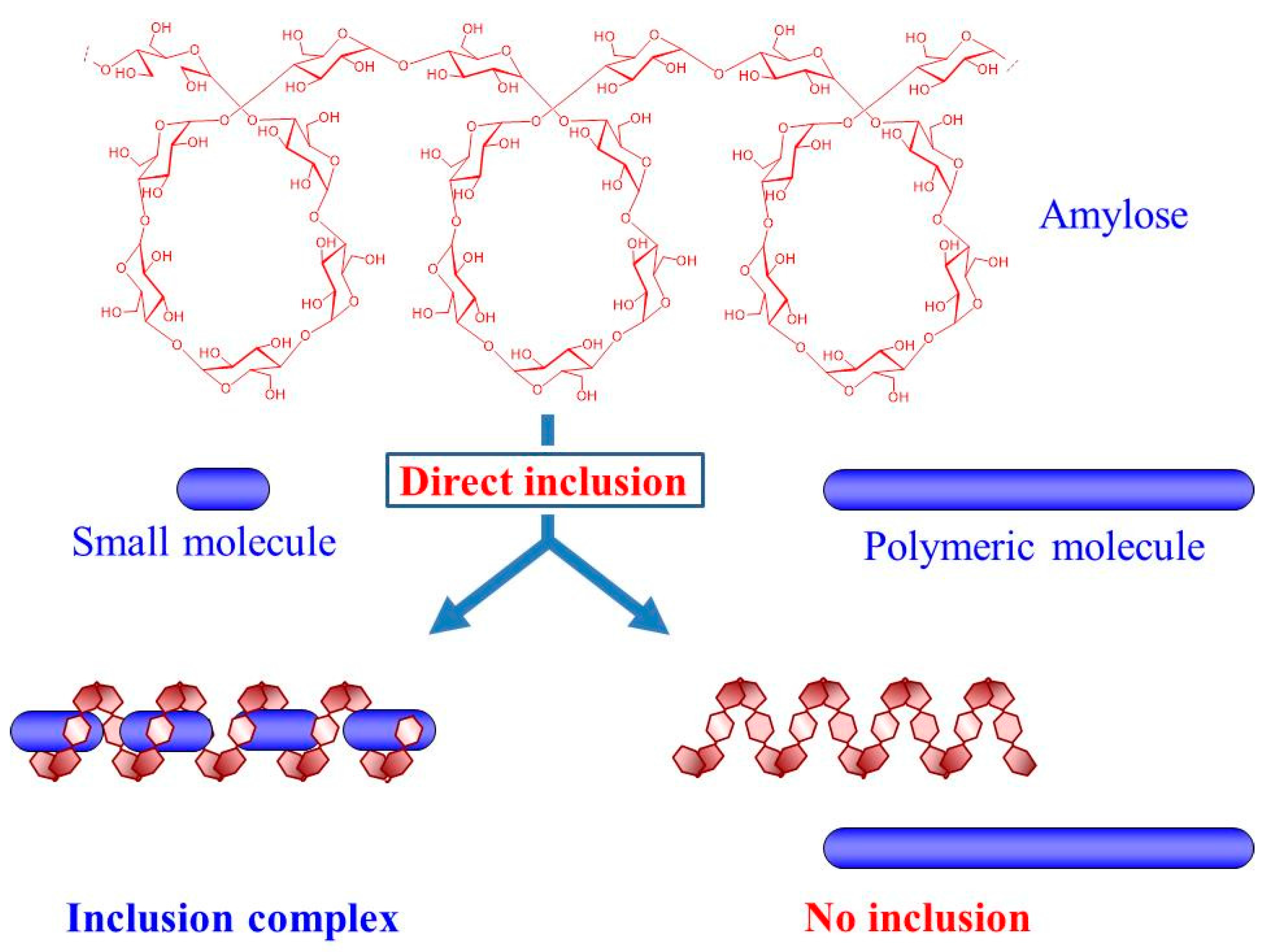
:1. Introduction
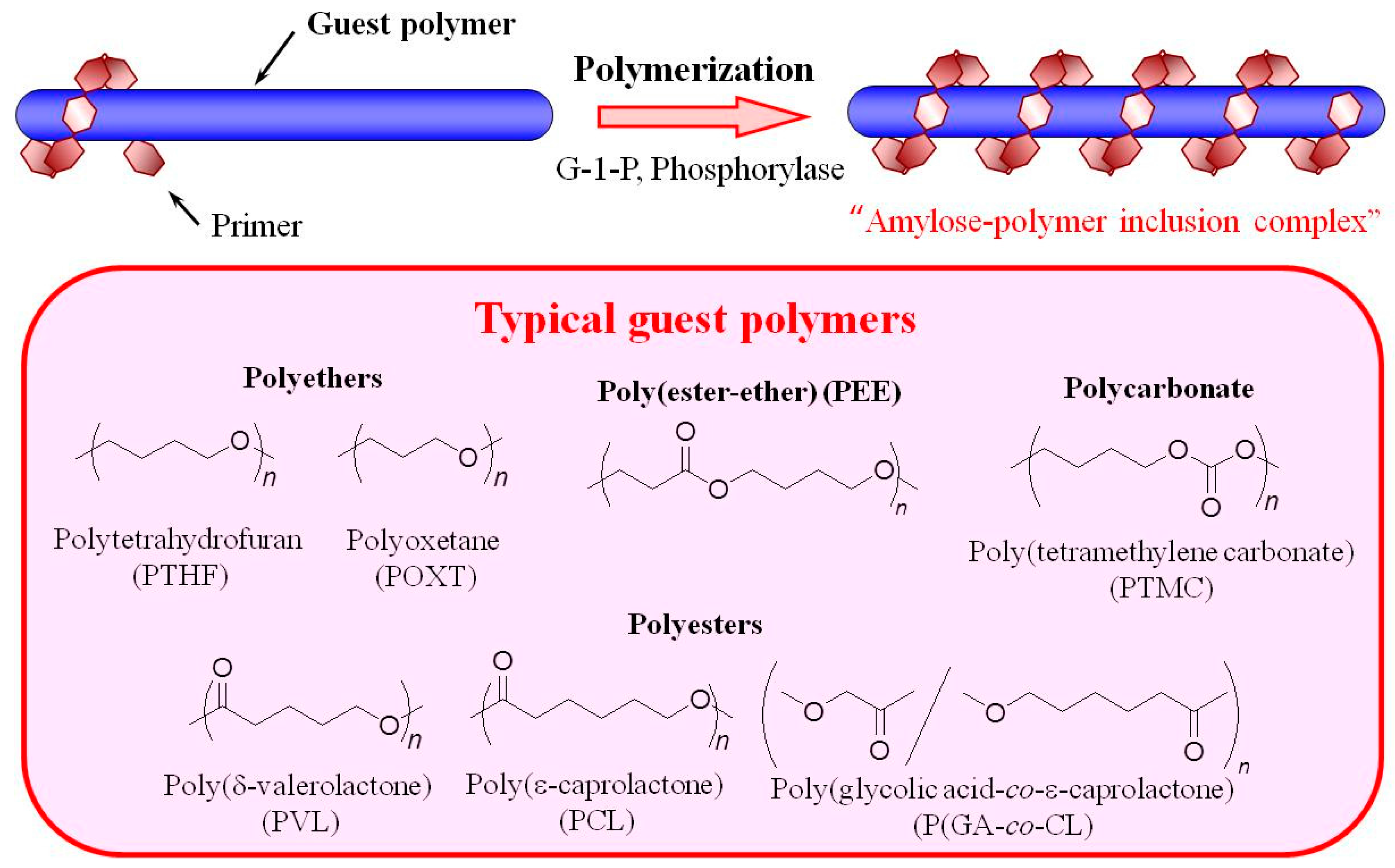
2. Preparation of Amylose-Polymer Inclusion Complexes by Enzymatic Polymerization Filed (Vine-Twining Polymerization)
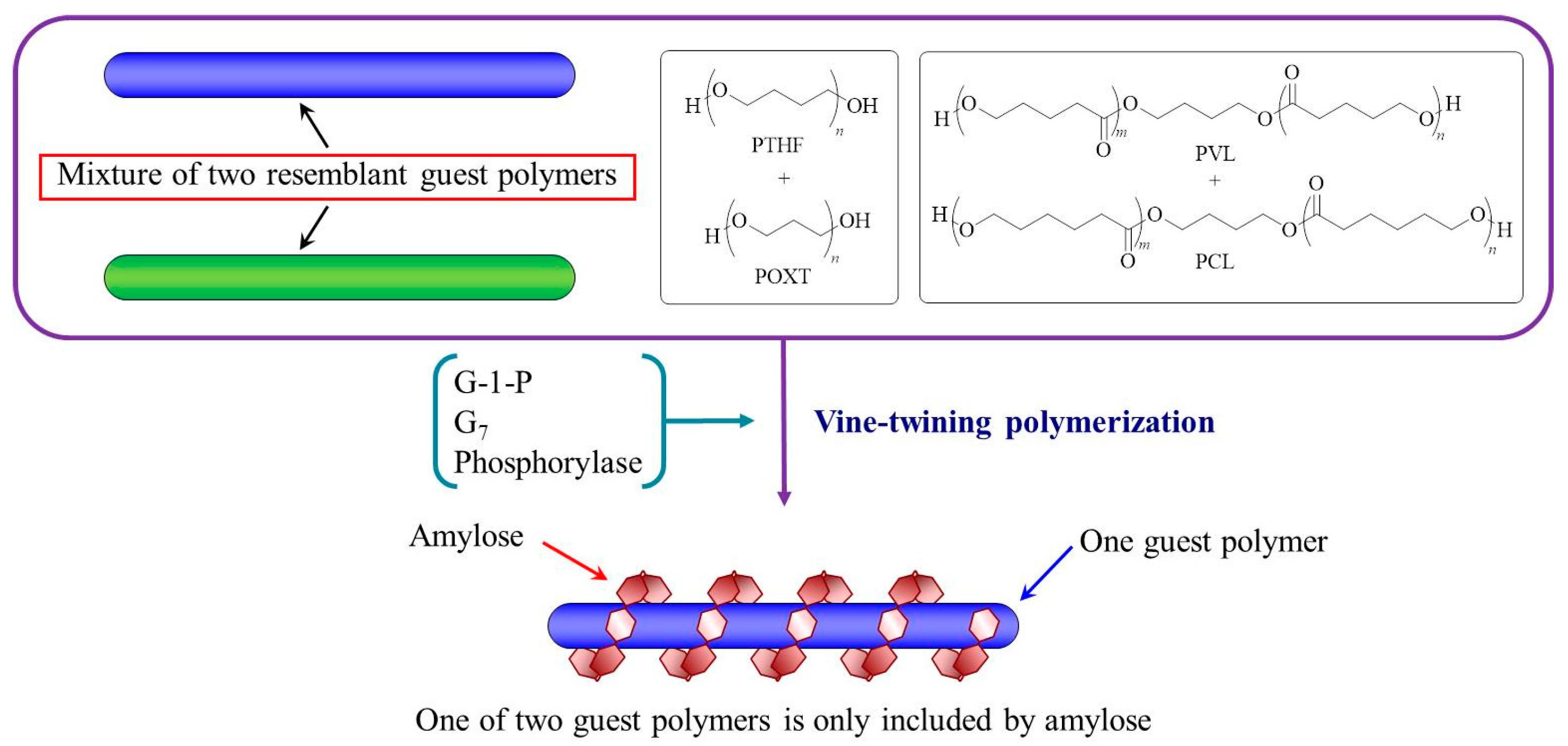
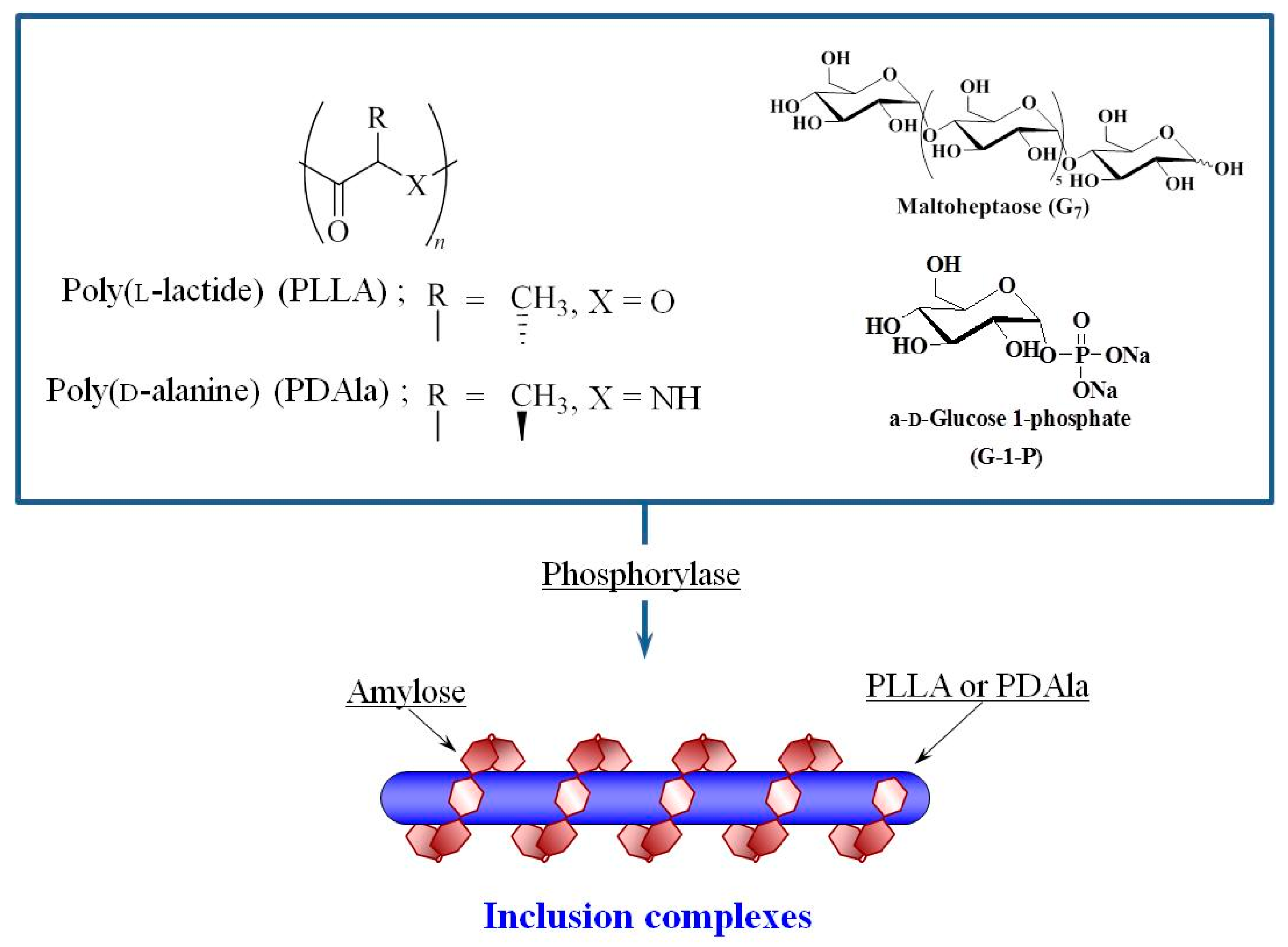
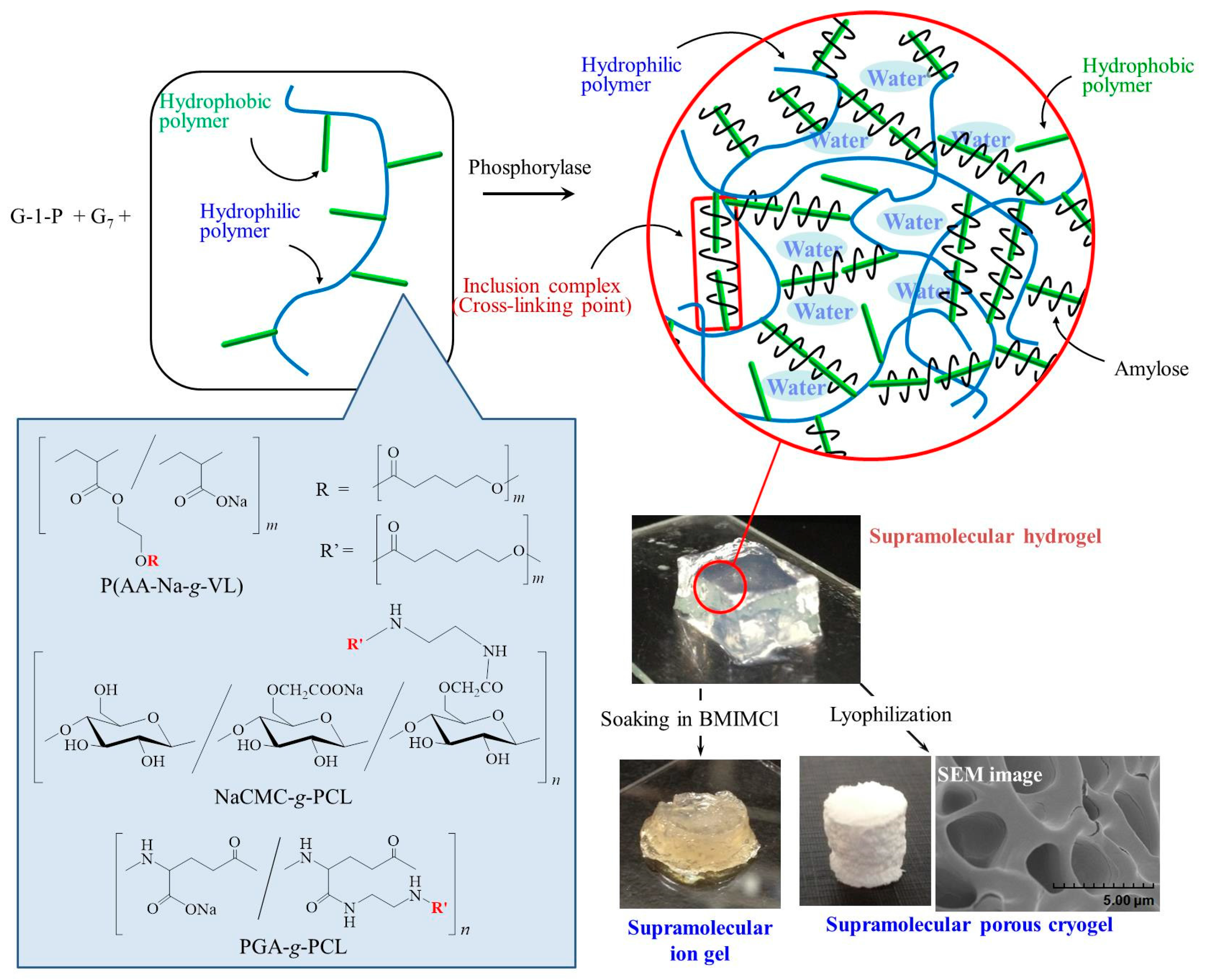
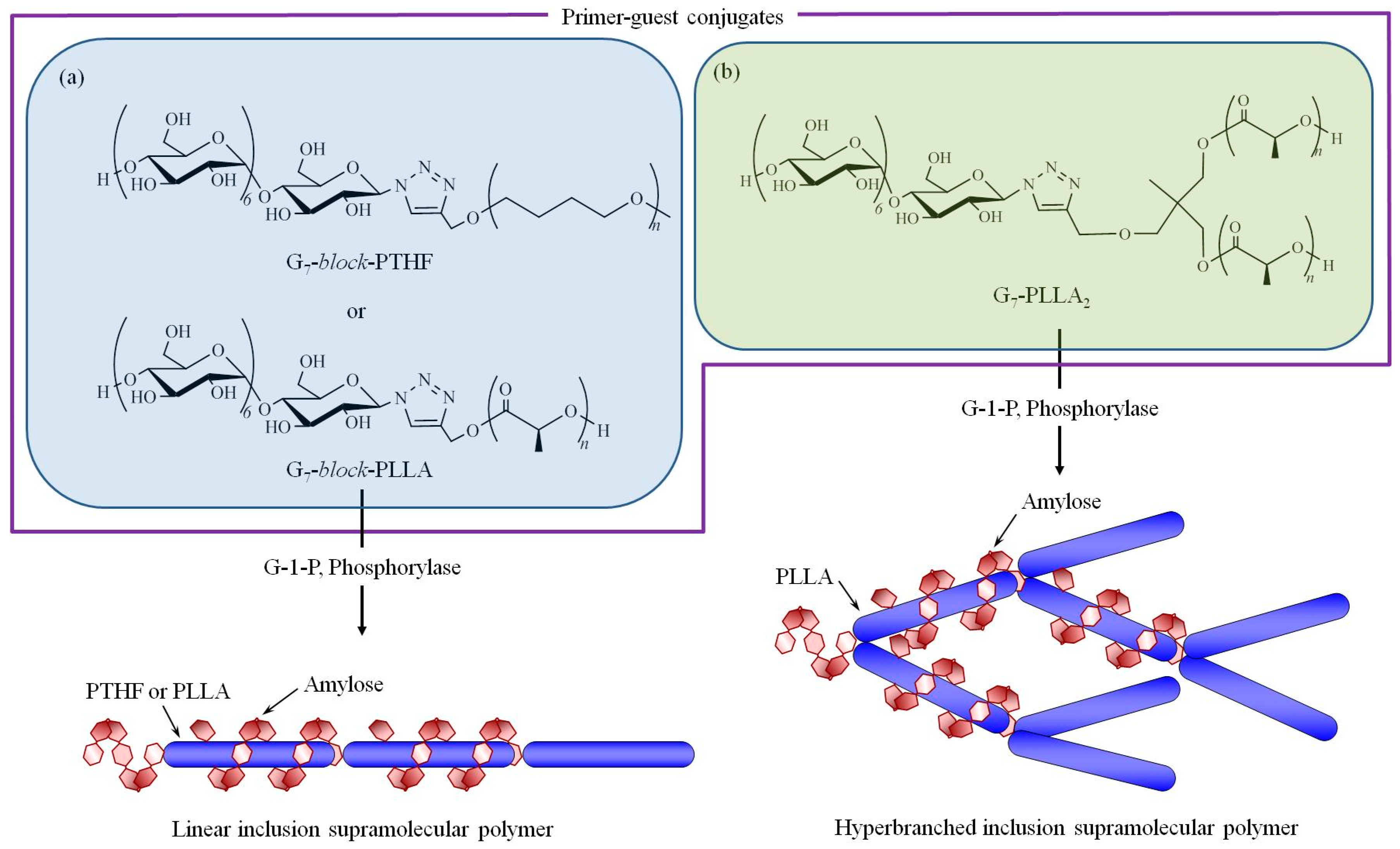
3. Hierarchical Structured Materials from Amylose-Polymer Inclusion Complexes by Vine-Twining Polymerization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 7th ed.; W.H. Freeman: New York, NY, USA, 2012; pp. 1041, 1043, 1048, 1054. [Google Scholar]
- Kasapis, S.; Norton, I.T.; Ubbink, J.B. Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Schuerch, C. Polysaccharides. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bilkales, N., Overberger, C.G., Eds.; John Wiley & Sons: New York, NY, USA, 1986; Volume 13, pp. 87–162. [Google Scholar]
- Sarko, A.; Zugenmaier, P. Crystal structures of amylose and its derivatives. In Fiber Diffraction Methods; ACS Symposium Series 141; French, A.D., Gardner, K.H., Eds.; American Chemical Society: Washington, DC, USA, 1980; pp. 459–482. [Google Scholar]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Shogren, R.L. Complexes of starch with telechelic poly(epsilon-caprolactone) phosphate. Carbohydr. Polym. 1993, 22, 93–98. [Google Scholar] [CrossRef]
- Shogren, R.L.; Greene, R.V.; Wu, Y.V. Complexes of starch polysaccharides and poly(ethylene coacrylic acid)—structure and stability in solution. J. Appl. Polym. Sci. 1991, 42, 1701–1709. [Google Scholar] [CrossRef]
- Star, A.; Steuerman, D.W.; Heath, J.R.; Stoddart, J.F. Starched carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 2508–2512. [Google Scholar] [CrossRef]
- Ikeda, M.; Furusho, Y.; Okoshi, K.; Tanahara, S.; Maeda, K.; Nishino, S.; Mori, T.; Yashima, E. A luminescent poly(phenylenevinylene)-amylose composite with supramolecular liquid crystallinity. Angew. Chem. Int. Ed. 2006, 45, 6491–6495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Woortman, A.J.J.; Loos, K. Synthesis of amylose-polystyrene inclusion complexes by a facile preparation route. Biomacromolecules 2013, 14, 1955–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef] [PubMed]
- Shoda, S.; Izumi, R.; Fujita, M. Green process in glycotechnology. Bull. Chem. Soc. Jpn. 2003, 76, 1–13. [Google Scholar] [CrossRef]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef] [PubMed]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. α-Glucan phosphorylase: A useful catalyst for precision enzymatic synthesis of oligo- and polysaccharides. Curr. Org. Chem. 2017, 21, 1192–1204. [Google Scholar] [CrossRef]
- Seibel, J.; Jordening, H.J.; Buchholz, K. Glycosylation with activated sugars using glycosyltransferases and transglycosidases. Biocatal. Biotransform. 2006, 24, 311–342. [Google Scholar] [CrossRef]
- Ziegast, G.; Pfannemüller, B. Linear and star-shaped hybrid polymers. 4. Phosphorolytic syntheses with di-functional, oligo-functional and multifunctional primers. Carbohydr. Res. 1987, 160, 185–204. [Google Scholar] [CrossRef]
- Fujii, K.; Takata, H.; Yanase, M.; Terada, Y.; Ohdan, K.; Takaha, T.; Okada, S.; Kuriki, T. Bioengineering and application of novel glucose polymers. Biocatal. Biotransform. 2003, 21, 167–172. [Google Scholar] [CrossRef]
- Yanase, M.; Takaha, T.; Kuriki, T. α-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006, 86, 1631–1635. [Google Scholar] [CrossRef]
- Kitamura, S. Starch polymers, natural and synthetic. In The Polymeric Materials Encyclopedia, Synthesis, Properties and Applications; Salamone, C., Ed.; CRC Press: New York, NY, USA, 1996; Volume 10, pp. 7915–7922. [Google Scholar]
- Kaneko, Y.; Kadokawa, J. Vine-twining polymerization: A new preparation method for well-defined supramolecules composed of amylose and synthetic polymers. Chem. Rec. 2005, 5, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kadokawa, J. Synthesis of nanostructured bio-related materials by hybridization of synthetic polymers with polysaccharides or saccharide residues. J. Biomater. Sci. Polym. Ed. 2006, 17, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Beppu, K.; Kadokawa, J. Amylose selectively includes a specific range of molecular weights in poly(tetrahydrofuran)s in vine-twining polymerization. Polym. J. 2009, 41, 792–796. [Google Scholar] [CrossRef]
- Kadokawa, J. Preparation and applications of amylose supramolecules by means of phosphorylase-catalyzed enzymatic polymerization. Polymers 2012, 4, 116–133. [Google Scholar] [CrossRef]
- Kadokawa, J. Architecture of amylose supramolecules in form of inclusion complexes by phosphorylase-catalyzed enzymatic polymerization. Biomolecules 2013, 3, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Chemoenzymatic synthesis of functional amylosic materials. Pure Appl. Chem. 2014, 86, 701–709. [Google Scholar] [CrossRef]
- Kadokawa, J. Hierarchically fabrication of amylosic supramolecular nanocomposites by means of inclusion complexation in phosphorylase-catalyzed enzymatic polymerization field. In Eco-Friendly Polymer Nanocomposites: Processing and Properties; Thakur, K.V., Thakur, K.M., Eds.; Springer India: New Delhi, India, 2015; pp. 513–525. [Google Scholar]
- Kadokawa, J.; Kaneko, Y.; Tagaya, H.; Chiba, K. Synthesis of an amylose-polymer inclusion complex by enzymatic polymerization of glucose 1-phosphate catalyzed by phosphorylase enzyme in the presence of polythf: A new method for synthesis of polymer-polymer inclusion complexes. Chem. Commun. 2001, 449–450. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kaneko, Y.; Nagase, S.; Takahashi, T.; Tagaya, H. Vine-twining polymerization: Amylose twines around polyethers to form amylose—polyether inclusion complexes. Chem. Eur. J. 2002, 8, 3321–3326. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kaneko, Y.; Nakaya, A.; Tagaya, H. Formation of an amylose-polyester inclusion complex by means of phosphorylase-catalyzed enzymatic polymerization of a-d-glucose 1-phosphate monomer in the presence of poly(e-caprolactone). Macromolecules 2001, 34, 6536–6538. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nakaya, A.; Kaneko, Y.; Tagaya, H. Preparation of inclusion complexes between amylose and ester-containing polymers by means of vine-twining polymerization. Macromol. Chem. Phys. 2003, 204, 1451–1457. [Google Scholar] [CrossRef]
- Nomura, S.; Kyutoku, T.; Shimomura, N.; Kaneko, Y.; Kadokawa, J. Preparation of inclusion complexes composed of amylose and biodegradable poly(glycolic acid-co-e-caprolactone) by vine-twining polymerization and their lipase-catalyzed hydrolysis behavior. Polym. J. 2011, 43, 971–977. [Google Scholar] [CrossRef]
- Kaneko, Y.; Beppu, K.; Kadokawa, J. Preparation of amylose/polycarbonate inclusion complexes by means of vine-twining polymerization. Macromol. Chem. Phys. 2008, 209, 1037–1042. [Google Scholar] [CrossRef]
- Kaneko, Y.; Beppu, K.; Kadokawa, J. Amylose selectively includes one from a mixture of two resemblant polyethers in vine-twining polymerization. Biomacromolecules 2007, 8, 2983–2985. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Beppu, K.; Kyutoku, T.; Kadokawa, J. Selectivity and priority on inclusion of amylose toward guest polyethers and polyesters in vine-twining polymerization. Polym. J. 2009, 41, 279–286. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ueno, K.; Yui, T.; Nakahara, K.; Kadokawa, J. Amylose’s recognition of chirality in polylactides on formation of inclusion complexes in vine-twining polymerization. Macromol. Biosci. 2011, 11, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Gotanda, R.; Yamamoto, K.; Kadokawa, J.-I. Amylose stereoselectively includes poly(d-alanine) to form inclusion complex in vine-twining polymerization: A novel saccharide-peptide supramolecular conjugate. Macromol. Chem. Phys. 2016. [Google Scholar] [CrossRef]
- Kaneko, Y.; Fujisaki, K.; Kyutoku, T.; Furukawa, H.; Kadokawa, J. Preparation of enzymatically recyclable hydrogels through the formation of inclusion complexes of amylose in a vine-twining polymerization. Chem. Asian J. 2010, 5, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Nomura, S.; Hatanaka, D.; Yamamoto, K. Preparation of polysaccharide supramolecular films by vine-twining polymerization approach. Carbohydr. Polym. 2013, 98, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Tanaka, K.; Hatanaka, D.; Yamamoto, K. Preparation of multiformable supramolecular gels through helical complexation by amylose in vine-twining polymerization. Polym. Chem. 2015, 6, 6402–6408. [Google Scholar] [CrossRef]
- Tanaka, T.; Sasayama, S.; Nomura, S.; Yamamoto, K.; Kimura, Y.; Kadokawa, J. An amylose-poly(l-lactide) inclusion supramolecular polymer: Enzymatic synthesis by means of vine-twining polymerization using a primer-guest conjugate. Macromol. Chem. Phys. 2013, 214, 2829–2834. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsutsui, A.; Gotanda, R.; Sasayama, S.; Yamamoto, K.; Kadokawa, J. Synthesis of amylose-polyether inclusion supramolecular polymers by vine-twining polymerization using maltoheptaose-functionalized poly(tetrahydrofuran) as a primer-guest conjugate. J. Appl. Glycosci. 2015, 62, 135–141. [Google Scholar] [CrossRef]
- Tanaka, T.; Gotanda, R.; Tsutsui, A.; Sasayama, S.; Yamamoto, K.; Kimura, Y.; Kadokawa, J. Synthesis and gel formation of hyperbranched supramolecular polymer by vine-twining polymerization using branched primer-guest conjugate. Polymer 2015, 73, 9–16. [Google Scholar] [CrossRef]
- Tanaka, T.; Sasayama, S.; Yamamoto, K.; Kimura, Y.; Kadokawa, J. Evaluating relative chain orientation of amylose and poly(l-lactide) in inclusion complexes formed by vine-twining polymerization using primer–guest conjugates. Macromol. Chem. Phys. 2015, 216, 794–800. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orio, S.; Yamamoto, K.; Kadokawa, J.-i. Preparation and Material Application of Amylose-Polymer Inclusion Complexes by Enzymatic Polymerization Approach. Polymers 2017, 9, 729. https://doi.org/10.3390/polym9120729
Orio S, Yamamoto K, Kadokawa J-i. Preparation and Material Application of Amylose-Polymer Inclusion Complexes by Enzymatic Polymerization Approach. Polymers. 2017; 9(12):729. https://doi.org/10.3390/polym9120729
Chicago/Turabian StyleOrio, Saya, Kazuya Yamamoto, and Jun-ichi Kadokawa. 2017. "Preparation and Material Application of Amylose-Polymer Inclusion Complexes by Enzymatic Polymerization Approach" Polymers 9, no. 12: 729. https://doi.org/10.3390/polym9120729




