Proton Conductive Channel Optimization in Methanol Resistive Hybrid Hyperbranched Polyamide Proton Exchange Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
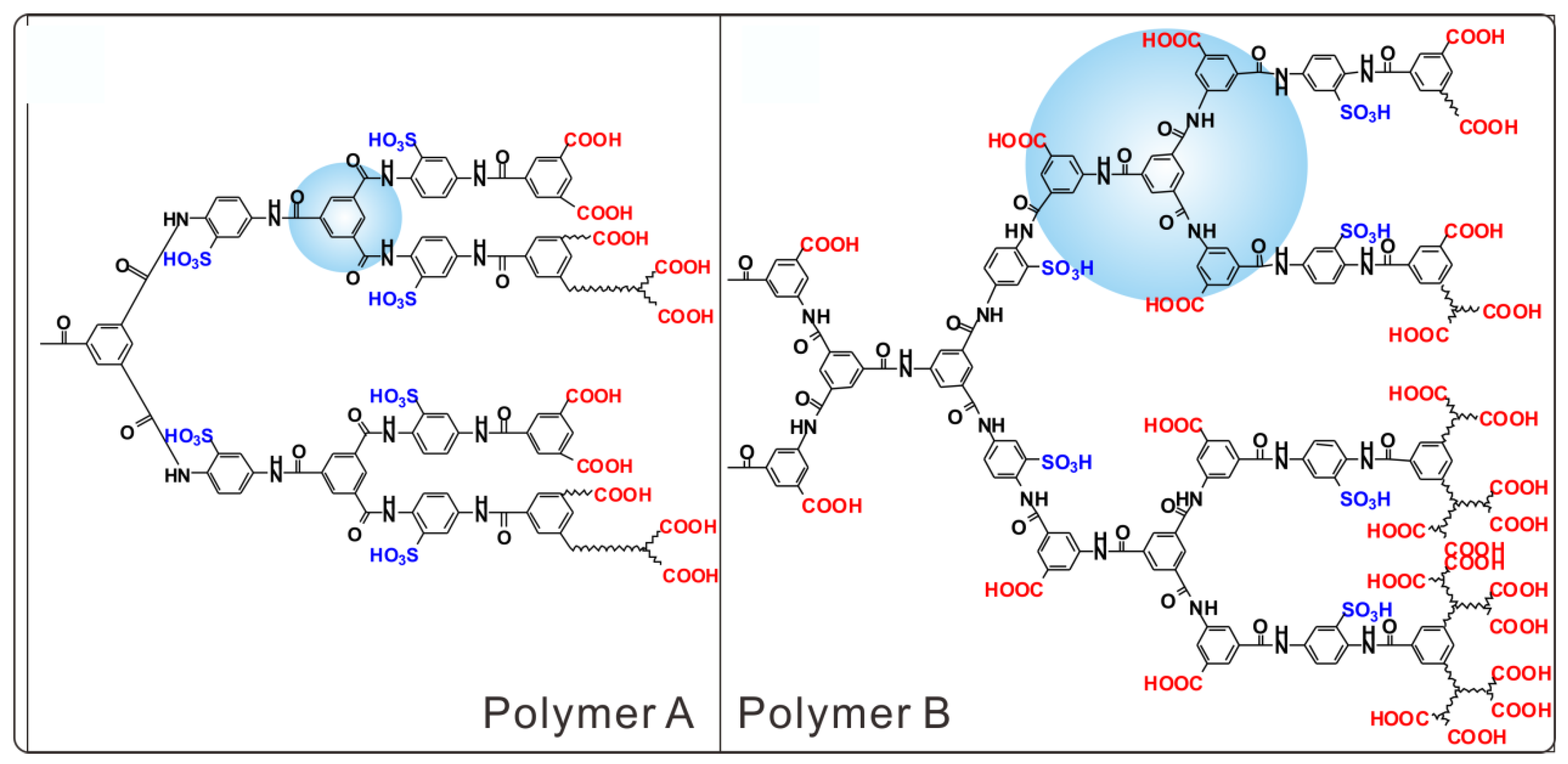
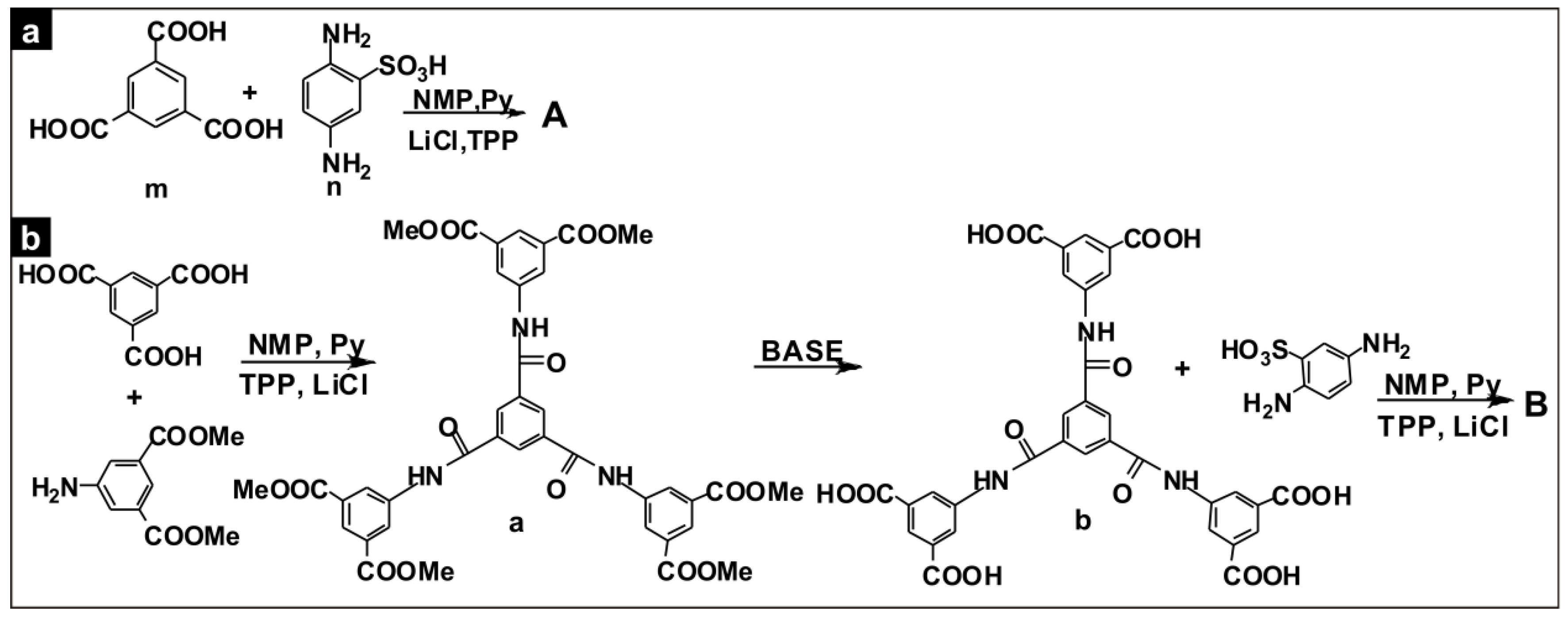
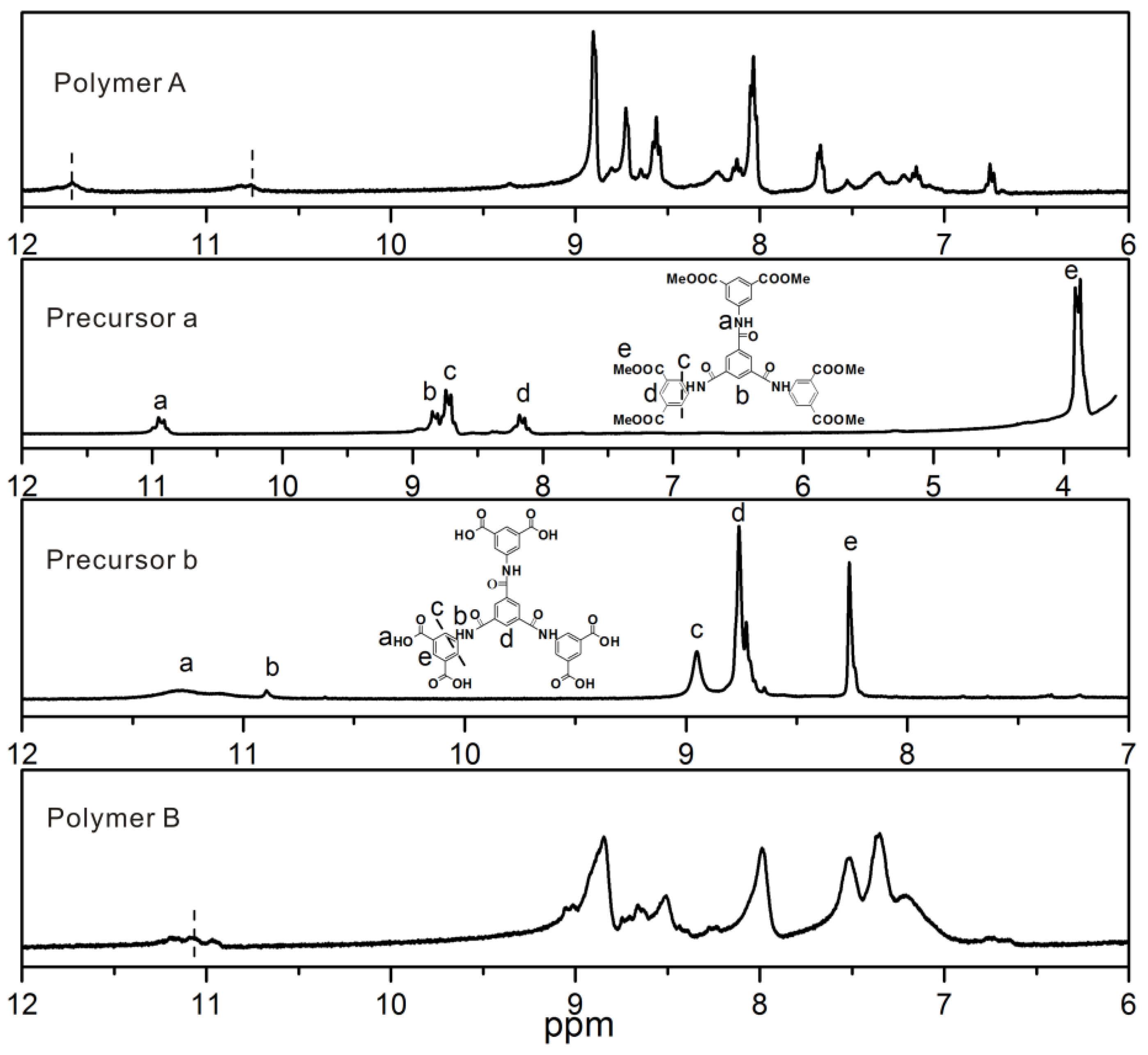
2.2.1. Polymer A
2.2.2. Polymer B
2.3. Membrane Preparation
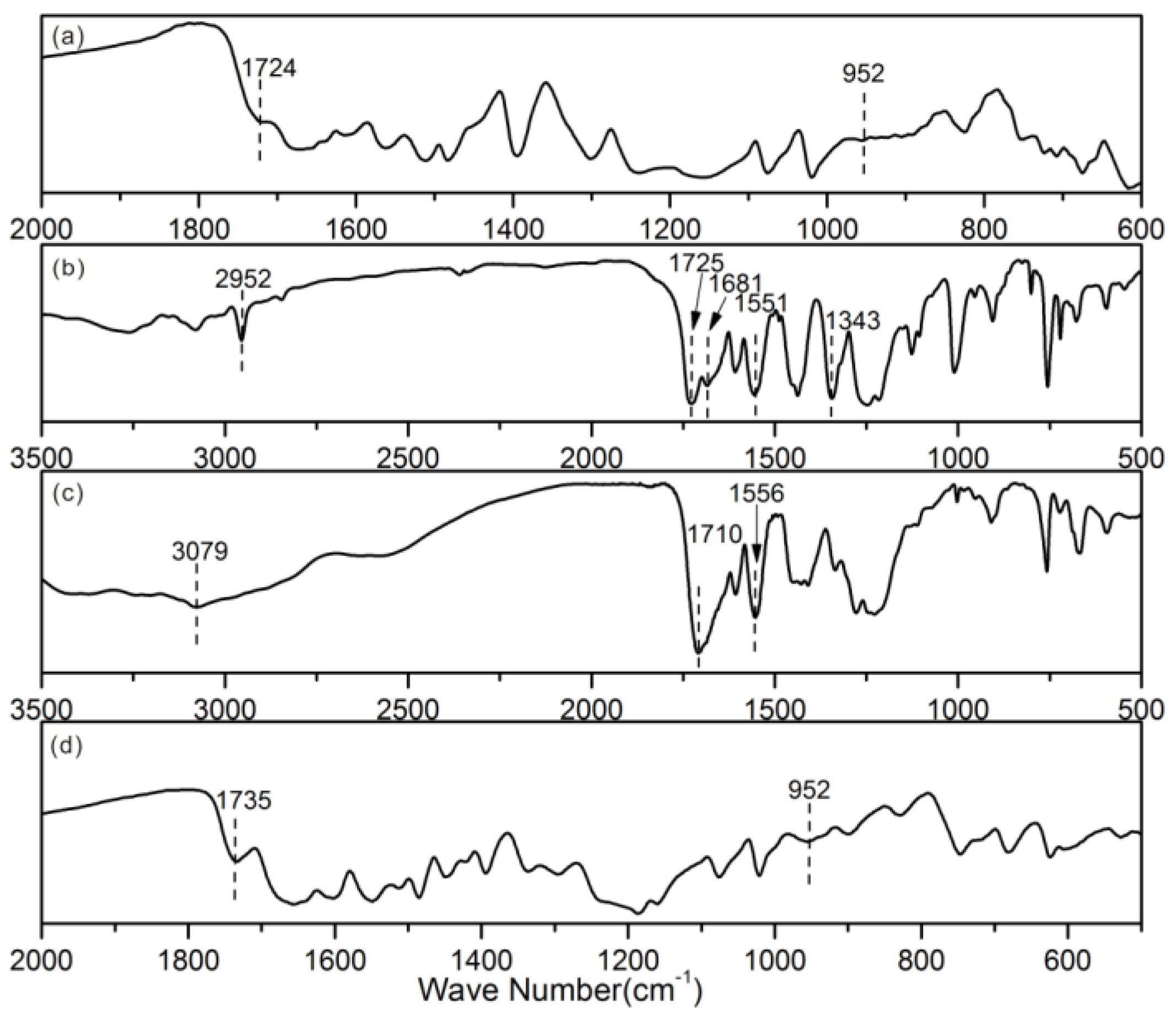
2.4. Measurements and Characterizations
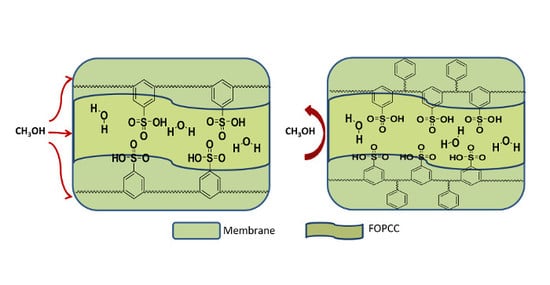
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4611. [Google Scholar] [CrossRef] [PubMed]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Staiti, P.; Antonucci, V.; Arico, A.S. Performance analysis of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2013, 243, 519–534. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 226, 223–240. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Kerres, J.A. Development of ionomer membranes for fuel cells. J. Membr. Sci. 2001, 185, 3–27. [Google Scholar] [CrossRef]
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymer 2009, 50, 5341–5357. [Google Scholar] [CrossRef]
- Inzelt, G.; Pineri, M.; Schultze, J.; Vorotyntsev, M. Electron and proton conducting polymers: Recent developments and prospects. Electrochim. Acta 2000, 45, 2403–2421. [Google Scholar] [CrossRef]
- Chikashige, Y.; Chikyu, Y.; Miyatake, K.; Watanabe, M. Poly(arylene ether) Ionomers Containing Sulfofluorenyl Groups for Fuel Cell Applications. Macromolecules 2005, 38, 7121–7126. [Google Scholar] [CrossRef]
- Cho, E.; Park, J.-S.; Sekhon, S.S.; Park, G.-G.; Yang, T.-H.; Lee, W.-Y.; Kim, C.-S.; Park, S.-B. A Study on Proton Conductivity of Composite Membranes with Various Ionic Liquids for High-Temperature Anhydrous Fuel Cells. J. Electrochem. Soc. 2009, 156, B197–B202. [Google Scholar] [CrossRef]
- Beattie, P.D.; Orfino, F.P.; Basura, V.I.; Zychowska, K.; Ding, J.; Chuy, C.; Schmeisser, J.; Holdcroft, S. Ionic conductivity of proton exchange membranes. J. Electroanal. Chem. 2001, 503, 45–56. [Google Scholar] [CrossRef]
- Ansari, S.; Kelarakis, A.; Estevez, L.; Giannelis, E.P. Oriented arrays of graphene in a polymer matrix by in situ reduction of graphite oxide nanosheets. Small 2010, 6, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Ramya, K.; Dhathathreyan, K.S. Direct methanol fuel cells: Determination of fuel crossover in a polymer electrolyte membrane. J. Electroanal. Chem. 2003, 542, 109–115. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Y.; Yaguchi, K.; Endo, N.; Higa, M.; Okamoto, K.-I. Synthesis and properties of sulfonated multiblock copolynaphthalimides. Polymer 2009, 50, 2933–2943. [Google Scholar] [CrossRef]
- Wu, L.; Huang, C.; Woo, J.-J.; Wu, D.; Yun, S.-H.; Seo, S.-J.; Xu, T.; Moon, S.-H. Hydrogen Bonding: A Channel for Protons to Transfer through Acid−Base Pairs. J. Phys. Chem. B 2009, 113, 12265–12270. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, C.; Woo, J.-J.; Wu, D.; Yun, S.-H.; Seo, S.-J.; Xu, T.; Moon, S.-H. Modifying a Proton Conductive Membrane by Embedding a “Barrier”. J. Phys. Chem. B 2010, 114, 13121–13127. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.G.; Kim, Y.S.; Yu, X.; Hill, M.; McGrath, J.E. Synthesis and Characterization of Poly(arylene ether sulfone) Copolymers with Sulfonimide Side Groups for a Proton-Exchange Membrane. J. Polym. Sci. 2006, 44, 6007–6014. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Portale, G.; Longo, A.; D’Ilario, L.; Passalacqua, E. Sulphonated poly(ether ether ketone) membranes for fuel cell application: Thermal and structural characterisation. J. Power Sources 2006, 163, 18–26. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Nasef, M.M.; Ghassemi, H.; Zakeri, M.; Ahmad, A.; Abdollahi, Y. Improved Methanol Barrier Property of Nafion Hybrid Membrane by Incorporating Nanofibrous Inter layer Self-Immobilized with High Level of Phosphotungstic Acid. ACS Appl. Mat. Interfaces 2015, 7, 17008–17015. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Li, N.; Zhang, S. Synthesis and properties of novel sulfonated poly(phenylquinoxaline)s as proton exchange membranes. Polymer 2009, 50, 6001–6008. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Choi, B.G.; Huh, Y.S.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Park, H. Enhanced transport properties in polymer electrolyte composite membranes with graphene oxide sheets. Carbon 2012, 50, 5395–5402. [Google Scholar] [CrossRef]
- Wycisk, R.; Lee, J.K.; Pintauro, P.N. Sulfonated polyphosphazene-polybenzimidazole membranes for DMFCs. J. Electrochem. Soc. 2005, 152, A892–A898. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Morohashi, N.; Nakagawa, N. Factors affecting methanol transport in a passive DMFC employing a porous carbon plate. J. Power Sources 2007, 172, 659–665. [Google Scholar] [CrossRef]
- Miyatake, K.; Zhou, H.; Watanabe, M. Proton Conductive Polyimide Electrolytes Containing Fluorenyl Groups: Synthesis, Properties, and Branching Effect. Macromolecules 2004, 37, 4956–4960. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Baker, A.P.; Xu, X.; Xiang, Y.; Wang, L.; Lavorgna, M.; Wu, J. Enhancement of Nafion based membranes for direct methanol fuel cell applications through the inclusion of ammonium-X zeolite fillers. J. Power Sources 2015, 294, 369–376. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir—Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2008, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, S.Y.; Liu, Y.-L.; Lee, Y.M.; Guiver, M.D. A new class of highly-conducting polymer electrolyte membranes: Aromatic ABA triblock copolymers. Energy Environ. Sci. 2012, 5, 5346–5355. [Google Scholar] [CrossRef]
- Tsang, E.M.W.; Zhang, Z.; Shi, Z.; Soboleva, T.; Holdcroft, S. Considerations of Macromolecular Structure in the Design of Proton Conducting Polymer Membranes: Graft versus Diblock Polyelectrolytes. J. Am. Chem. Soc. 2007, 129, 15106–15107. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Lee, S.Y.; Park, C.H.; Lee, Y.M.; Guiver, M.D. Enhancement of Proton Transport by Nanochannels in Comb-Shaped Copoly(arylene ether sulfone)s. Angew. Chem. Int. Ed. 2011, 50, 9158–9161. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Kamarudin, S.K.; Hasran, U.A.; Daud, W.R.W. Overview of hybrid membranes for direct-methanol fuel-cell applications. Int. J. Hydrog. Energy 2010, 35, 2160–2175. [Google Scholar] [CrossRef]
- Ma, L.; Cai, W.; Li, J.; Fan, K.; Jiang, Y.; Ma, L.; Cheng, H. A high performance polyamide-based proton exchange membrane fabricated via construction of hierarchical proton conductive channels. J. Power Source 2016, 302, 189–194. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Cai, W.; Fan, K.; Jiang, Y.; Cheng, H. A facile method to construct highly efficient methanol resistive polyamide-based proton exchange membrane. Int. J. Hydrog. Energy 2016, 41, 16205–16211. [Google Scholar] [CrossRef]
- Ansari, Y.; Tucker, T.G.; Huang, W.; Klein, I.S.; Lee, S.Y.; Yarger, J.L.; Angell, C.A. A flexible all-inorganic fuel cell membrane with conductivity above Nafion, and durable operation at 150 °C. J. Power Sources 2016, 303, 142–149. [Google Scholar] [CrossRef]
- Yeager, H.L. Cation and Water Diffusion in Nafion Ion Exchange Membranes: Influence of Polymer Structure. J. Electrochem. Soc. 1981, 128, 1880–1884. [Google Scholar] [CrossRef]

| Membranes | CC-80 | CC-85 | CC-90 | CC-95 | CC-100 |
|---|---|---|---|---|---|
| Water uptake (%) | 37.56 | 36.27 | 35.25 | 33.52 | 31.71 |
| Volume swelling (%) | 34.95 | 32.02 | 31.19 | 30.41 | 27.48 |
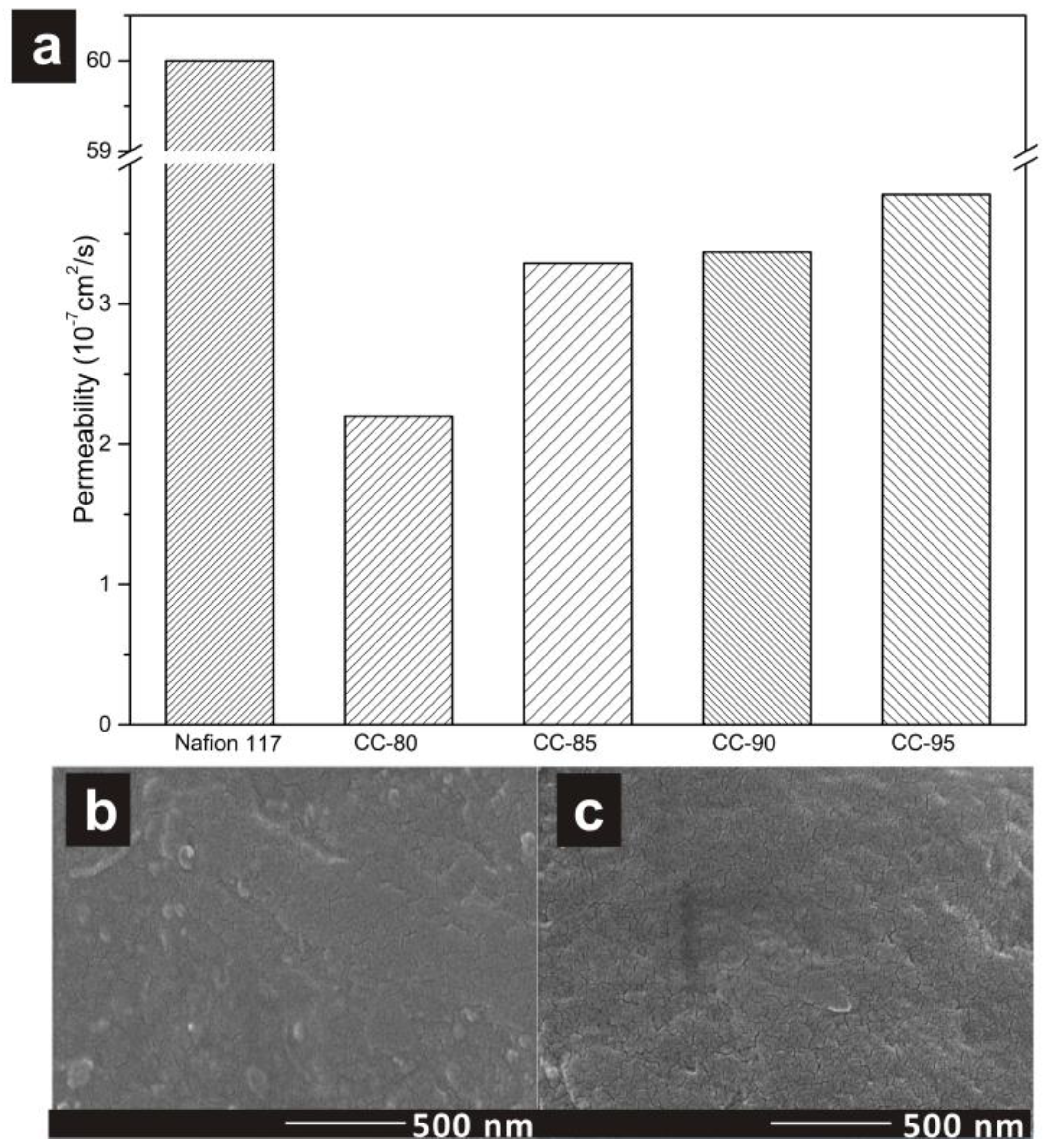
| Permeability (10−7 cm2/s) | 2.20 | 3.29 | 3.37 | 3.78 | 4.86 |
| IEC (mmol/g) | 1.5243 | 1.7293 | 1.8905 | 1.9263 | 2.33 |
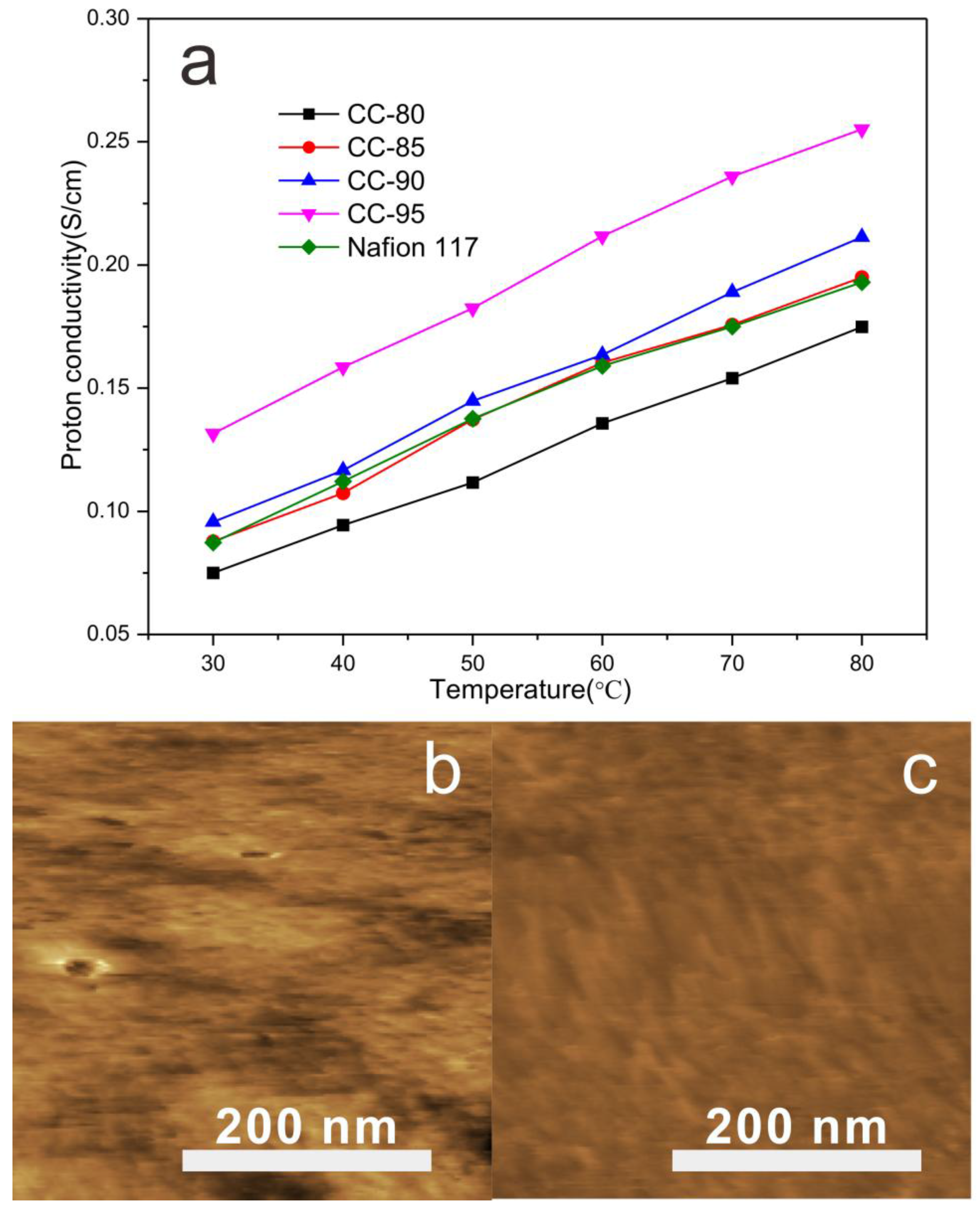
| Proton conductivity (S/cm, 30 °C) | 0.075 | 0.088 | 0.096 | 0.132 | 0.113 |
| Selectivity (104 S·s/cm3) | 34.09 | 26.75 | 28.49 | 34.92 | 23.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Li, J.; Xiong, J.; Xu, G.; Liu, Z.; Cai, W. Proton Conductive Channel Optimization in Methanol Resistive Hybrid Hyperbranched Polyamide Proton Exchange Membrane. Polymers 2017, 9, 703. https://doi.org/10.3390/polym9120703
Ma L, Li J, Xiong J, Xu G, Liu Z, Cai W. Proton Conductive Channel Optimization in Methanol Resistive Hybrid Hyperbranched Polyamide Proton Exchange Membrane. Polymers. 2017; 9(12):703. https://doi.org/10.3390/polym9120703
Chicago/Turabian StyleMa, Liying, Jing Li, Jie Xiong, Guoxiao Xu, Zhao Liu, and Weiwei Cai. 2017. "Proton Conductive Channel Optimization in Methanol Resistive Hybrid Hyperbranched Polyamide Proton Exchange Membrane" Polymers 9, no. 12: 703. https://doi.org/10.3390/polym9120703