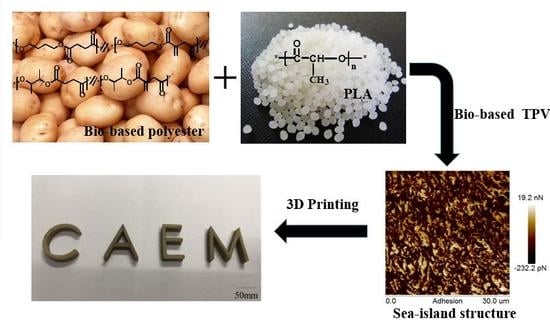
Preparation and Properties of Novel Thermoplastic Vulcanizate Based on Bio-Based Polyester/Polylactic Acid, and Its Application in 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
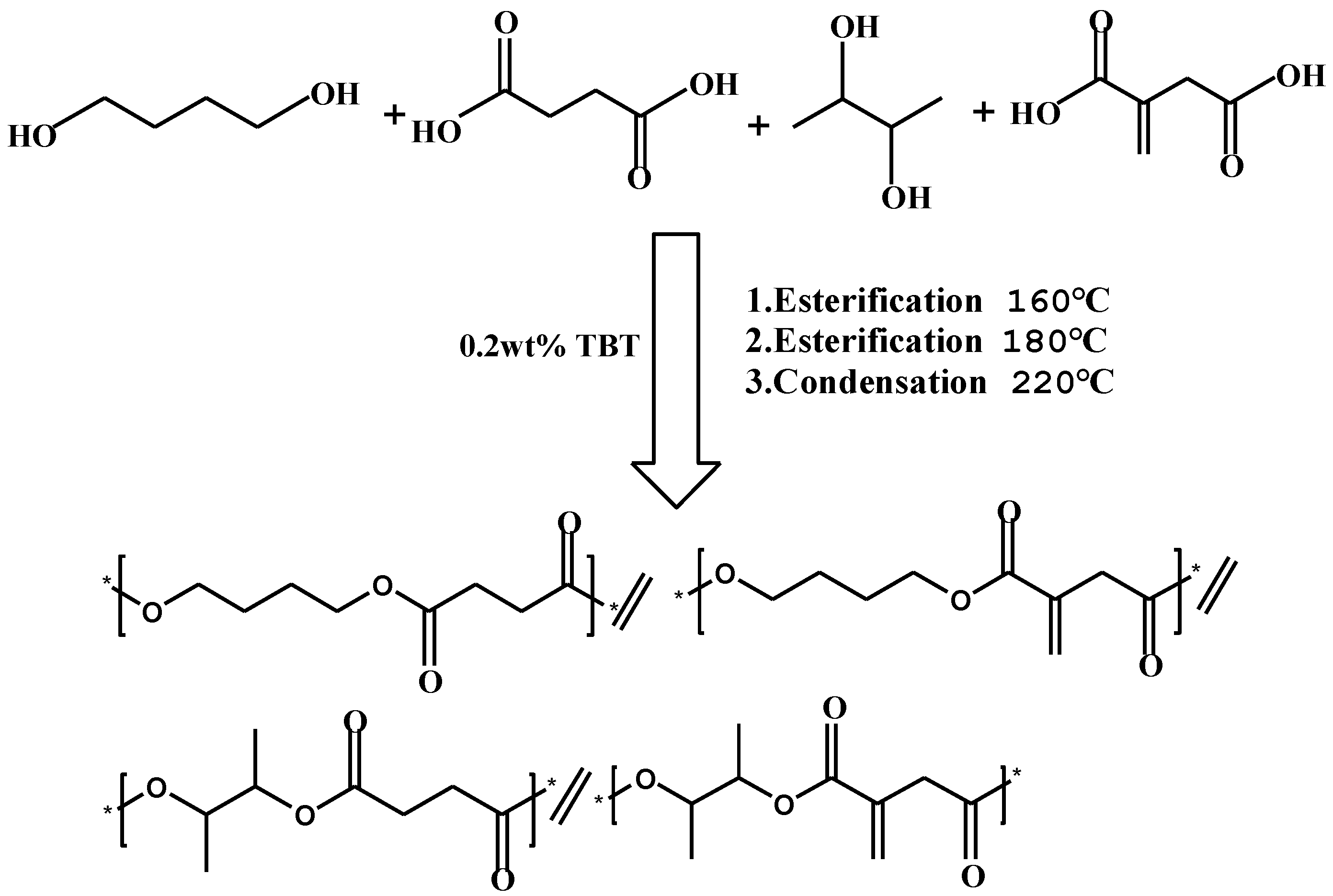
2.2. Synthesis of PBBSI Copolyesters
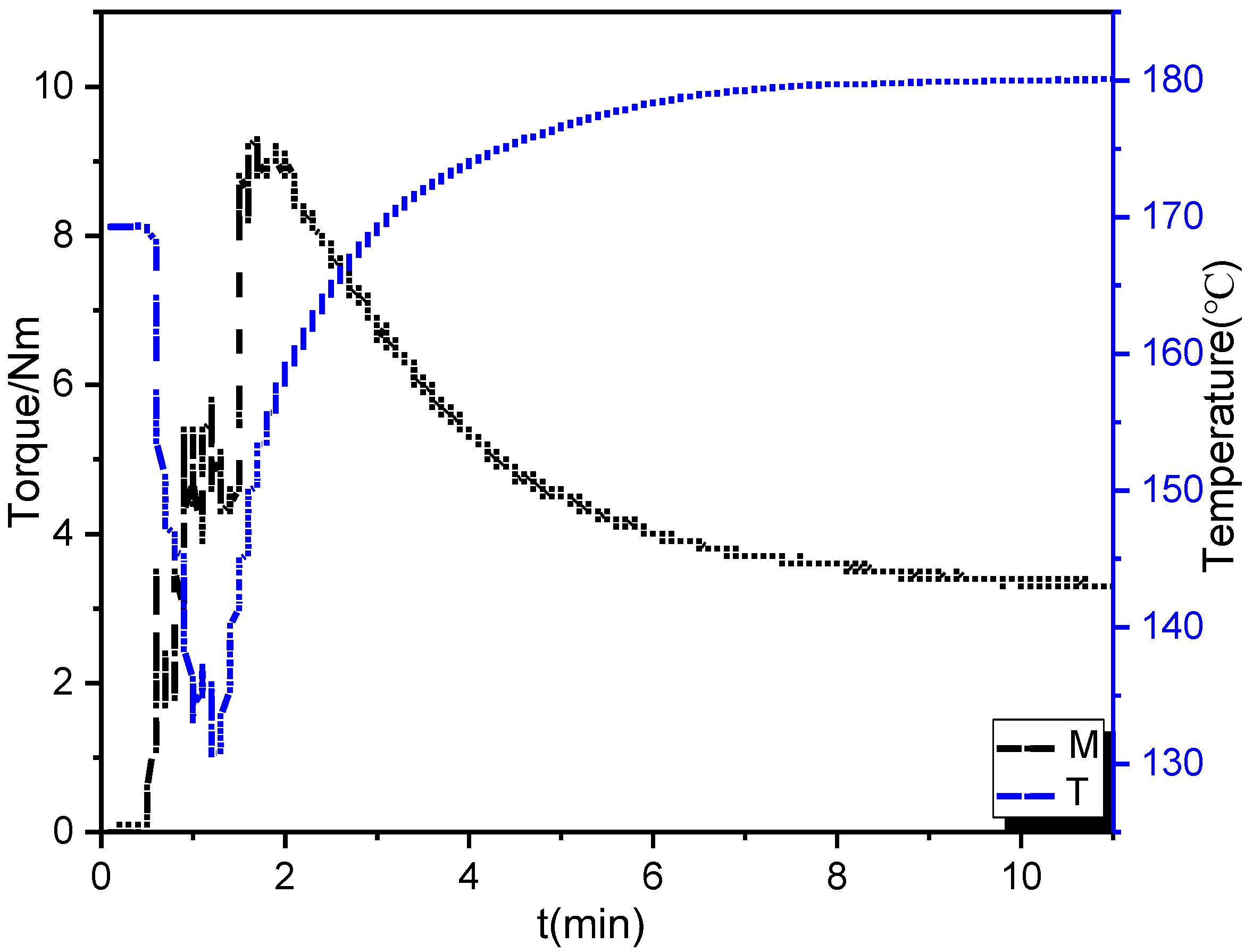
2.3. Preparation of PBBSI/PLA TPV
2.4. Characterization and Measurements
3. Results and Discussion
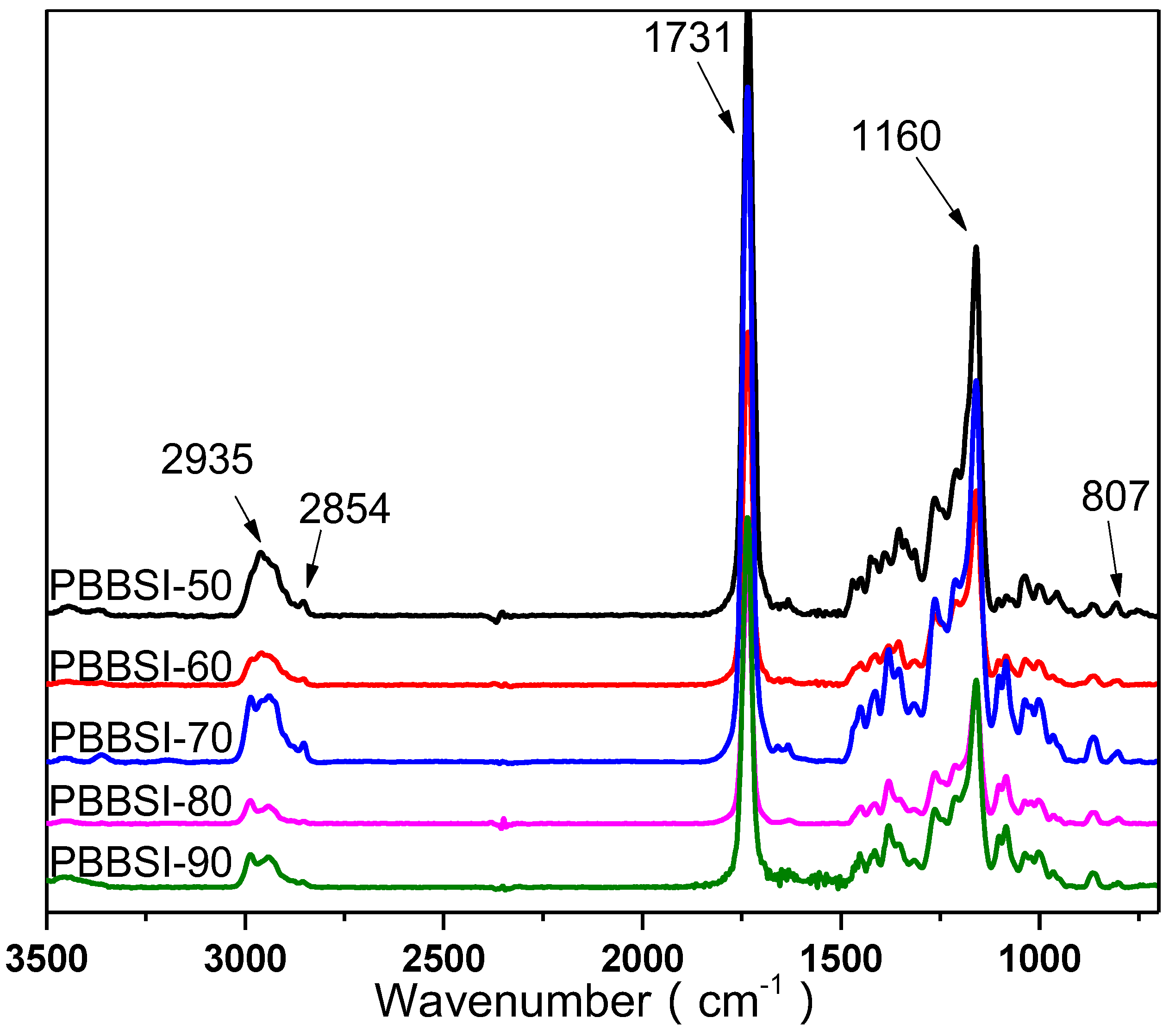
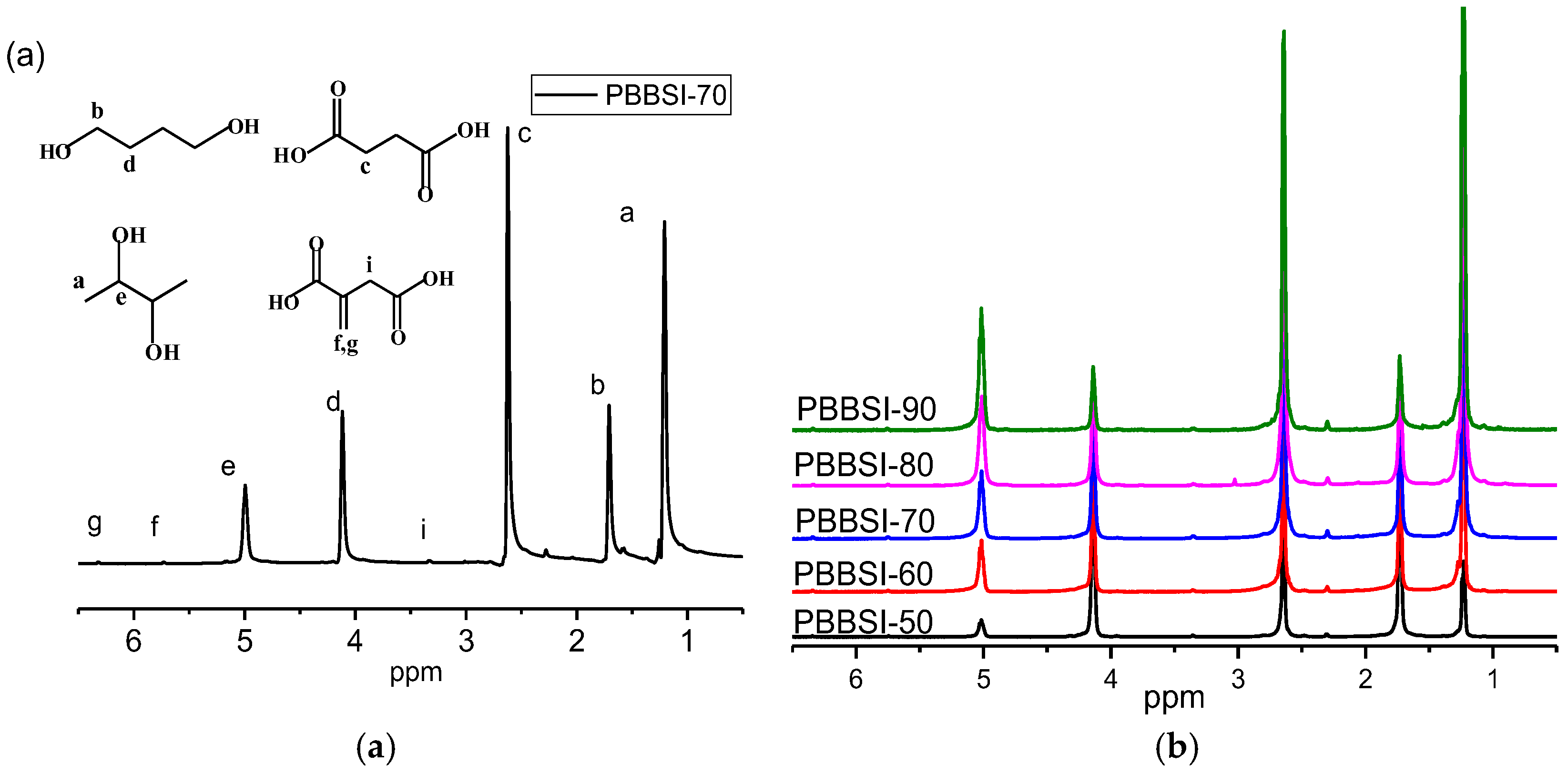
3.1. Synthesis and Characterization of PBBSI Copolyesters
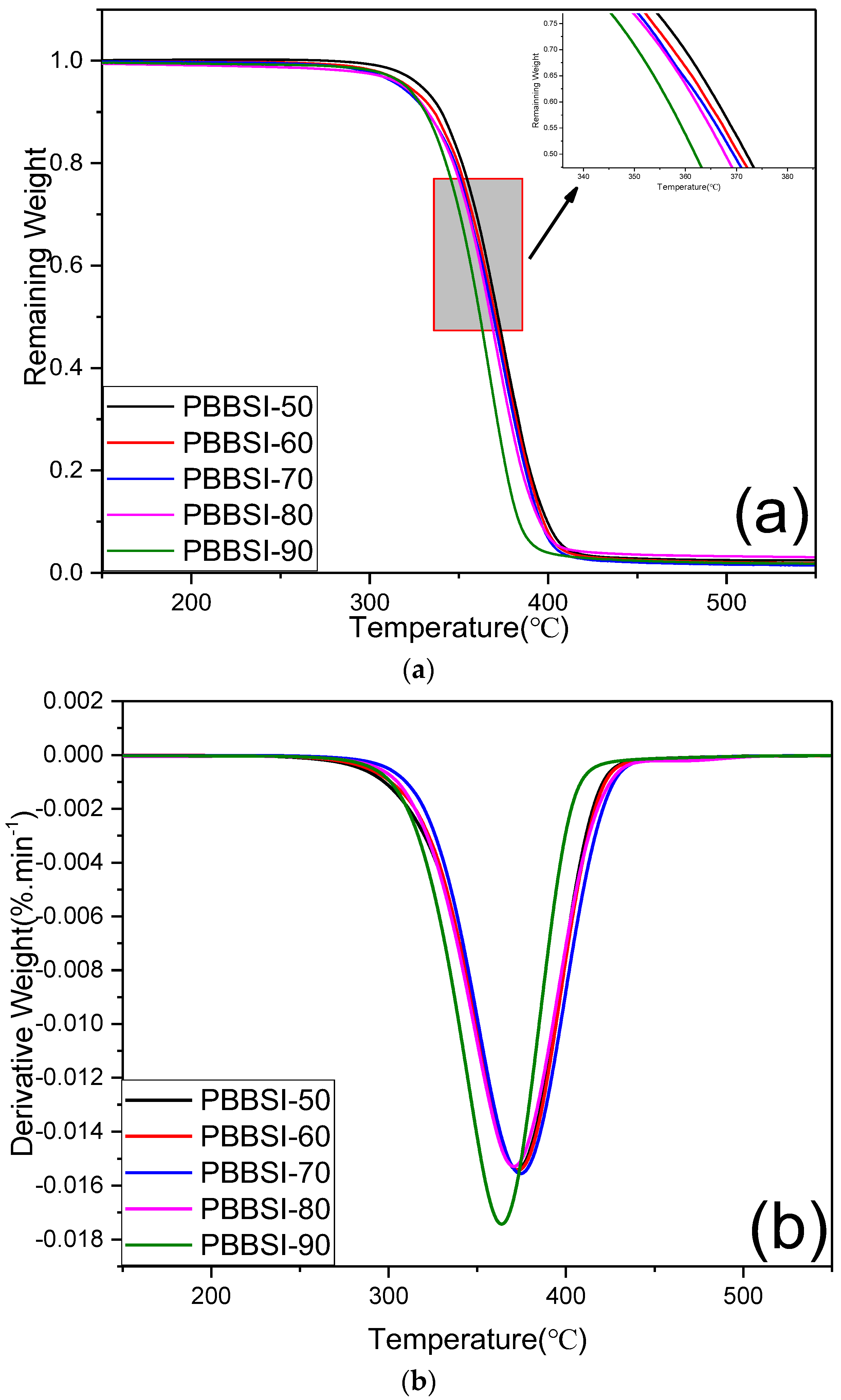
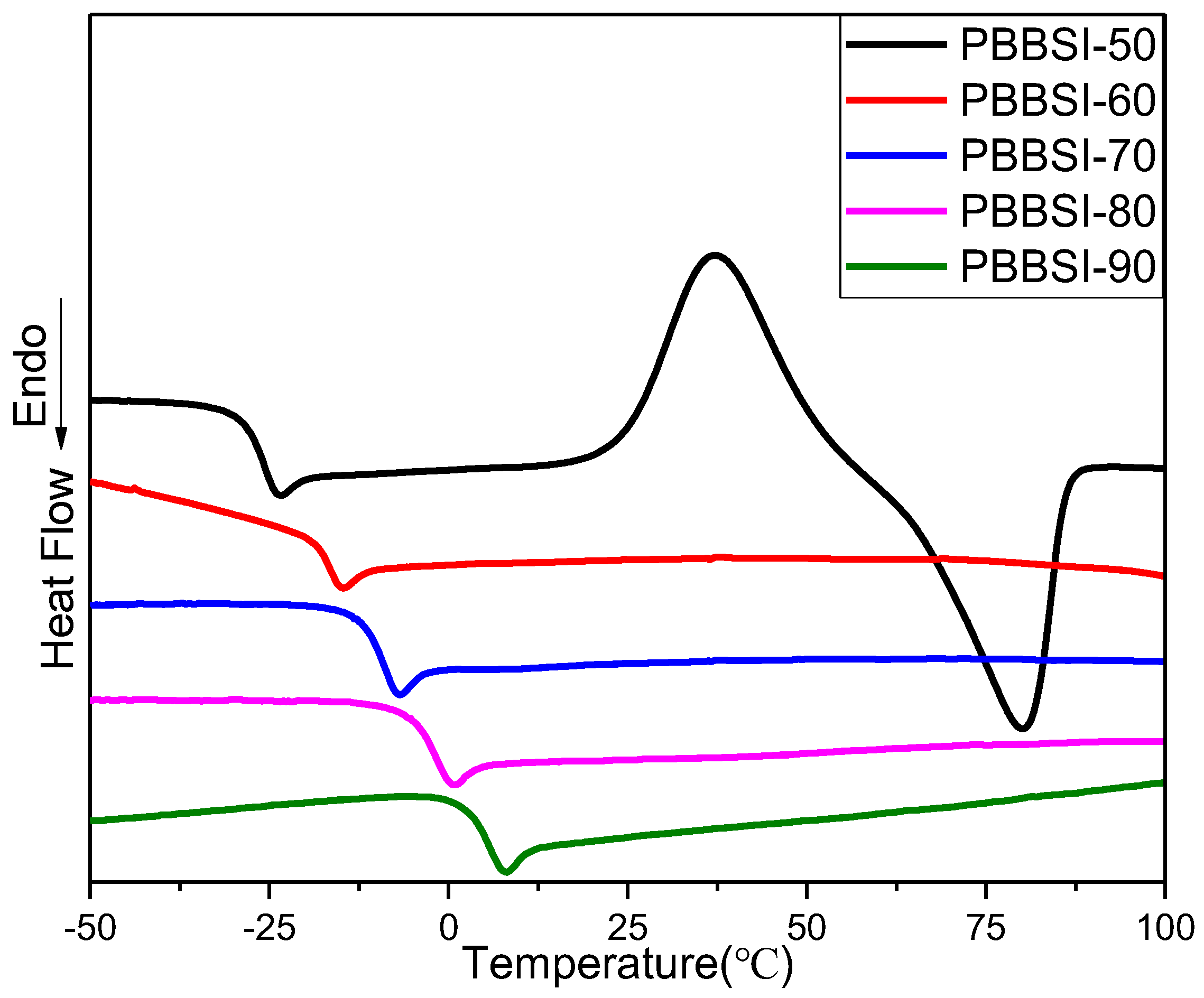
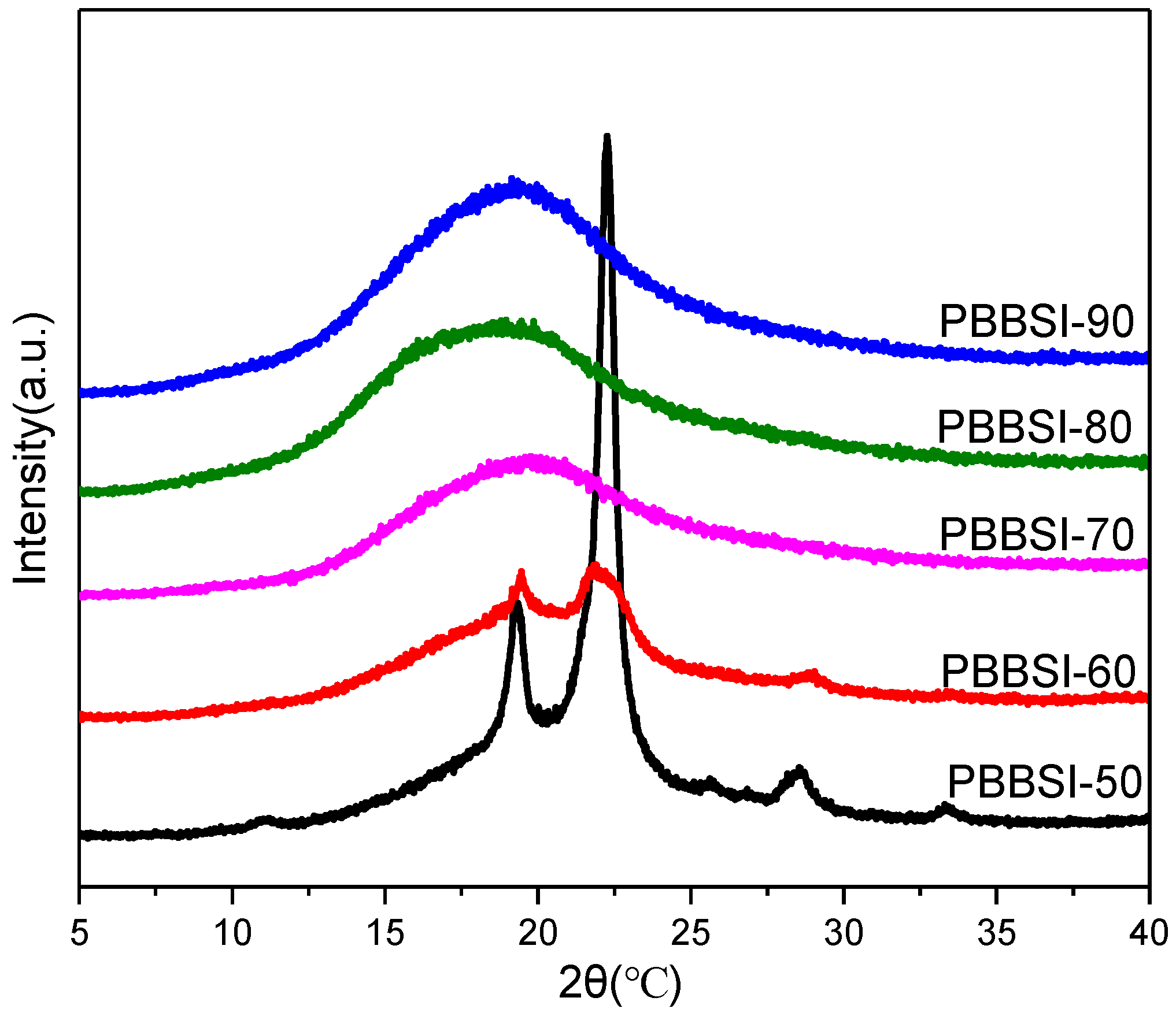
3.2. Thermal and Crystalline Properties of PBBSI Copolyesters
3.3. Mechanical Properties of PBBSI Copolyesters
3.4. Morphology of PBBSI/PLA TPV
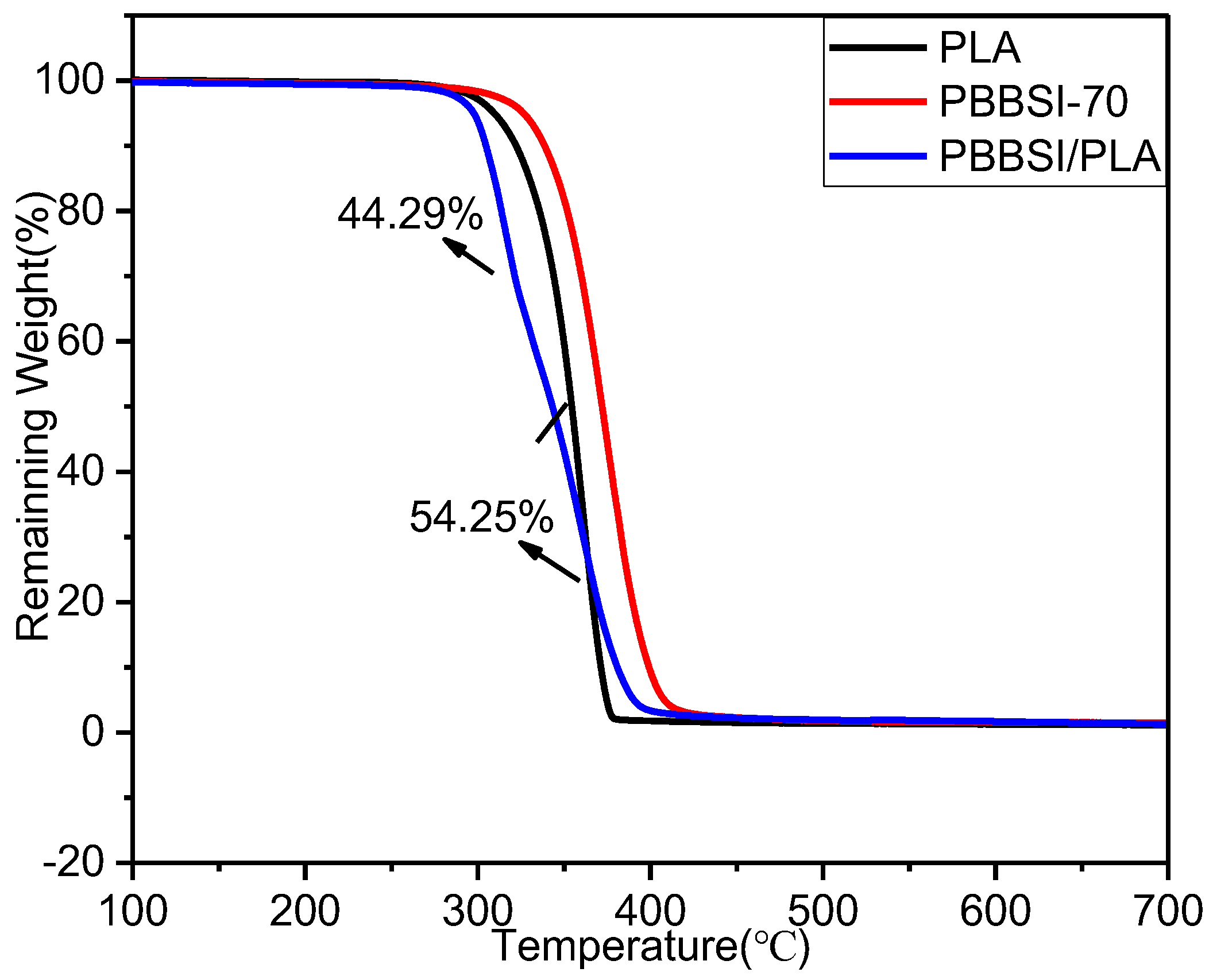
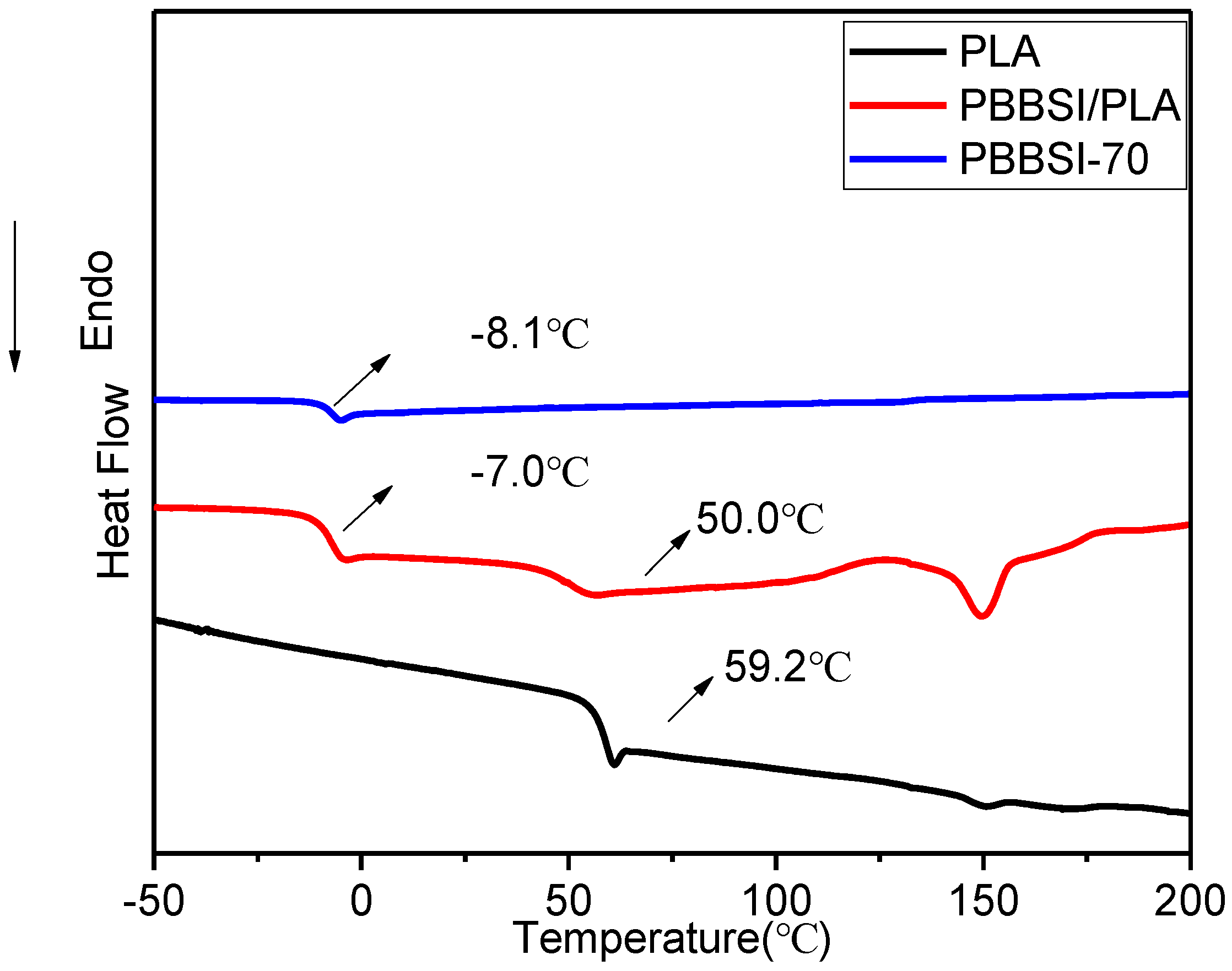
3.5. Thermal and Crystalline Properties of PBBSI/PLA TPV
3.6. Mechanical Properties of the PBBSI/PLA TPV
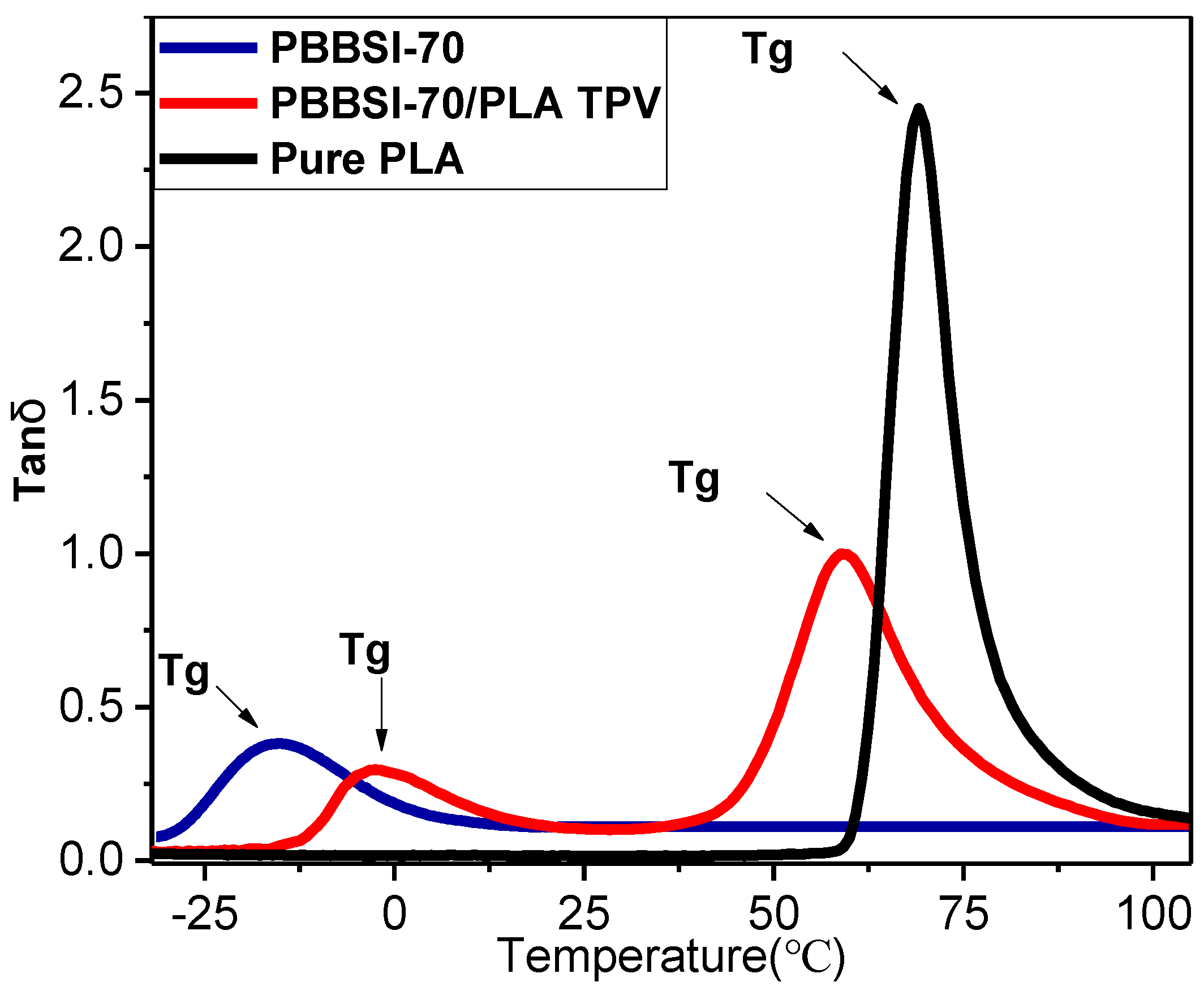
3.7. Thermomechanical Properties of the PBBSI/PLA TPV
3.8. Rheological Properties of the PBBSI/PLA TPV
3.9. 3D-Printing Study of the PBBSI/PLA TPV
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.; Lv, Y.; Sheng, J.; Tian, H.; Ning, N.; Zhang, L.; Wu, H.; Tian, M. Morphology development of POE/PP thermoplastic vulcanizates (TPVs) during dynamic vulcanization. Eur. Polym. J. 2017, 96, 590–601. [Google Scholar] [CrossRef]
- Antunes, C.; van Duin, M.; Machado, A. Effect of crosslinking on morphology and phase inversion of EPDM/PP blends. Mater. Chem. Phys. 2012, 133, 410–418. [Google Scholar] [CrossRef]
- Lu, K.; van Duin, M.; Loos, J.; de With, G. On the volume organisation of thermoplastic vulcanisates (TPVs) as revealed by scanning transmission electron microscopy (STEM). Polymer 2012, 53, 4171–4177. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, D.; Xu, C. Dynamically vulcanized biobased polylactide/natural rubber blend material with continuous cross-linked rubber phase. ACS Appl. Mater. Interfaces 2014, 6, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; van Duin, M. Dynamic vulcanisation of EPDM/PE-based thermoplastic vulcanisates studied along the extruder axis. Polymer 2005, 46, 6575–6586. [Google Scholar] [CrossRef]
- Naskar, K.; Chatterjee, T.; Wiessner, S.; Heinrich, G. Novel thermoplastic vulcanizates(TPVs)based on silicone rubber and polyamide exploring peroxide cross-linking. eXPRESS Polym. Lett. 2014, 8, 220–231. [Google Scholar]
- Ma, L.-F.; Bao, R.-Y.; Dou, R.; Zheng, S.-D.; Liu, Z.-Y.; Zhang, R.-Y.; Yang, M.-B.; Yang, W. Conductive thermoplastic vulcanizates (TPVs) based on polypropylene (PP)/ethylene-propylene-diene rubber (EPDM) blend: From strain sensor to highly stretchable conductor. Compos. Sci. Technol. 2016, 128, 176–184. [Google Scholar] [CrossRef]
- Coran, A. Thermoplastic elastomers based on dynamically vulcanized elastomer/thermoplastic blends. In Thermoplastic Elastomers; Hanser Publishers: Munich, Germany, 1996. [Google Scholar]
- Karger-Kocsis, J. Thermoplastic rubbers via dynamic vulcanization. Plast. Eng. N. Y. 1999, 52, 125–154. [Google Scholar]
- De, S.; Bhowmick, A.K. Thermoplastic Elastomers from Rubber-Plastic Blends; Ellis Horwood: Hertfordshire, UK, 1990; Volume 32, pp. 349–394. [Google Scholar]
- Rajeshbabu, R.; Gohs, U.; Naskar, K.; Thakur, V.; Wagenknecht, U.; Heinrich, G. Preparation of polypropylene (PP)/ethylene octene copolymer (EOC) thermoplastic vulcanizates (TPVs) by high energy electron reactive processing. Radiat. Phys. Chem. 2011, 80, 1398–1405. [Google Scholar] [CrossRef]
- Uthaipan, N.; Jarnthong, M.; Peng, Z.; Junhasavasdikul, B.; Nakason, C.; Thitithammawong, A. Effects of cooling rates on crystallization behavior and melting characteristics of isotactic polypropylene as neat and in the TPVs EPDM/PP and EOC/PP. Polym. Test. 2015, 44, 101–111. [Google Scholar] [CrossRef]
- Marques, A.; Reis, R.; Hunt, J. The biocompatibility of novel starch-based polymers and composites: in vitro studies. Biomaterials 2002, 23, 1471–1478. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.J.; Augusta, J.; Widdecke, H.; Deckwer, W.D. Synthesis, properties and biodegradability of polyesters based on 1,3-propanediol. Macromol. Chem. Phys. 1994, 195, 793–802. [Google Scholar] [CrossRef]
- Kurian, J.V. A new polymer platform for the future—Sorona® from corn derived 1,3-propanediol. J. Polym. Environ. 2005, 13, 159–167. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly (butylene succinate) and its copolymers: research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.; Kang, H.; Zhang, L. Design, preparation and properties of bio-based elastomer composites aiming at engineering applications. Compos. Sci. Technol. 2016, 133, 136–156. [Google Scholar] [CrossRef]
- Kim, S.; Hahn, J.-S. Efficient production of 2,3-butanediol in Saccharomyces cerevisiae by eliminating ethanol and glycerol production and redox. Metab. Eng. 2015, 31, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Hu, H.-Y.; Liu, D.-H.; Song, Y.-Q. The implementation of high fermentative 2,3-butanediol production from xylose by simultaneous additions of yeast extract, Na 2 EDTA, and acetic acid. New Biotechnol. 2016, 33, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Woortman, A.J.; van Ekenstein, G.O.A.; Loos, K. Environmentally benign synthesis of saturated and unsaturated aliphatic polyesters via enzymatic polymerization of biobased monomers derived from renewable resources. Polym. Chem. 2015, 6, 5451–5463. [Google Scholar] [CrossRef]
- Hoffmann, C.; Stuparu, M.C.; Daugaard, A.; Khan, A. Aza-Michael addition reaction: Post-polymerization modification and preparation of PEI/PEG-based polyester hydrogels from enzymatically synthesized reactive polymers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 745–749. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- ASTM International. ASTM International; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Tran, T.N.; Bayer, I.S.; Heredia-Guerrero, J.A.; Frugone, M.; Lagomarsino, M.; Maggio, F.; Athanassiou, A. Cocoa Shell Waste Biofilaments for 3D Printing Applications. Macromol. Mater. Eng. 2017, 302, 11. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Holmes, B.; Faucett, S.; Zhang, L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. Part B Rev. 2014, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Jin, Q.; Bland, M.E.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010, 31, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Taut, A.D.; Padial-Molina, M.; Flanagan, C.L.; Pilipchuk, S.P.; Hollister, S.J.; Giannobile, W.V. Image-based, fiber guiding scaffolds: a platform for regenerating tissue interfaces. Tissue Eng. Part C Methods 2013, 20, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.-H.; Fischer, K.; Edminster, K.; Park, J.-K.; Yoo, S.-S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2013, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Li, M.; Tang, Z.; Xue, J.; Hu, X.; Zhang, L.; Guo, B. Synthesis and characterization of biobased isosorbide-containing copolyesters as shape memory polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 7877–7886. [Google Scholar] [CrossRef]
- Hu, X.; Kang, H.; Li, Y.; Geng, Y.; Wang, R.; Zhang, L. Preparation, morphology and superior performances of biobased thermoplastic elastomer by in situ dynamical vulcanization for 3D-printed materials. Polymer 2017, 108, 11–20. [Google Scholar] [CrossRef]
- Hu, X.; Kang, H.; Li, Y.; Li, M.; Wang, R.; Xu, R.; Qiao, H.; Zhang, L. Direct copolycondensation of biobased elastomers based on lactic acid with tunable and versatile properties. Polym. Chem. 2015, 6, 8112–8123. [Google Scholar] [CrossRef]
- Hu, X.; Shen, X.; Huang, M.; Liu, C.; Geng, Y.; Wang, R.; Xu, R.; Qiao, H.; Zhang, L. Biodegradable unsaturated polyesters containing2,3-butanediol for engineering applications: Synthesis, characterization and performances. Polymer 2016, 84, 343–354. [Google Scholar] [CrossRef]
- Shen, X.; Lin, Y.; Jain, R.; Yuan, Q.; Yan, Y. Inhibition of acetate accumulation leads to enhanced production of (R,R)-2,3-butanediol from glycerol in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2012, 39, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, W.; Wang, X.; Yuan, Q. Separation of 2,3-Butanediol Using ZSM-5 Zeolite Modified with Hydrophobic Molecular Spaces. Chem. Lett. 2013, 43, 411–413. [Google Scholar] [CrossRef]




| Molecular Weights | |||||
|---|---|---|---|---|---|
| PBBSI-50 | PBBSI-60 | PBBSI-70 | PBBSI-80 | PBBSI-90 | |
| Mn | 39,944 | 26,174 | 25,376 | 23,396 | 21,907 |
| PDI | 4.5 | 5.0 | 3.7 | 3.2 | 4.7 |
| Molar Compositions 1,4-BDO/2,3-BDO/SuA/IA | ||
|---|---|---|
| Samples | Feed a | Copolyester b |
| PBBSI-50 | 0.25/0.25/0.45/0.05 | 0.27/0.23/0.48/0.02 |
| PBBSI-60 | 0.20/0.30/0.45/0.05 | 0.22/0.27/0.49/0.02 |
| PBBSI-70 | 0.15/0.35/0.45/0.05 | 0.18/0.32/0.48/0.02 |
| PBBSI-80 | 0.10/0.40/0.45/0.05 | 0.13/0.35/0.50/0.02 |
| PBBSI-90 | 0.05/0.45/0.45/0.05 | 0.09/0.40/0.49/0.02 |
| Samples | PBBSI-50 | PBBSI-60 | PBBSI-70 | PBBSI-80 | PBBSI-90 |
|---|---|---|---|---|---|
| Td,10% a (°C) | 398.5 | 396.6 | 394.9 | 394.1 | 382.8 |
| Td,max b (°C) | 377.3 | 375.9 | 374.9 | 372.8 | 366 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, Y.; Hu, X.; Wu, W.; Wang, Z.; Wang, R.; Zhang, L. Preparation and Properties of Novel Thermoplastic Vulcanizate Based on Bio-Based Polyester/Polylactic Acid, and Its Application in 3D Printing. Polymers 2017, 9, 694. https://doi.org/10.3390/polym9120694
Gao Y, Li Y, Hu X, Wu W, Wang Z, Wang R, Zhang L. Preparation and Properties of Novel Thermoplastic Vulcanizate Based on Bio-Based Polyester/Polylactic Acid, and Its Application in 3D Printing. Polymers. 2017; 9(12):694. https://doi.org/10.3390/polym9120694
Chicago/Turabian StyleGao, Yu, Yan Li, Xiaoran Hu, Weidong Wu, Zhao Wang, Runguo Wang, and Liqun Zhang. 2017. "Preparation and Properties of Novel Thermoplastic Vulcanizate Based on Bio-Based Polyester/Polylactic Acid, and Its Application in 3D Printing" Polymers 9, no. 12: 694. https://doi.org/10.3390/polym9120694