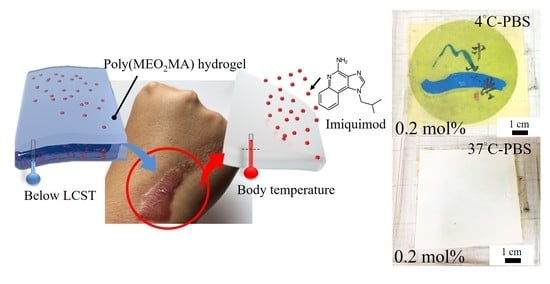
Development and Characterisation of the Imiquimod Poly(2-(2-methoxyethoxy)ethyl Methacrylate) Hydrogel Dressing for Keloid Therapy
Abstract
:1. Introduction
2. Fabrication and Characterisation
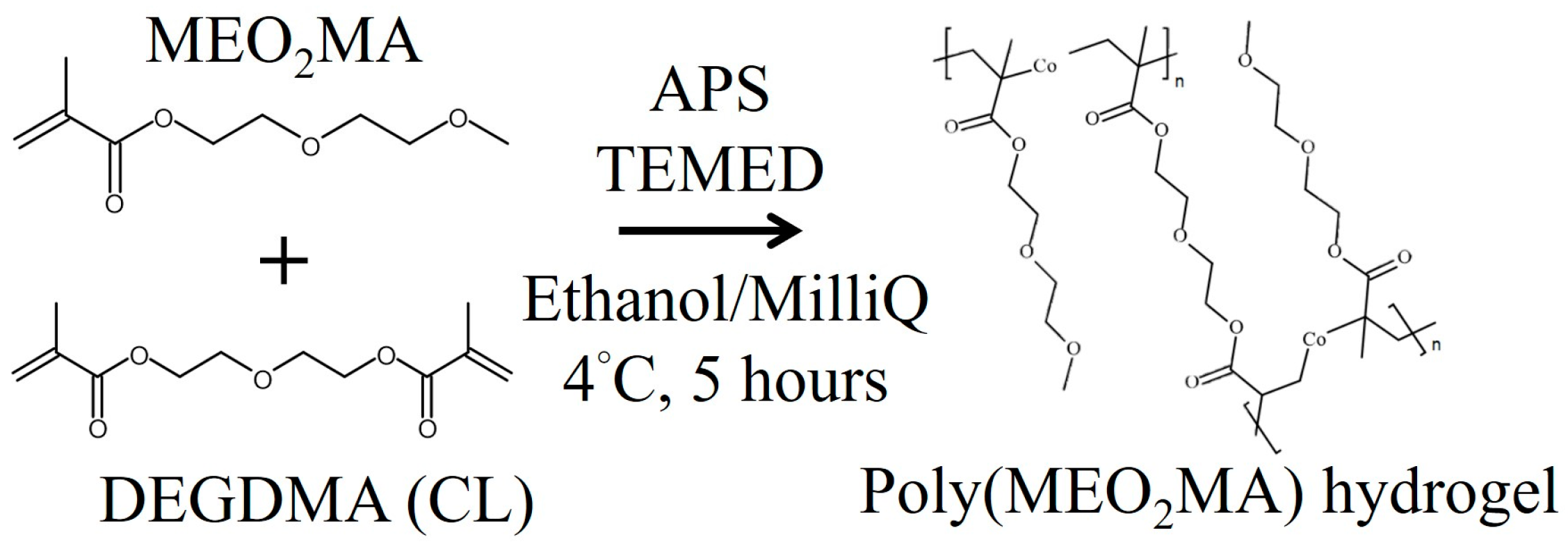
2.1. Preparation of Poly(MEO2MA) Hydrogel Samples
2.2. Preliminary Study of Poly(MEO2MA) Hydrogel Properties
2.2.1. Lower Critical Solution Temperature of Poly(MEO2MA) Hydrogel
2.2.2. Characterisation of Swelling Property
2.2.3. Characterisation of Deswelling Property
2.3. Fabrication of Imiquimod-Poly(MEO2MA) Hydrogel
2.3.1. Imiquimod Loading
2.3.2. Study of Imiquimod Releasing Profile
2.4. Performance Evaluation of Fabricated Imiquimod-Poly(MEO2MA) Hydrogel
3. Results and Discussion
3.1. Synthesis of Poly(MEO2MA) Hydrogels
3.2. Characterisations of Poly(MEO2MA) Hydrogel
3.2.1. Measurements of the LCST
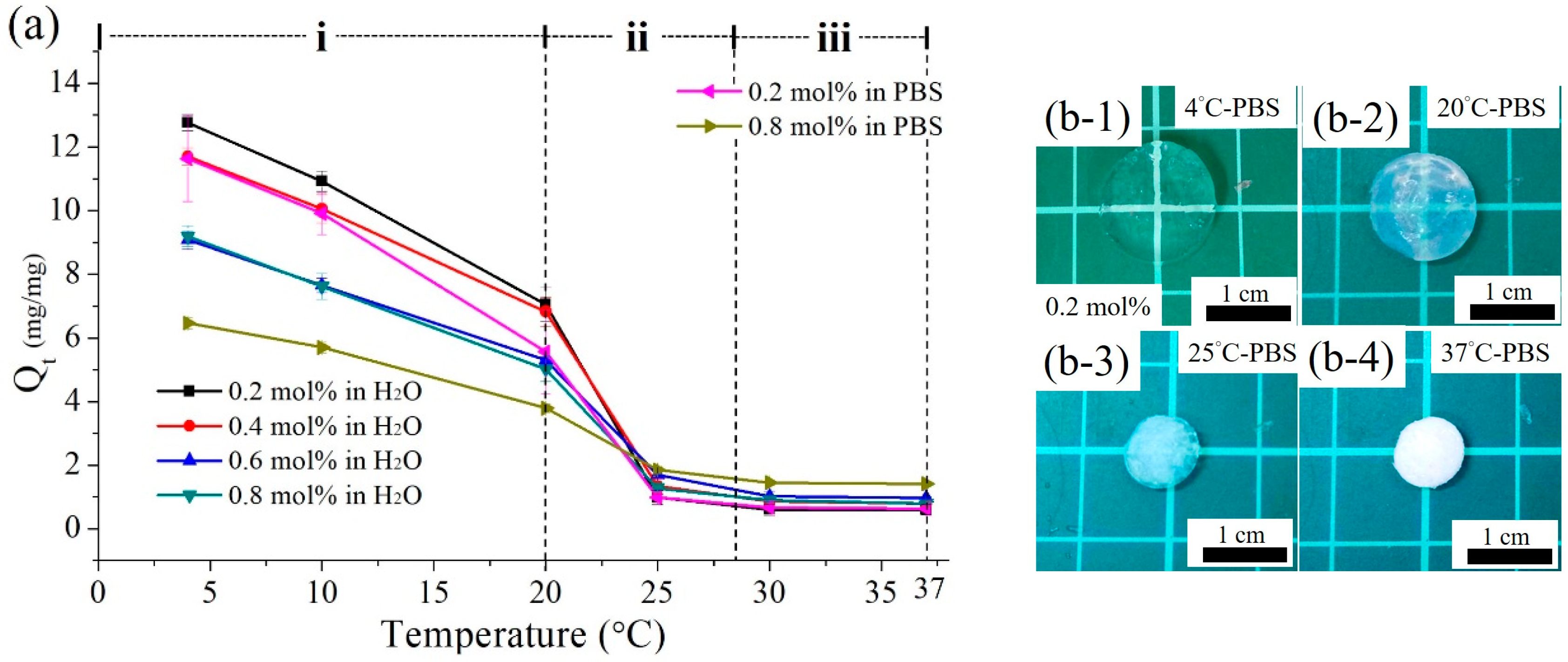
3.2.2. Swelling Profiles of the Poly(MEO2MA) Hydrogels
3.2.3. Deswelling Profiles of the Poly(MEO2MA) Hydrogels
3.3. Study of Imiquimod Loading and Releasing
3.3.1. Imiquimod Loading Profile
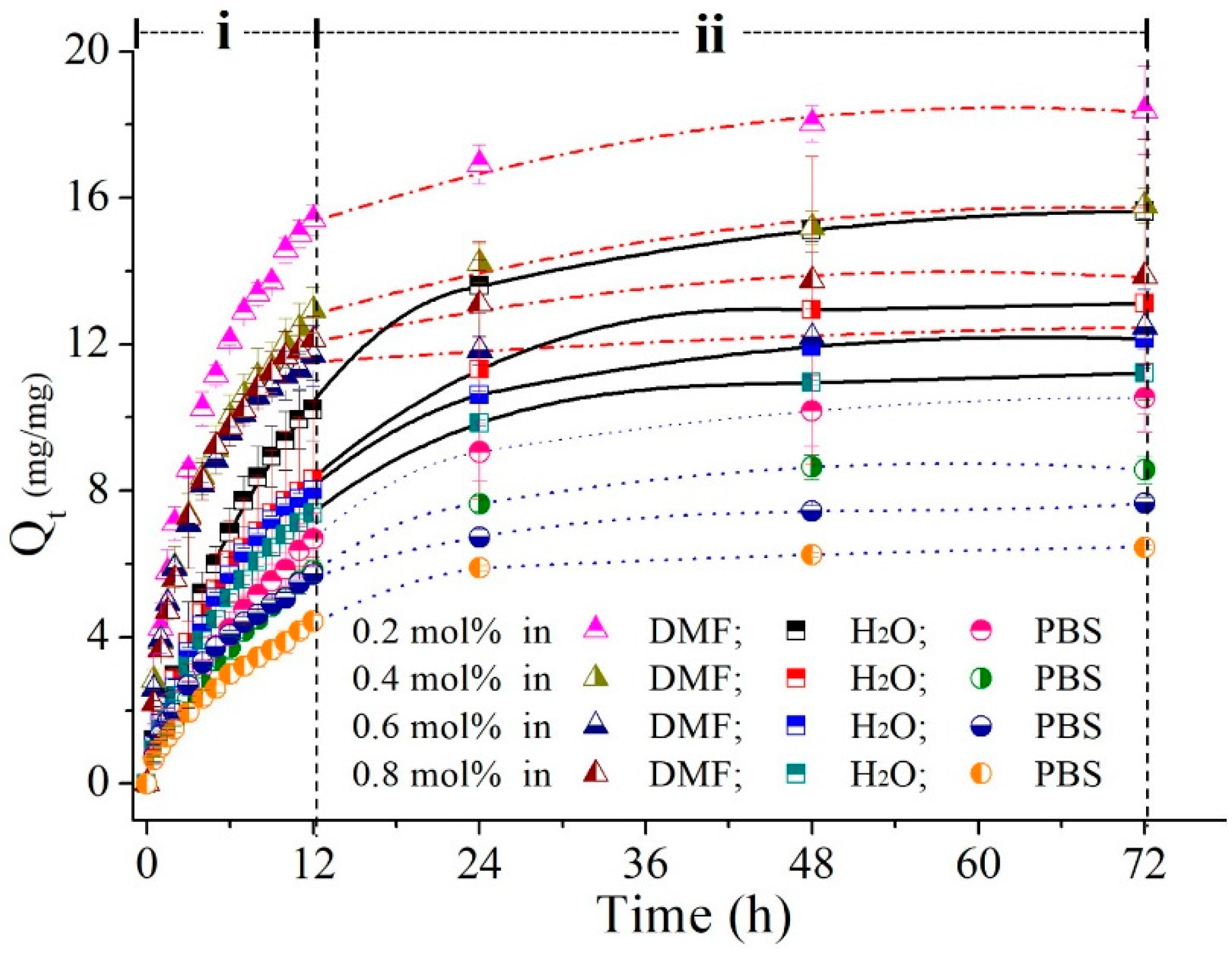
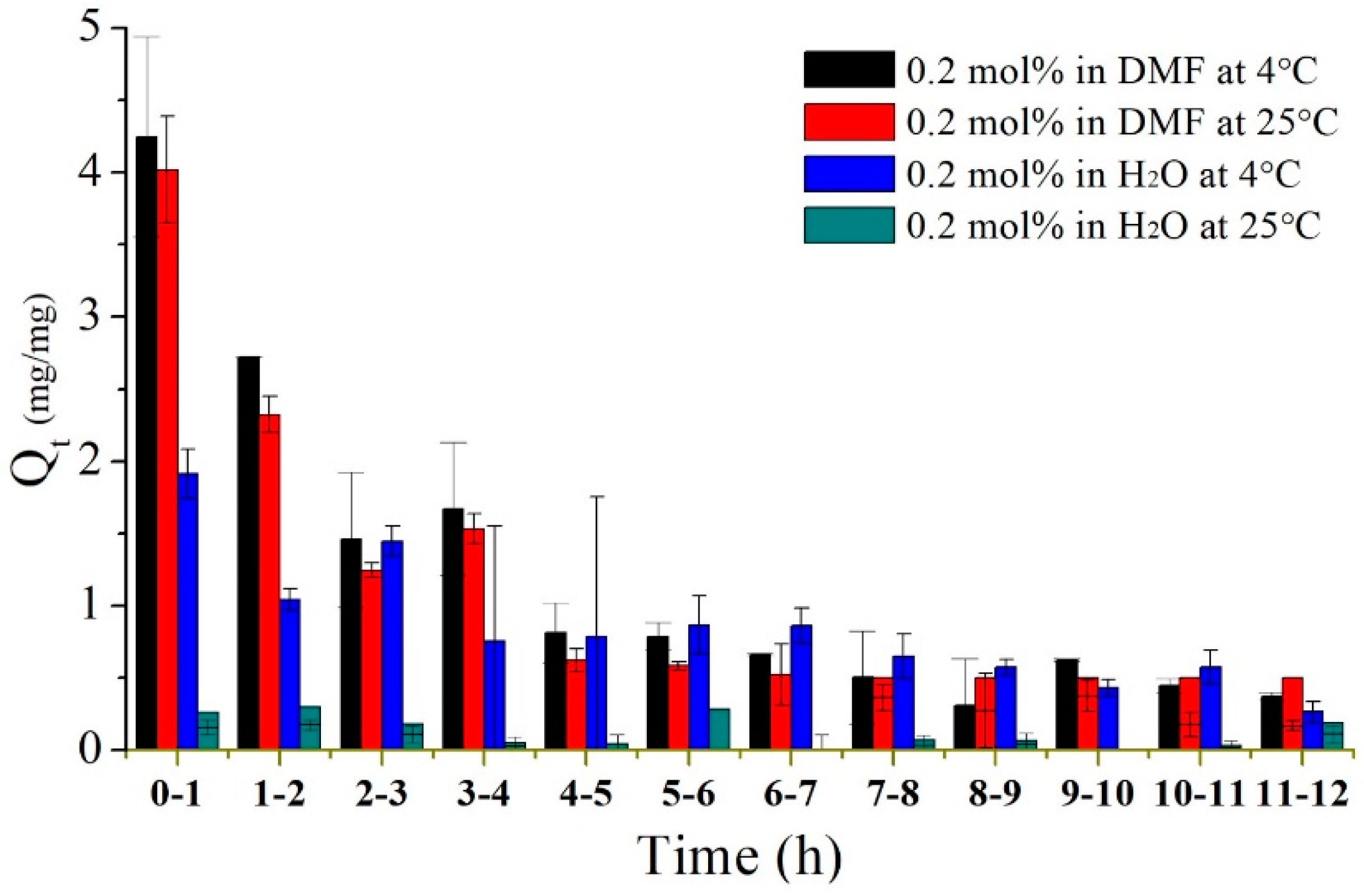
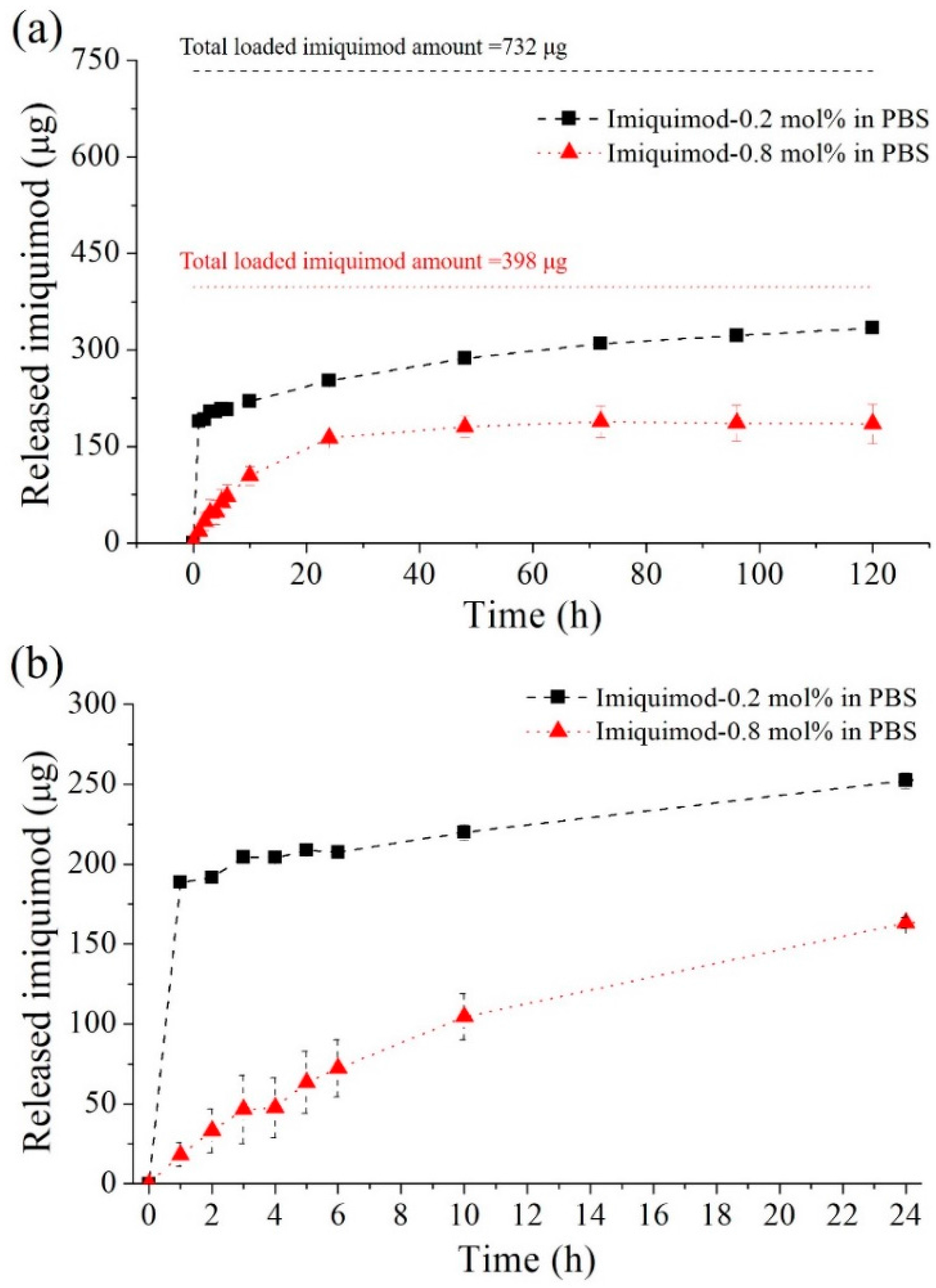
3.3.2. Imiquimod-Release Profiles
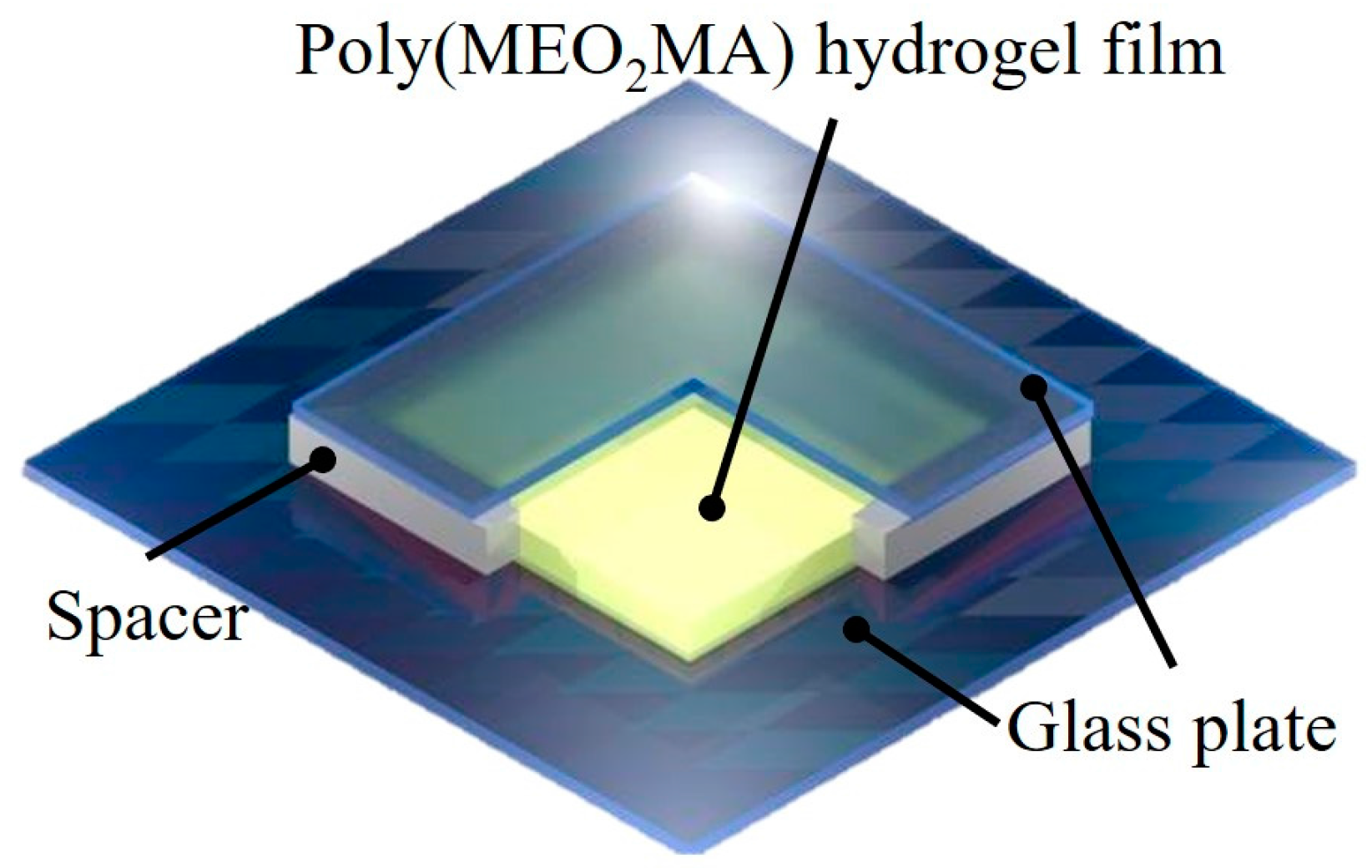
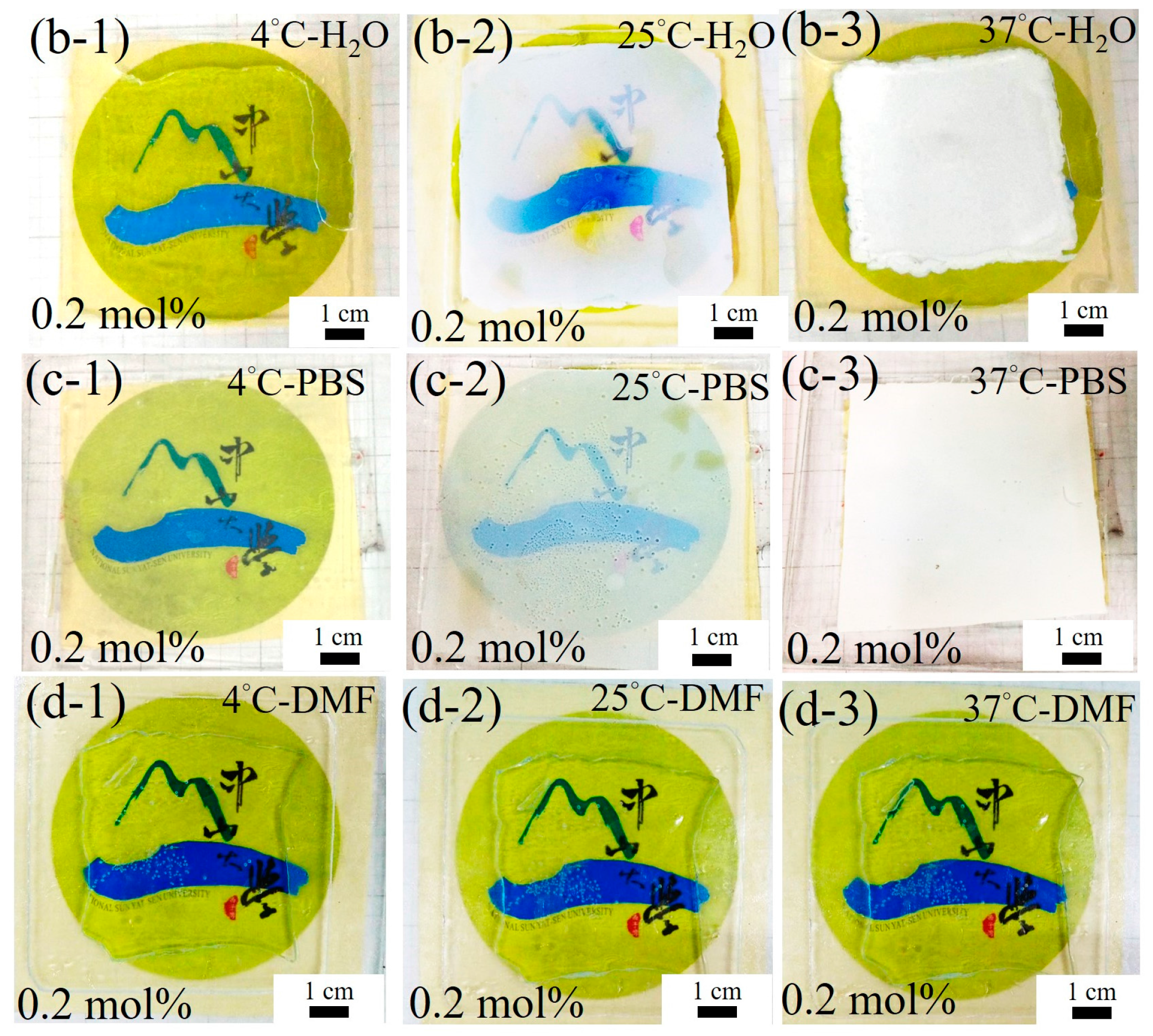
3.4. Characterisation of Fabricated Imiquimod-Poly(MEO2MA) Hydrogel Pad
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hunt, T.K. The physiology of wound healing. Ann. Emerg. Med. 1988, 17, 1265–1273. [Google Scholar] [CrossRef]
- Degreef, H.J. How to heal a wound fast. Dermatol. Clin. 1998, 16, 365–375. [Google Scholar] [CrossRef]
- Zaidi, Z.; Lanigan, S.W. Skin: Structure and Function, Dermatology in Clinical Practice; Springer: London, UK, 2010; pp. 1–15. [Google Scholar]
- Andrews, J.P.; Marttala, J.; Macarak, E.; Rosenbloom, J.; Uitto, J. Keloids: The paradigm of skin fibrosis—Pathomechanisms and treatment. Matrix Biol. 2016, 51, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Constam, D.B.; Philipp, J.; Malipiero, U.V.; Dijke, P.T.; Schachner, M.; Fontana, A. Differential expression of transforming growth factor-β 1, -β 2, and -β 3 by glioblastoma cells, astrocytes, and microglia. J. Immunol. 1992, 148, 1404–1410. [Google Scholar] [PubMed]
- Chike-Obi, C.J.; Cole, P.D.; Brissett, A.E. Keloids: Pathogenesis, Clinical Features, and Management, SEMINARS in Plastic Surgery; Thieme Medical Publishers: Houston, TX, USA, 2009; pp. 178–184. [Google Scholar]
- Jalali, M.; Bayat, A. Current use of steroids in management of abnormal raised skin scars. Surgeon 2007, 5, 175–180. [Google Scholar] [CrossRef]
- Froelich, K.; Staudenmaier, R.; Kleinsasser, N.; Hagen, R. Therapy of auricular keloids: Review of different treatment modalities and proposal for a therapeutic algorithm. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Kaufman, J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J. Am. Acad. Dermatol. 2002, 47, S209–S211. [Google Scholar] [CrossRef] [PubMed]
- Kamperman, M.; Synytska, A. Switchable adhesion by chemical functionality and topography. J. Mater. Chem. 2012, 22, 19390–19401. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Lu, T.-P.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. Part B Eng. 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Uhlig, K.; Wischerhoff, E.; Lutz, J.-F.; Laschewsky, A.; Jaeger, M.S.; Lankenau, A.; Duschl, C. Monitoring cell detachment on PEG-based thermoresponsive surfaces using TIRF microscopy. Soft Matter 2010, 6, 4262–4267. [Google Scholar] [CrossRef]
- Harding, K.; Jones, V.; Price, P. Topical treatment: Which dressing to choose. Diabetes Metab. Res. Rev. 2000, 16, S47–S50. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ding, G.; Shi, X.; Guo, W.; Ni, Z.; Fu, H.; Fu, Z. A bioengineered drug-Eluting scaffold accelerated cutaneous wound healing in diabetic mice. Colloids Surf. B Biointerfaces 2016, 145, 226–231. [Google Scholar] [PubMed]
- Sheridan, M.; Shea, L.; Peters, M.; Mooney, D. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control. Release 2000, 64, 91–102. [Google Scholar] [CrossRef]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Vaughan, D. Hydrogel dressings in the management of a variety of wound types: A review. J. Orthop. Nurs. 2005, 9, S1–S11. [Google Scholar]
- Wischerhoff, E.; Uhlig, K.; Lankenau, A.; Börner, H.G.; Laschewsky, A.; Duschl, C.; Lutz, J.F. Controlled cell adhesion on PEG-based switchable surfaces. Angew. Chem. Int. Ed. 2008, 47, 5666–5668. [Google Scholar]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-isopropylacrylamide)-cellulose nanocrystals hybrid ydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- París, R.; Quijada-Garrido, I. Swelling behaviour of thermo-sensitive hydrogels based on oligo (ethylene glycol) methacrylates. Eur. Polym. J. 2009, 45, 3418–3425. [Google Scholar] [CrossRef]
- García-García, J.M.; Liras, M.; Quijada-Garrido, I.; Gallardo, A.; París, R. Swelling control in thermo-responsive hydrogels based on 2-(2-methoxyethoxy) ethyl methacrylate by crosslinking and copolymerization with N-isopropylacrylamide. Polym. J. 2011, 43, 887–892. [Google Scholar] [CrossRef]
- Ali, M.E.; Lamprecht, A. Polyethylene glycol as an alternative polymer solvent for nanoparticle preparation. Int. J. Pharm. 2013, 456, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F. Polymerization of oligo (ethylene glycol)(meth) acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Almomani, A.; Hong, W.; Hong, W.; Montazami, R. Influence of Temperature on the Electromechanical Properties of Ionic Liquid-Doped Ionic Polymer-Metal Composite Actuators. Polymers 2017, 9, 358. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Weichenhan, K.; Akdemir, Ö.; Hoth, A. About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy) ethyl methacrylate and oligo (ethylene glycol) methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Roy, S.G.; Kumar, A.; De, P. Amino acid containing cross-linked co-polymer gels: pH, thermo and salt responsiveness. Polymer 2016, 85, 1–9. [Google Scholar] [CrossRef]
- Xia, M.; Cheng, Y.; Meng, Z.; Jiang, X.; Chen, Z.; Theato, P.; Zhu, M. A novel nanocomposite hydrogel with precisely tunable UCST and LCST. Macromol. Rapid Commun. 2015, 36, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, K.; Yang, D.; Nie, J. Photopolymerization of methacrylated chitosan/PNIPAAm hybrid dual-sensitive hydrogels as carrier for drug delivery. Int. J. Biol. Macromol. 2009, 44, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yang, T.-M.; Chang, H.-P.; Wang, Y.-M. Dual-responsive polymer–drug nanoparticles for drug delivery. React. Funct. Polym. 2015, 86, 27–36. [Google Scholar] [CrossRef]
- Li, Q.; Gan, L.; Tao, H.; Wang, Q.; Ye, L.; Zhang, A.; Feng, Z. The synthesis and application of heparin-based smart drug carrier. Carbohydr. Polym. 2016, 140, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Koňák, Č.R.; Ganchev, B.; Teodorescu, M.; Matyjaszewski, K.; Kopečková, P.; Kopeček, J. Poly[N-(2-hydroxypropyl)methacrylamide-block-n-butyl acrylate] micelles in water/DMF mixed solvents. Polymer 2002, 43, 3735–3741. [Google Scholar] [CrossRef]
- Ramineni, S.K.; Cunningham, L.L.; Dziubla, T.D.; Puleo, D.A. Development of imiquimod-loaded mucoadhesive films for oral dysplasia. J. Pharm. Sci. 2013, 102, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Wang, B.; Shivji, G.M.; Toto, P.; Amerio, P.; Tomai, M.A.; Miller, R.L.; Sauder, D.N. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J. Investig. Dermatol. 2000, 114, 135–141. [Google Scholar] [CrossRef] [PubMed]




| Samples | 0.2 mol % | 0.4 mol % | 0.6 mol % | 0.8 mol % |
|---|---|---|---|---|
| Imiquimod (μg/mm3) | 27.4 ± 0.55 | 21.7 ± 3.95 | 19.2 ± 5.44 | 14.1 ± 2.49 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Liou, S.-H.; Kotsuchibashi, Y. Development and Characterisation of the Imiquimod Poly(2-(2-methoxyethoxy)ethyl Methacrylate) Hydrogel Dressing for Keloid Therapy. Polymers 2017, 9, 579. https://doi.org/10.3390/polym9110579
Lin W-C, Liou S-H, Kotsuchibashi Y. Development and Characterisation of the Imiquimod Poly(2-(2-methoxyethoxy)ethyl Methacrylate) Hydrogel Dressing for Keloid Therapy. Polymers. 2017; 9(11):579. https://doi.org/10.3390/polym9110579
Chicago/Turabian StyleLin, Wei-Chih, Sin-Han Liou, and Yohei Kotsuchibashi. 2017. "Development and Characterisation of the Imiquimod Poly(2-(2-methoxyethoxy)ethyl Methacrylate) Hydrogel Dressing for Keloid Therapy" Polymers 9, no. 11: 579. https://doi.org/10.3390/polym9110579