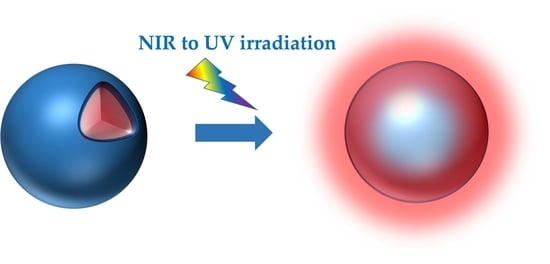
Light-Responsive Polymer Micro- and Nano-Capsules
Abstract
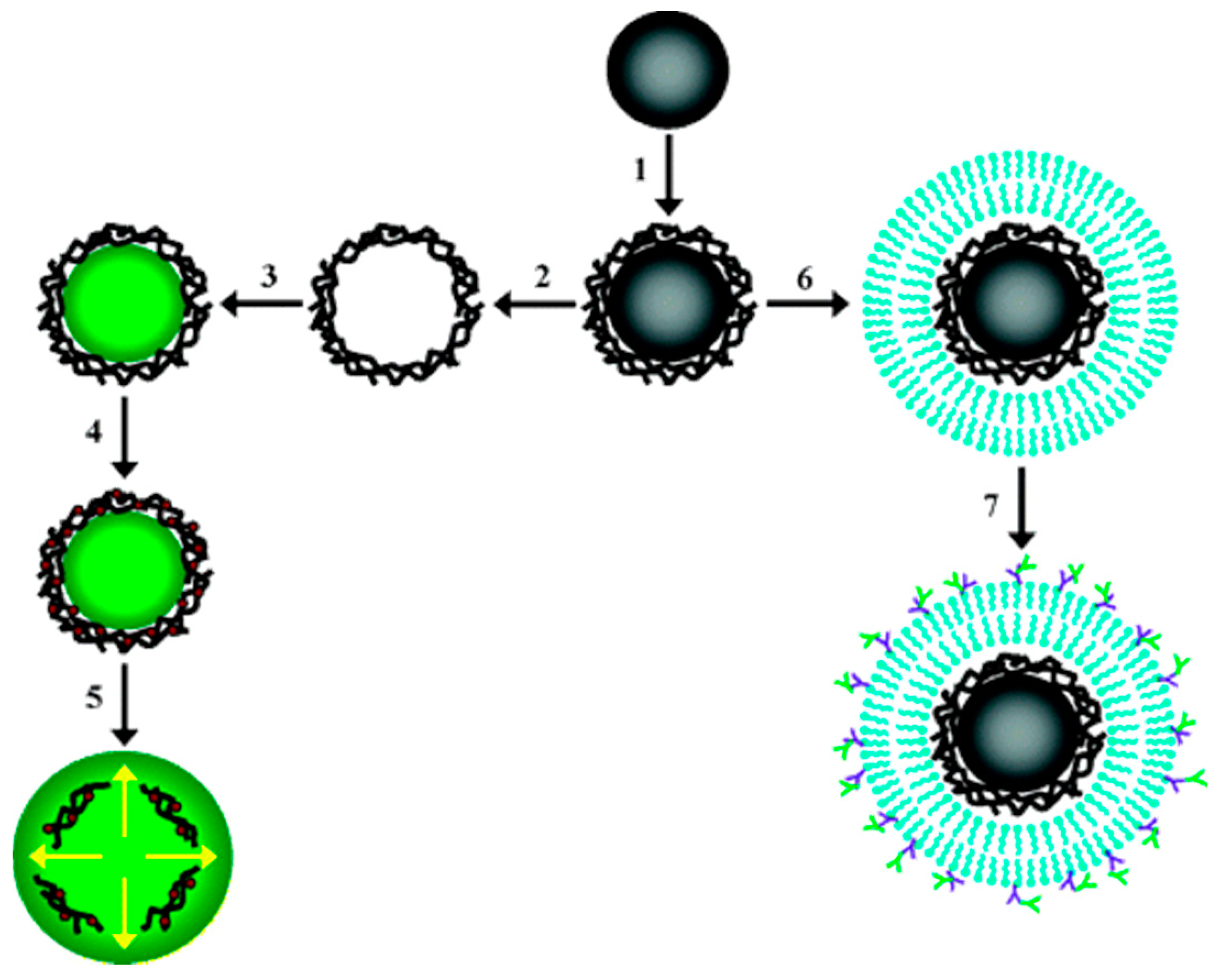
:1. Introduction
2. Interfacial Methods for Capsules Formation
2.1. Emulsion Polymerization
2.2. Phase Inversion Precipitation
3. Templating Methods
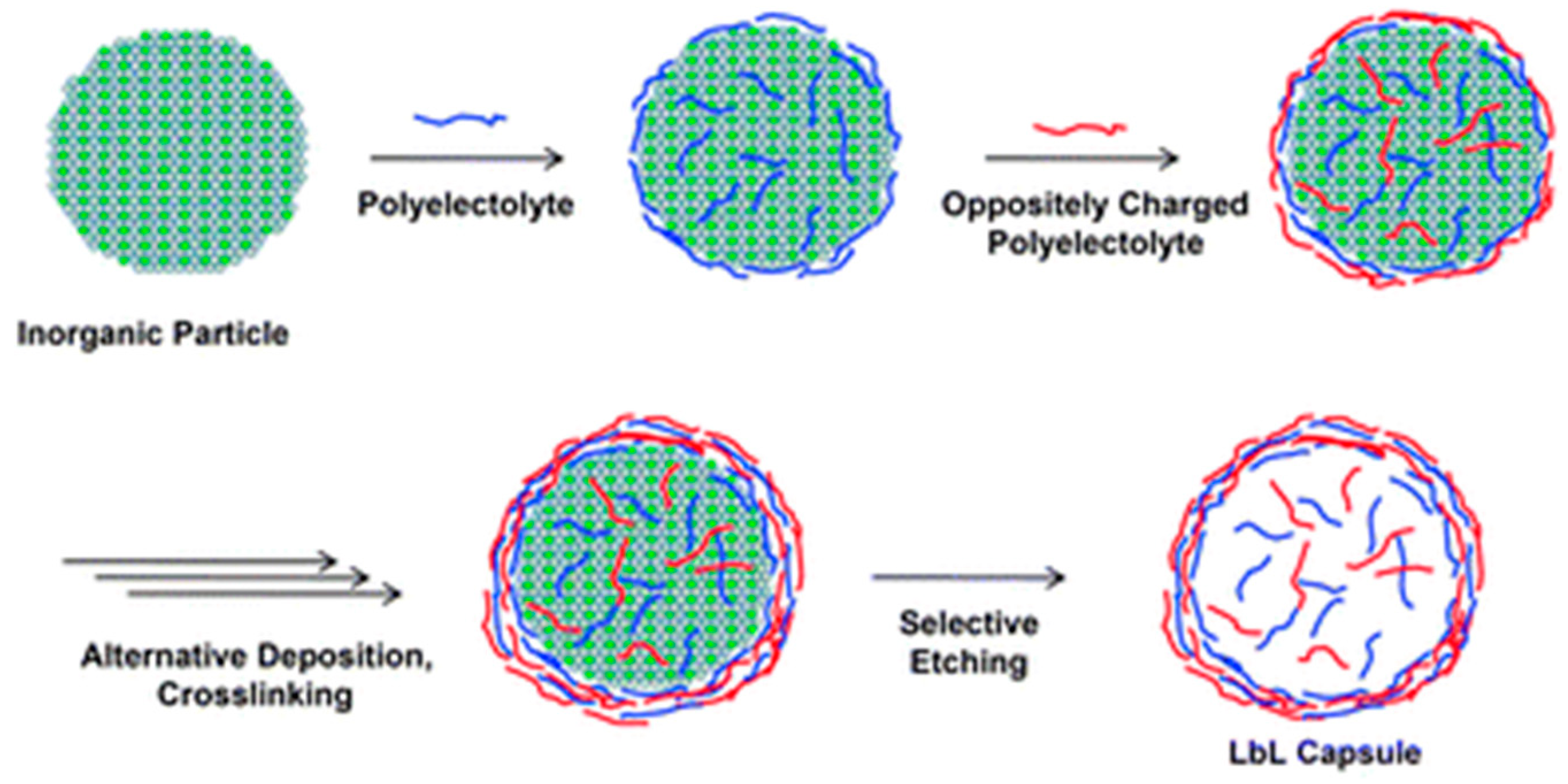
3.1. Layer-by-Layer (LbL) Using Polyelectrolytes
3.2. Layer-by-Layer (LbL) Using Host-Guest Systems
3.3. Other Templating Methods
4. Self-Assembly Methods
4.1. Block Copolymers Self-Assembly
4.2. Liposomes
5. Characterization Methods of Photo-Responsive Capsules
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blume, G.; Cevc, G. Liposomes for the sustained drug release in vivo. Biochim. Biophys. Acta 1990, 1029, 91–97. [Google Scholar] [CrossRef]
- Peteu, S.F.; Oancea, F.; Sicuia, O.A.; Constantinescu, F.; Dinu, S. Responsive polymers for crop protection. Polymers 2010, 2, 229–251. [Google Scholar] [CrossRef]
- Hastings, C.J.; Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Enzymelike catalysis of the Nazarov cyclization by supramolecular encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Zondanos, H.S.; Farrugia, J.M.; Serelis, A.K.; Such, C.H.; Hawkett, B.S. Pigment encapsulation by emulsion polymerization using macro-RAFT copolymers. Langmuir 2008, 24, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Sanlés-Sobrido, M.; Pérez-Lorenzo, M.; Rodríguez-González, B.; Salgueiriño, V.; Correa-Duarte, M.A. Highly active nanoreactors: Nanomaterial encapsulation based on confined catalysis. Angew. Chem. Int. Ed. 2012, 51, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Theato, P.; Sumerlin, B.S.; O’Reilly, R.K.; Epps, T.H., III. Stimuli responsive materials. Chem. Soc. Rev. 2013, 42, 7055–7056. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Gupta, M.N. Smart polymeric materials: Emerging biochemical applications. Chem. Biol. 2003, 10, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Déjugnat, C.; Sukhorukov, G.B. pH-responsive properties of hollow polyelectrolyte microcapsules templated on various cores. Langmuir 2004, 20, 7265–7269. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.B.; Cai, T.; Hu, Z.; Marquez, M.; Dinsmore, A.D. Temperature-responsive semipermeable capsules composed of colloidal microgel spheres. Langmuir 2007, 23, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Ultrasound-triggered smart drug release from multifunctional core−shell capsules one-step fabricated by coaxial electrospray method. Langmuir 2010, 27, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tong, W.; Gao, C. Molecular-engineered polymeric microcapsules assembled from Concanavalin A and glycogen with specific responses to carbohydrates. Soft Matter 2011, 7, 5805–5815. [Google Scholar] [CrossRef]
- Lv, L.P.; Zhao, Y.; Vilbrandt, N.; Gallei, M.; Vimalanandan, A.; Rohwerder, M.; Landfester, K.; Crespy, D. Redox responsive release of hydrophobic self-healing agents from polyaniline capsules. J. Am. Chem. Soc. 2013, 135, 14198–14205. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, R.; Zhu, X.; Yan, D. Photo-responsive polymeric micelles. Soft Matter 2014, 10, 6121–6138. [Google Scholar] [CrossRef] [PubMed]
- Ercole, F.; Davis, T.P.; Evans, R.A. Photo-responsive systems and biomaterials: Photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym. Chem. 2010, 1, 37–54. [Google Scholar] [CrossRef]
- Yager, K.G.; Barrett, C.J. Azobenzene Polymers for photonic applications. In Smart Light-Responsive Materials: Azobenzene-Containing Polymers and Liquid Crystals; Zhao, Y., Ikeda, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Görner, H.; Kuhn, H.J. Cis-Trans photoisomerization of stilbenes and stilbene-like molecules. In Advances in Photochemistry; Neckers, D.C., Volman, D.H., von Bünau, G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VHCA: Zurich, Switzerland, 2006. [Google Scholar]
- El Halabieh, R.H.; Mermut, O.; Barrett, C.J. Using light to control physical properties of polymers and surfaces with azobenzene chromophores. Pure Appl. Chem. 2004, 76, 1445–1465. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Baumann, E. Ueber eine einfache Methode der Darstellung von Benzoësäureäthern. Ber. Dtsch. Chem. Ges. 1886, 19, 3218–3222. [Google Scholar] [CrossRef]
- Schotten, C. Ueber die Oxydation des Piperidins. Ber. Dtsch. Chem. Ges. 1884, 17, 2544–2547. [Google Scholar] [CrossRef]
- Wittbecker, E.L.; Morgan, P.W. Interfacial polycondensation-I. J. Polym. Sci. 1959, 40, 289–297. [Google Scholar] [CrossRef]
- Torini, L.; Argillier, J.F.; Zydowicz, N. Interfacial polycondensation encapsulation in miniemulsion. Macromolecules 2005, 38, 3225–3236. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.H.; Hoek, E.M.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, T.; Han, B.; Wang, Y.; Du, J.; Liu, Z.; Zhang, J. Aqueous/ionic liquid interfacial polymerization for preparing polyaniline nanoparticles. Polymer 2004, 45, 3017–3019. [Google Scholar] [CrossRef]
- Cho, J.S.; Kwon, A.; Cho, C.G. Microencapsulation of octadecane as a phase-change material by interfacial polymerization in an emulsion system. Colloid Polym. Sci 2002, 280, 260–266. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsions for nanoparticle synthesis. In Colloid Chemistry II; Antonietti, M., Ed.; Springer: Berlin, Germany, 2003; pp. 75–123. [Google Scholar]
- Tylkowski, B.; Pregowska, M.; Jamowska, E.; Garcia-Valls, R.; Giamberini, M. Preparation of a new lightly cross-linked liquid crystalline polyamide by interfacial polymerization. Application to the obtainment of microcapsules with photo-triggered release. Eur. Polym. J. 2009, 45, 1420–1432. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Carfagna, C.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Photo-responsive polymer nanocapsules. Polymer 2015, 70, 222–230. [Google Scholar] [CrossRef]
- Bizzarro, V.; Carfagna, C.; Cerruti, P.; Marturano, V.; Ambrogi, V. Light-responsive polymer microcapsules as delivery systems for natural active agents. AIP Conf. Proc. 2016, 1736, 020078. [Google Scholar]
- Wachtveitl, J.; Zumbusch, A. Azobenzene: An optical switch for in vivo experiments. ChemBioChem 2011, 12, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene photoswitching without ultraviolet light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef] [PubMed]
- Wegner, H.A. Azobenzenes in a new light—Switching in vivo. Angew. Chem. Int. Ed. 2012, 51, 4787–4788. [Google Scholar] [CrossRef] [PubMed]
- Tylkowski, B.; Giamberini, M.; Underiner, T.; Prieto, S.F.; Smets, J. Photo-Triggered Microcapsules. Macromol. Symp. 2016, 360, 192–198. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, E.; Steinbacher, J.; McQuade, D.T. Interfacial polymerization within a simplified microfluidic device: Capturing capsules. J. Am. Chem. Soc. 2005, 127, 10498–10499. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, Z.; Parker, R.M.; Wu, Y.; Abell, C.; Scherman, O.A. Interfacial assembly of dendritic microcapsules with host–guest chemistry. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, S. Fabrication of ultraviolet-responsive microcapsules via Pickering emulsion polymerization using modified nano-silica/nano-titania as Pickering agents. RSC Adv. 2015, 5, 13850–13856. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Tylkowski, B.; Giamberini, M. Preparation and characterization of light-sensitive microcapsules based on a liquid crystalline polyester. Langmuir 2013, 29, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Peña, B.; Panisello, C.; Areste, G.; Garcia-Valls, R.; Gumí, T. Preparation and characterization of polysulfone microcapsules for perfume release. Chem. Eng. J. 2012, 179, 394–403. [Google Scholar] [CrossRef]
- Peyratout, C.S.; Dähne, L. Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Susha, L.; Caruso, F. Multilayered Titania, silica and laponite nanoparticle coatings on polystyrene colloidal templates and resulting inorganic hollow spheres. Chem. Mater. 2001, 13, 400–409. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.A.; Möhwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Jang, W.D. Polymeric supramolecular systems for drug delivery. J. Mater. Chem. 2010, 20, 211–222. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.; Cortez, C.; Angelatos, A.S.; Caruso, F. Layer-by-layer engineered capsules and their applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 203–209. [Google Scholar] [CrossRef]
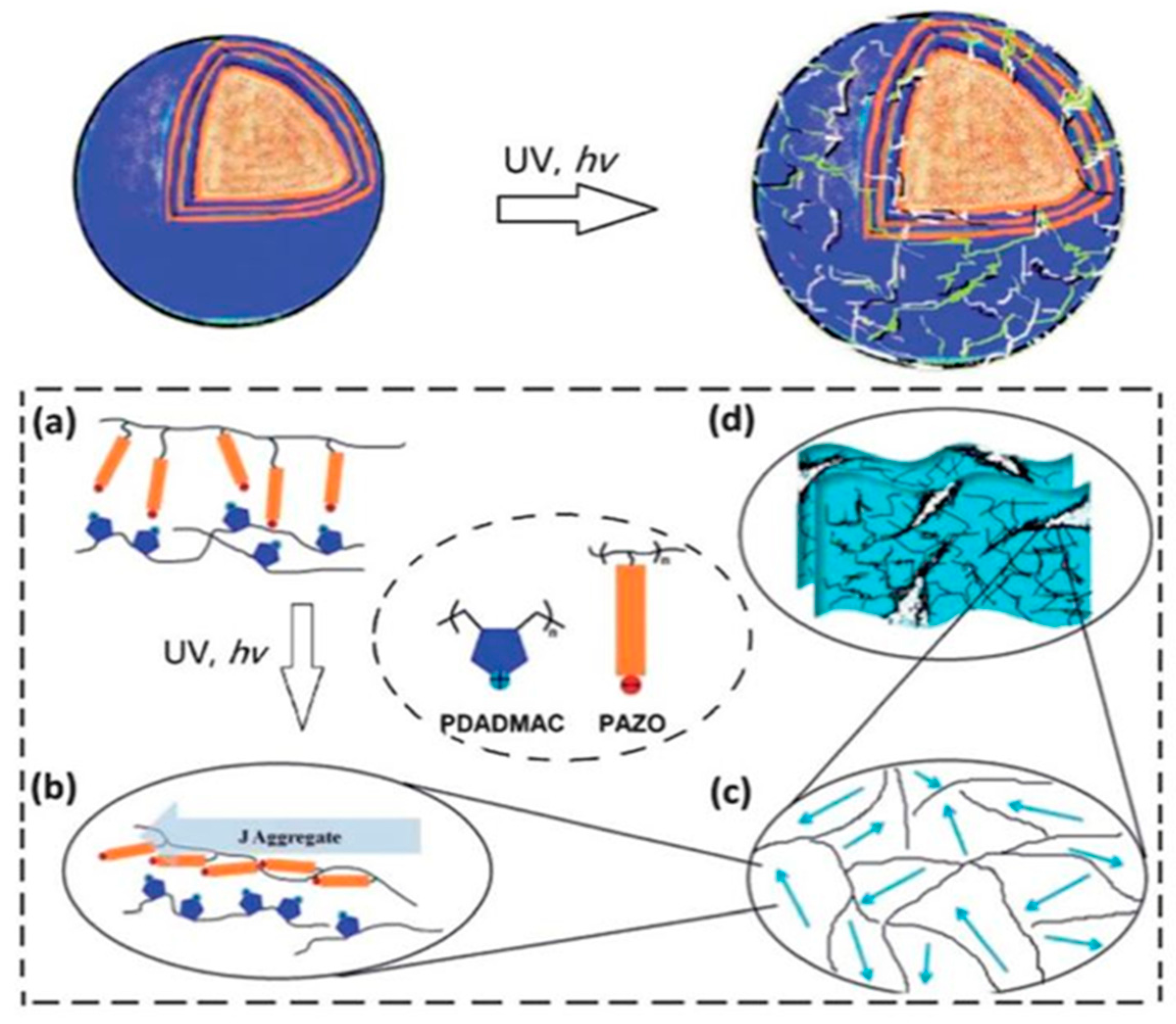
- Tao, X.; Li, J.; Möhwald, H. Self-Assembly, Optical Behavior, and Permeability of a Novel Capsule Based on an Azo Dye and Polyelectrolytes. Chem. Eur. J. 2004, 10, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Bédard, M.; Skirtach, A.G.; Sukhorukov, G. Optically driven encapsulation using novel polymeric hollow shells containing an azobenzene polymer. Macromol. Rapid Commun. 2007, 28, 1517–1521. [Google Scholar] [CrossRef]
- Yi, Q.; Sukhorukov, G.B. UV-induced disruption of microcapsules with azobenzene groups. Soft Matter 2014, 10, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol. 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Yi, Q.; Sukhorukov, G.B. Photolysis triggered sealing of multilayer capsules to entrap small molecules. ACS Appl. Mater. Interfaces 2013, 5, 6723–6731. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Sukhorukov, G.B. Single component diazo-resin microcapsules for encapsulation and triggered release of small molecules. Part. Part. Syst. Charact. 2013, 30, 989–995. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Shen, J.; Möhwald, H. Multilayer microcapsules as anti-cancer drug delivery vehicle: deposition, sustained release, and in vitro bioactivity. Macromol. Biosci. 2005, 5, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, G.L.; Dähne, L.; Hartmann, J.; Donath, E.; Möhwald, H. Controlled precipitation of dyes into hollow polyelectrolyte capsules based on colloids and biocolloids. Adv. Mater. 2000, 12, 112–115. [Google Scholar] [CrossRef]
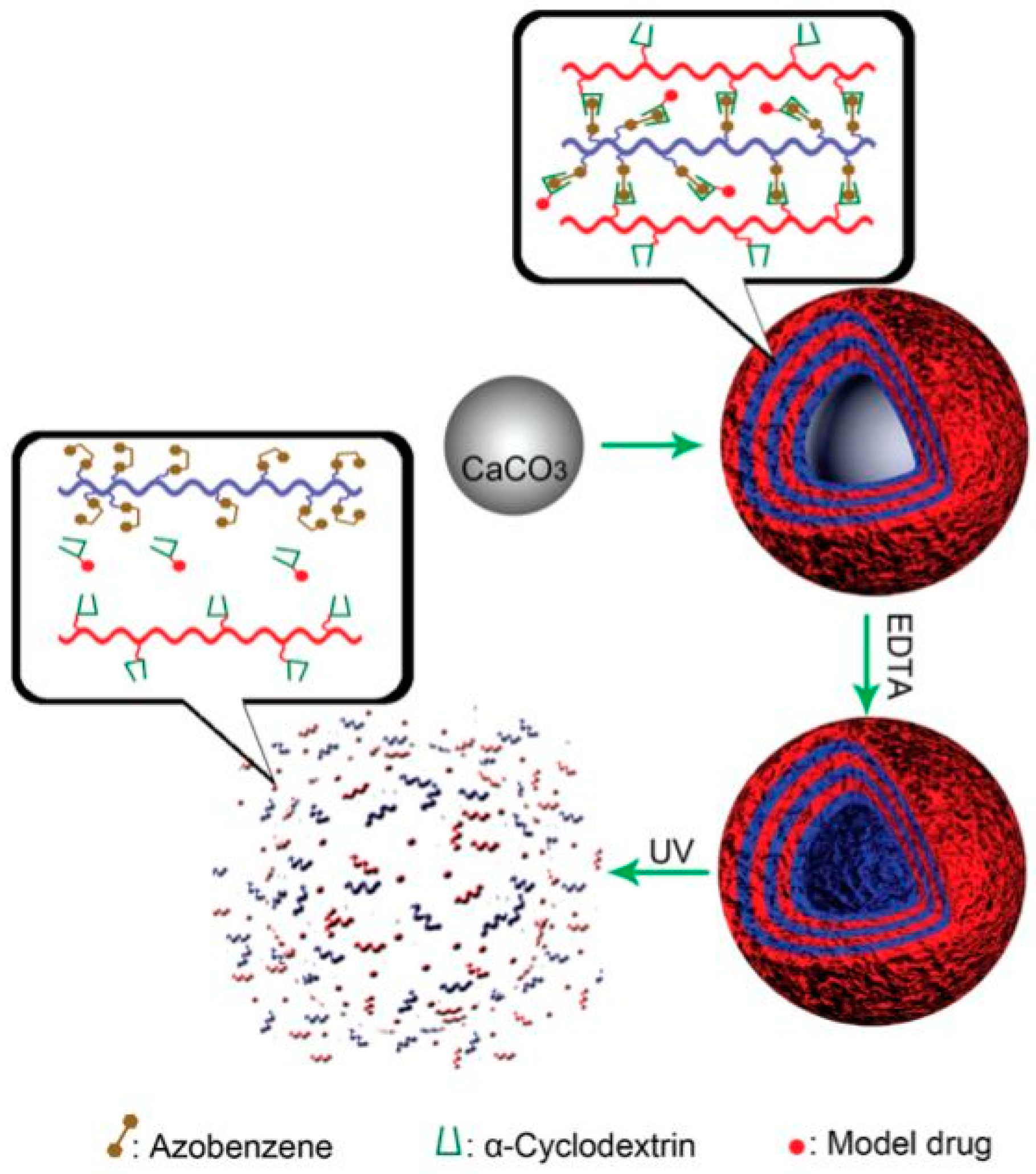
- Qu, D.H.; Wang, Q.C.; Zhang, Q.W.; Ma, X.; Tian, H. Photoresponsive host–guest functional systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, W.H.; Zhang, J.; Li, C.; Zhuo, R.X.; Zhang, X.Z. Design of a photoswitchable hollow microcapsular drug delivery system by using a supramolecular drug-loading approach. J. Phys. Chem. B 2011, 115, 13796–13802. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xiao, W.; Qin, S.Y.; Cheng, S.X.; Zhang, X.Z. Switch on/off microcapsules for controllable photosensitive drug release in a ‘release-cease-recommence’ mode. Polym. Chem. 2014, 5, 4437–4440. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Hu, J.; Li, C.; Liu, S. Multi-responsive supramolecular double hydrophilic diblock copolymer driven by host-guest inclusion complexation between β-cyclodextrin and adamantyl moieties. Macromol. Chem. Phys. 2009, 210, 2125–2137. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.Z. Effect of α-cyclodextrin on the photoisomerization of azobenzene functionalized hydroxypropyl methylcellulose in aqueous solution. Polym. Bull. 2007, 59, 537–544. [Google Scholar] [CrossRef]
- Achilleos, D.S.; Hatton, T.A.; Vamvakaki, M. Light-regulated supramolecular engineering of polymeric nanocapsules. J. Am. Chem. Soc. 2012, 134, 5726–5729. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef] [PubMed]
- Wajs, E.; Nielsen, T.T.; Larsen, K.L.; Fragoso, A. Preparation of stimuli-responsive nano-sized capsules based on cyclodextrin polymers with redox or light switching properties. Nano Res. 2016, 9, 2070–2078. [Google Scholar] [CrossRef]
- Li, H.; Tong, W.; Gao, C. Photo-responsive polyethyleneimine microcapsules cross-linked by ortho-nitrobenzyl derivatives. J. Colloid Interface Sci. 2016, 463, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Margerum, J.D.; Miller, L.J.; Saito, E.; Brown, M.S.; Mosher, H.S.; Hardwick, R. Phototropism of ortho-NitroBenzyl derivatives. J. Phys. Chem. 1962, 66, 2434–2438. [Google Scholar] [CrossRef]
- Kim, M.S.; Diamond, S.L. Photocleavage of o-nitrobenzyl ether derivatives for rapid biomedical release applications. Bioorg. Med. Chem. Lett. 2006, 16, 4007–4010. [Google Scholar] [CrossRef] [PubMed]
- Roggan, A.; Friebel, M.; Do, K.; Hahn, A.; Mu, G. Optical properties of circulating human blood in the wavelength range 400–2500 nm. J. Biomed. Opt. 1999, 4, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Radt, B.; Smith, T.A.; Caruso, F. Optically addressable nanostructured capsules. Adv. Mater. 2004, 16, 2184–2189. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Muñoz Javier, A.; Kreft, O.; Köhler, K.; Piera Alberola, A.; Möhwald, H.; Parak, W.J.; Sukhorukov, G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed. 2006, 45, 4612–4617. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Karageorgiev, P.; Bédard, M.F.; Sukhorukov, G.B.; Möhwald, H. Reversibly permeable nanomembranes of polymeric microcapsules. J. Am. Chem. Soc. 2008, 130, 11572–11573. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Takai, M. Bioinspired interface for nanobiodevices based on phospholipid polymer chemistry. J. R. Soc. Interface 2009, 6, S279–S291. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, A.; Marchesano, V.; Carregal-Romero, S.; Intartaglia, D.; Parak, W.J.; Tortiglione, C. Control of Wnt/β-catenin signaling pathway in vivo via light responsive capsules. ACS Nano 2016, 10, 4828–4834. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Tuzar, Z.; Kratochvil, P. Block and graft copolymer micelles in solution. Adv. Colloid Interface Sci. 1976, 6, 201–232. [Google Scholar] [CrossRef]
- Koblenz, T.S.; Wassenaar, J.; Reek, J.N. Reactivity within a confined self-assembled nanospace. Chem. Soc. Rev. 2008, 37, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Serrano, J.L.; Piñol, M.; Oriol, L. Light responsive vesicles based on linear–dendritic block copolymers using azobenzene–aliphatic codendrons. Macromolecules 2013, 46, 5951–5960. [Google Scholar] [CrossRef]
- Blasco, E.; Schmidt, B.V.; Barner-Kowollik, C.; Piñol, M.; Oriol, L. A novel photoresponsive azobenzene-containing miktoarm star polymer: Self-assembly and photoresponse properties. Macromolecules 2014, 47, 3693–3700. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V.; Suzdaltseva, Y.G.; Alakhov, V.Y. Water-soluble block polycations as carriers for oligonucleotide delivery. Bioconjug. Chem. 1995, 6, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, P.; Xu, H.; Wang, Z.; Zhang, X.; Kabanov, A.V. Photocontrolled self-assembly and disassembly of block ionomer complex vesicles: A facile approach toward supramolecular polymer nanocontainers. Langmuir 2009, 26, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. The carrier potential of liposomes in biology and medicine. N. Engl. J. Med. 1976, 295, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Puri, A. Phototriggerable liposomes: Current research and future perspectives. Pharmaceutics 2013, 6, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. J. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Shum, P.; Kim, J.M.; Thompson, D.H. Phototriggering of liposomal drug delivery systems. Adv. Drug Deliv. Rev. 2001, 53, 273–284. [Google Scholar] [CrossRef]
- Leung, S.J.; Romanowski, M. Light-activated content release from liposomes. Theranostics 2012, 2, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Bondurant, B.; Mueller, A.; O’Brien, D.F. Photoinitiated destabilization of sterically stabilized liposomes. Biochim. Biophys. Acta 2001, 1511, 113–122. [Google Scholar] [CrossRef]
- Thompson, D.H.; Gerasimov, O.V.; Wheeler, J.J.; Rui, Y.; Anderson, V.C. Triggerable plasmalogen liposomes: Improvement of system efficiency. Biochim. Biophys. Acta Biomembr. 1996, 1279, 25–34. [Google Scholar] [CrossRef]
- Luo, D.; Li, N.; Carter, K.A.; Lin, C.; Geng, J.; Shao, S.; Huang, W.C.; Qin, Y.; Atilla-Gokcumen, G.E.; Lovell, J.F. Rapid Light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin–phospholipids. Small 2016, 12, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Sine, J.; Urban, C.; Thayer, D.; Charron, H.; Valim, N.; Tata, D.B.; Schiff, R.; Blumenthal, R.; Joshi, A.; Puri, A. Photo activation of HPPH encapsulated in “Pocket” liposomes triggers multiple drug release and tumor cell killing in mouse breast cancer xenografts. Int. J. Nanomed. 2015, 10, 125–145. [Google Scholar]
- Bisby, R.H.; Mead, C.; Morgan, C.G. Wavelength-programmed solute release from photosensitive liposomes. Biochem. Biophys. Res. Commun. 2000, 276, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.K.; Phoeung, T.; Rousseau, P.A.; Rydzek, G.; Zhang, Q.; Bazuin, C.G.; Lafleur, M. Nonphospholipid fluid liposomes with switchable photocontrolled release. Langmuir 2014, 30, 10818–10825. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Fang, R.H.; Chen, Y.W.; Liao, B.J.; Chen, I.W.; Chen, S.Y. Photoresponsive protein–graphene–protein hybrid capsules with dual targeted heat-triggered drug delivery approach for enhanced tumor therapy. Adv. Funct. Mater. 2014, 24, 4144–4155. [Google Scholar] [CrossRef]
- Kurapati, R.; Raichur, A.M. Near-infrared light-responsive graphene oxide composite multilayer capsules: A novel route for remote controlled drug delivery. Chem. Commun. 2013, 49, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Smets, J.; Fernández-Prieto, S.; Underiner, T.L.; Wos, J.A.; Huhn, W.E.; Frederick, H.A.; Giamberini, M.; Tylkowski, B. Encapsulates. U.S. Patent 20130039962, 14 February 2013. [Google Scholar]
- Giamberini, M.; Fernandez Prieto, S.; Tylkowski, B. Microencapsulation: Innovative Applications; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2015. [Google Scholar]
- Tylkowski, B.; Tsibranska, I. Overview of main techniques used for membrane characterization. J. Chem. Technol. Metall. 2015, 50, 3–12. [Google Scholar]








| Capsules properties | Method |
|---|---|
| Capsule shape and size | Optical microscopy |
| Dynamic Light Scattering (DLS) | |
| Particle size analyzer | |
| Capsule shape, size and surface/cross-section morphology | Environmental/Scanning Electron Microscopy (ESEM/SEM) |
| Transmission Electron Microscopy (TEM) | |
| Capsule surface physical properties | Atomic force microscopy (AFM) |
| Contact angle measurement (CA) | |
| Nanoindentation | |
| Capsule surface chemical properties | SEM + X-ray microanalysis (EDS) |
| X-ray photoelectron spectroscopy (XPS) | |
| Nuclear magnetic resonance spectroscopy (NMR) | |
| Attenuated total reflectance infrared spectroscopy (ATR-IR) | |
| Thermodynamic properties of shell and/or encapsulated materials | Differential scanning calorimetry (DSC) |
| Thermogravimetry (TG) | |
| Active material stability and release | Ultraviolet-visible spectrophotometry (UV–Vis) |
| Gas chromatography–mass spectrometry (GC–MS) | |
| High-performance liquid chromatography (HPLC) | |
| Spectrofluorimetry | |
| Olfactive Evaluation |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2017, 9, 8. https://doi.org/10.3390/polym9010008
Marturano V, Cerruti P, Giamberini M, Tylkowski B, Ambrogi V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers. 2017; 9(1):8. https://doi.org/10.3390/polym9010008
Chicago/Turabian StyleMarturano, Valentina, Pierfrancesco Cerruti, Marta Giamberini, Bartosz Tylkowski, and Veronica Ambrogi. 2017. "Light-Responsive Polymer Micro- and Nano-Capsules" Polymers 9, no. 1: 8. https://doi.org/10.3390/polym9010008