Rapid and Effective Removal of Cu2+ from Aqueous Solution Using Novel Chitosan and Laponite-Based Nanocomposite as Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbent
2.3. Characterization
2.4. Adsorption Experiments
2.5. Desorption Experiments
2.6. Model to Experimental Data
3. Results and Discussion
3.1. Preparation and Characterization of Adsorbents
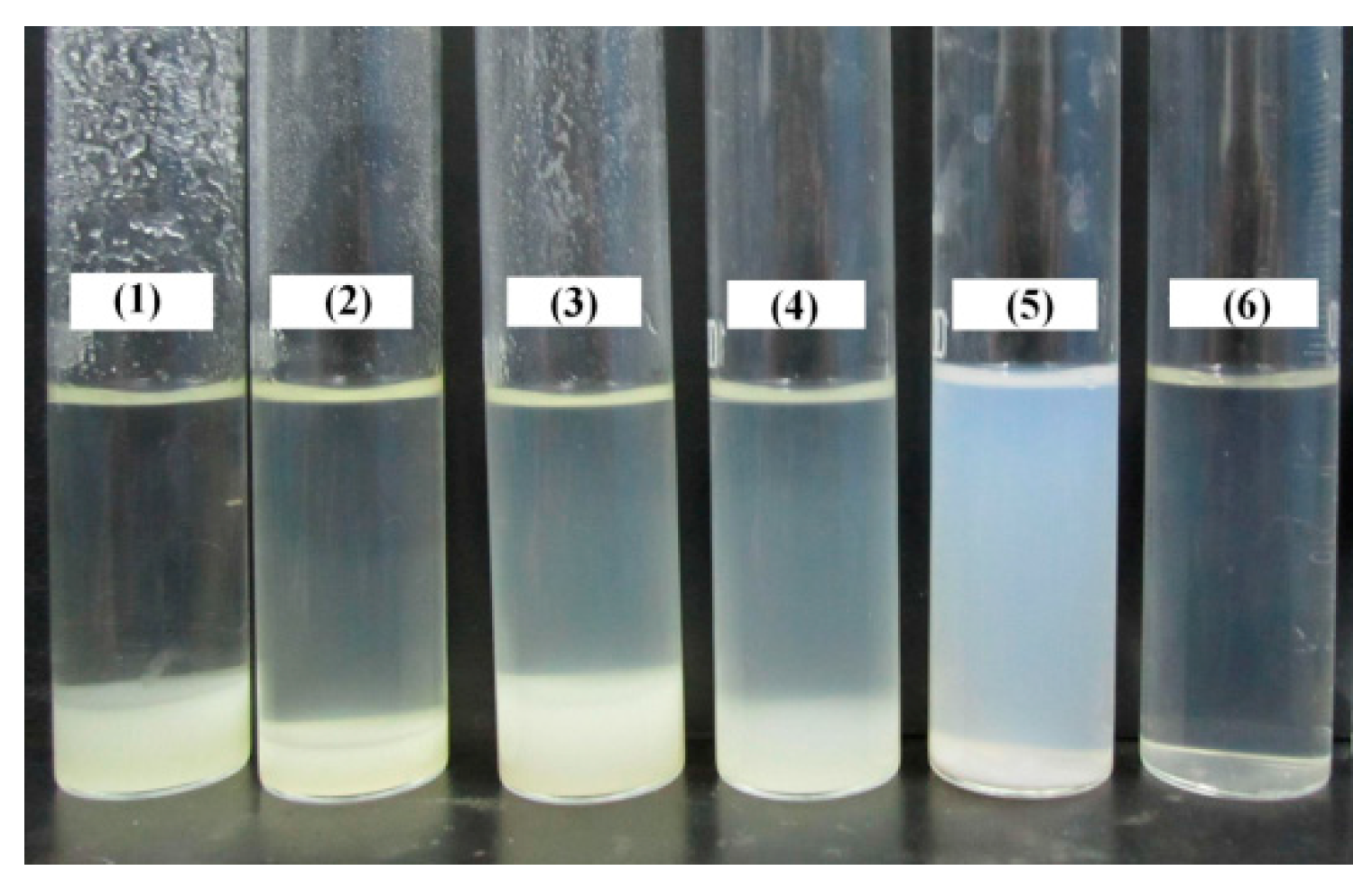
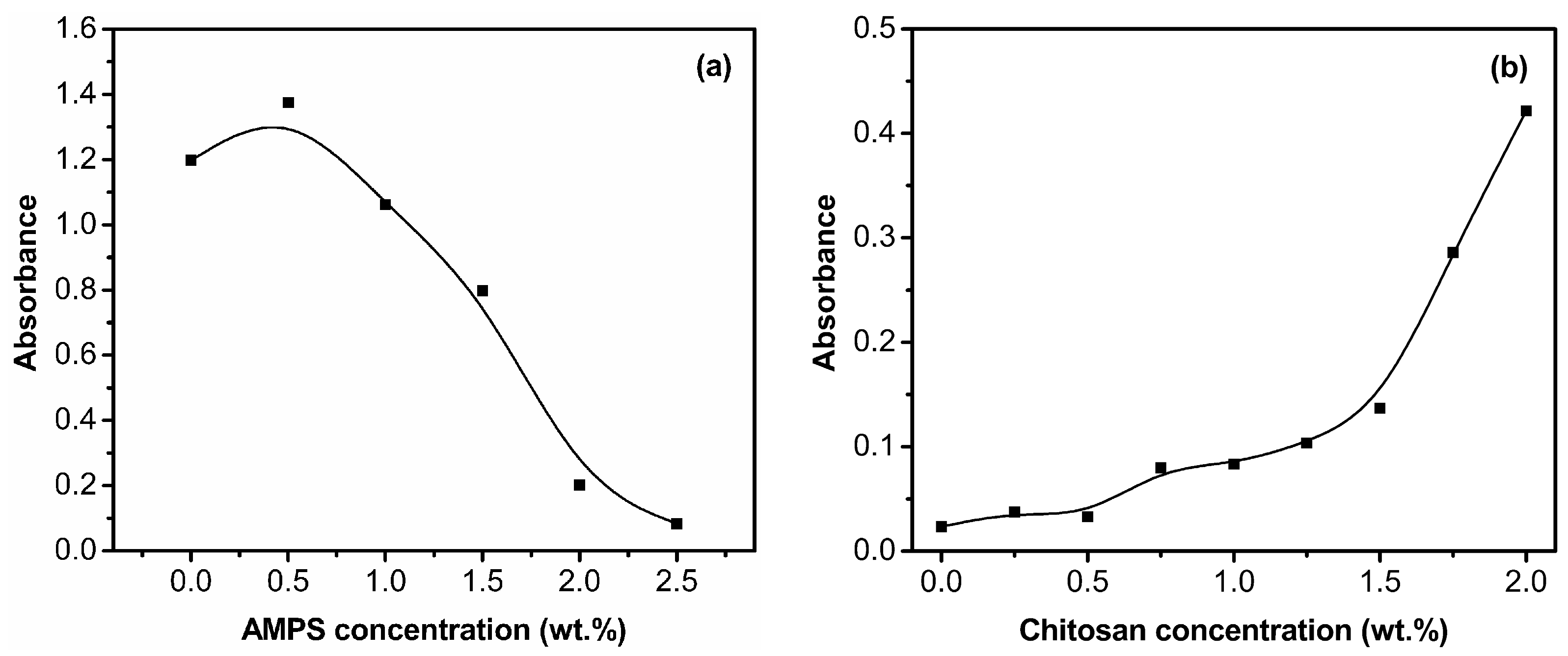
3.1.1. Effects of AMPS and Chitosan Concentrations on the Dispersion Property of Laponite
3.1.2. FTIR Analysis of Four Samples
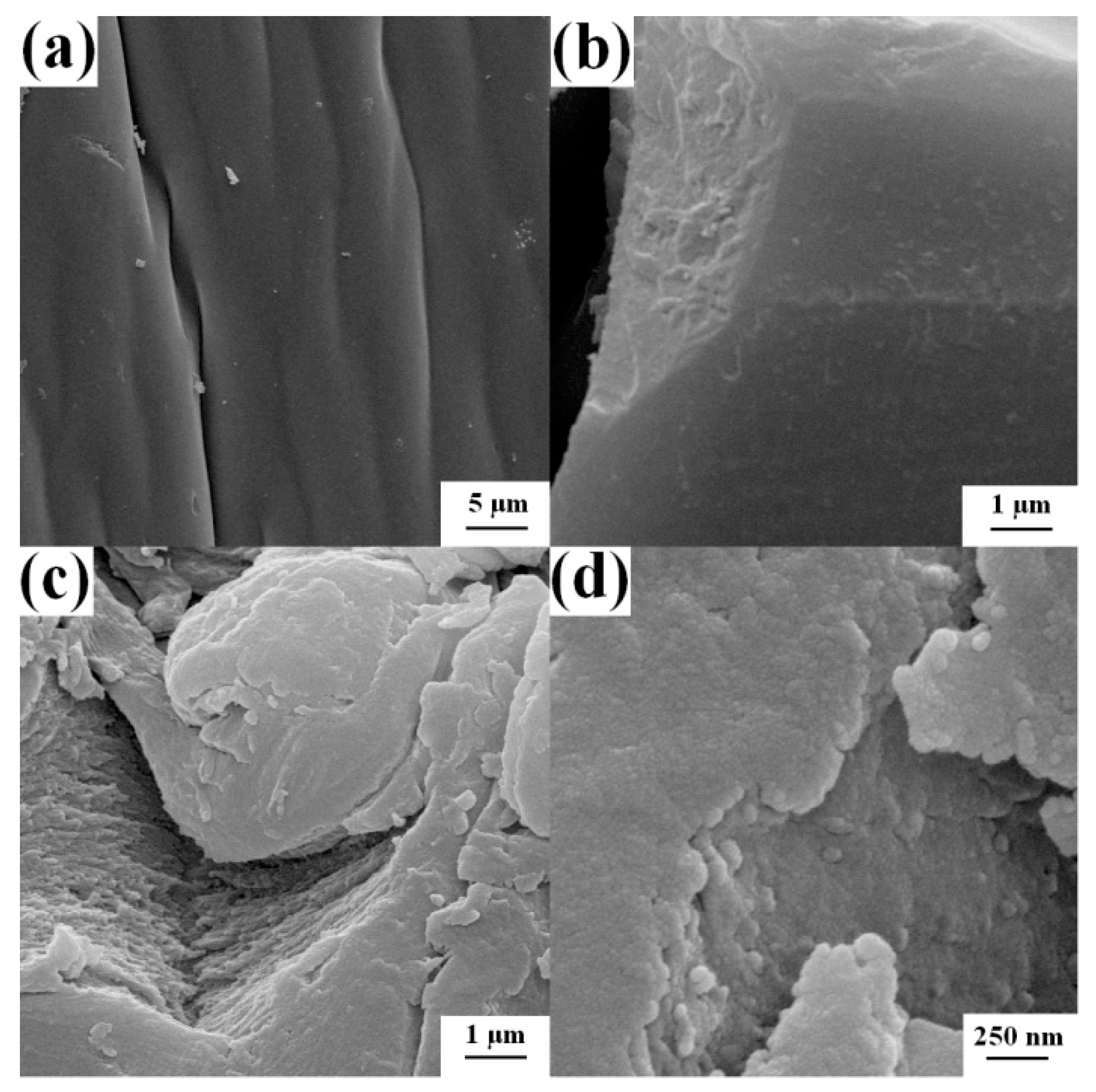
3.1.3. SEM Analysis of Four Samples
3.1.4. BET Analysis of Four Samples
3.2. Adsorption Studies
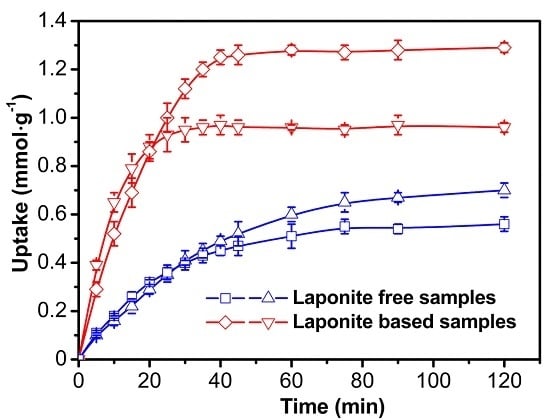
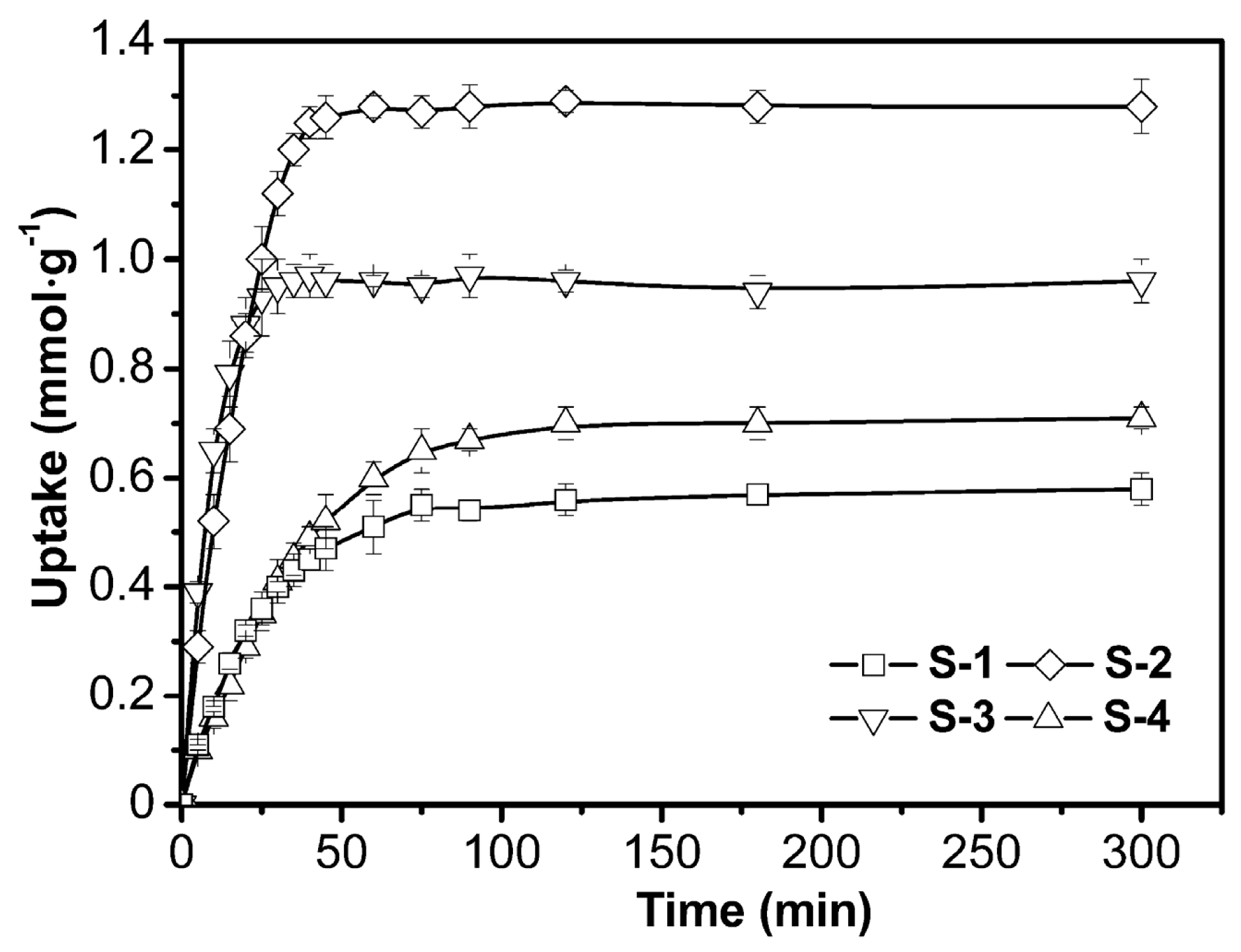
3.2.1. Adsorption Kinetics
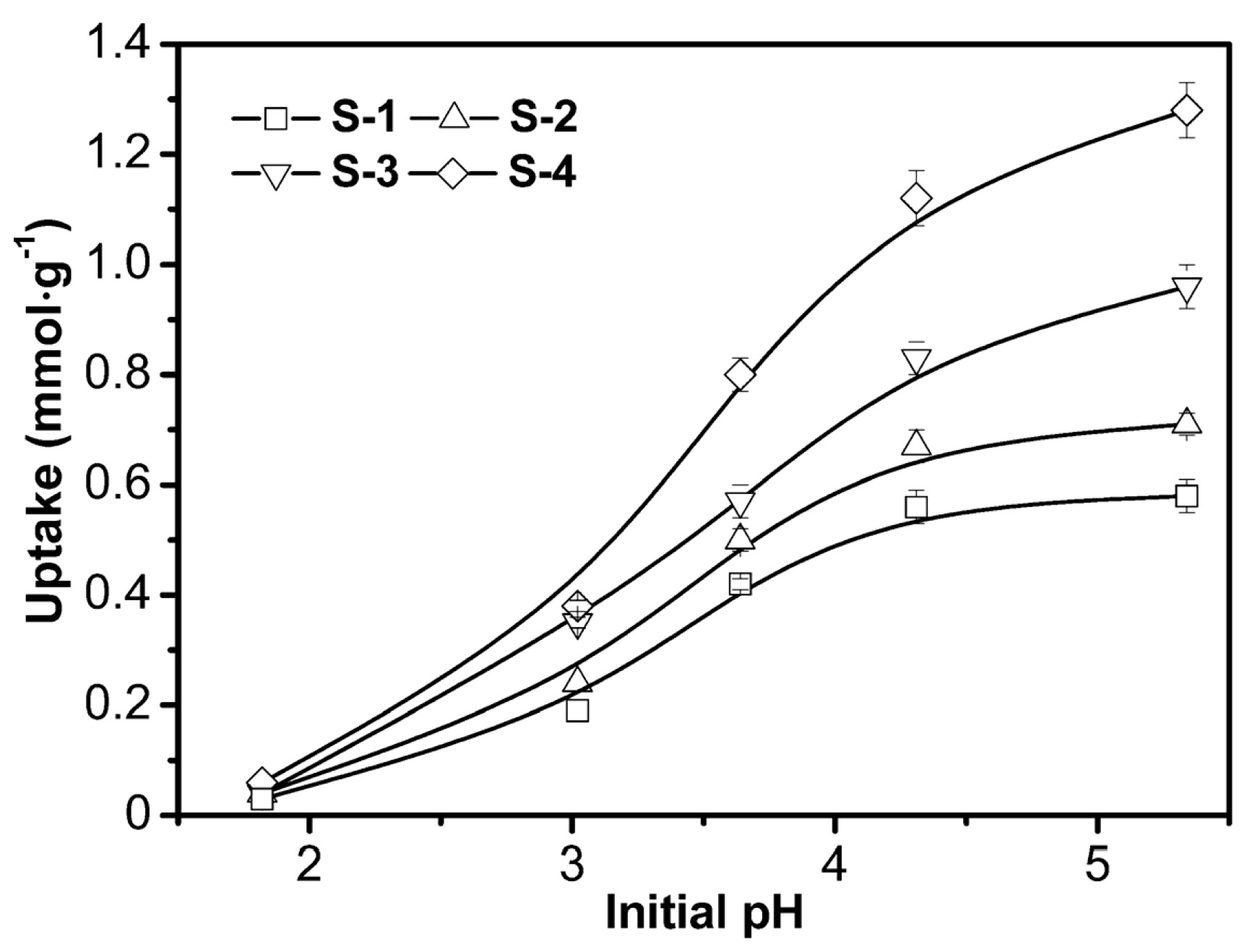
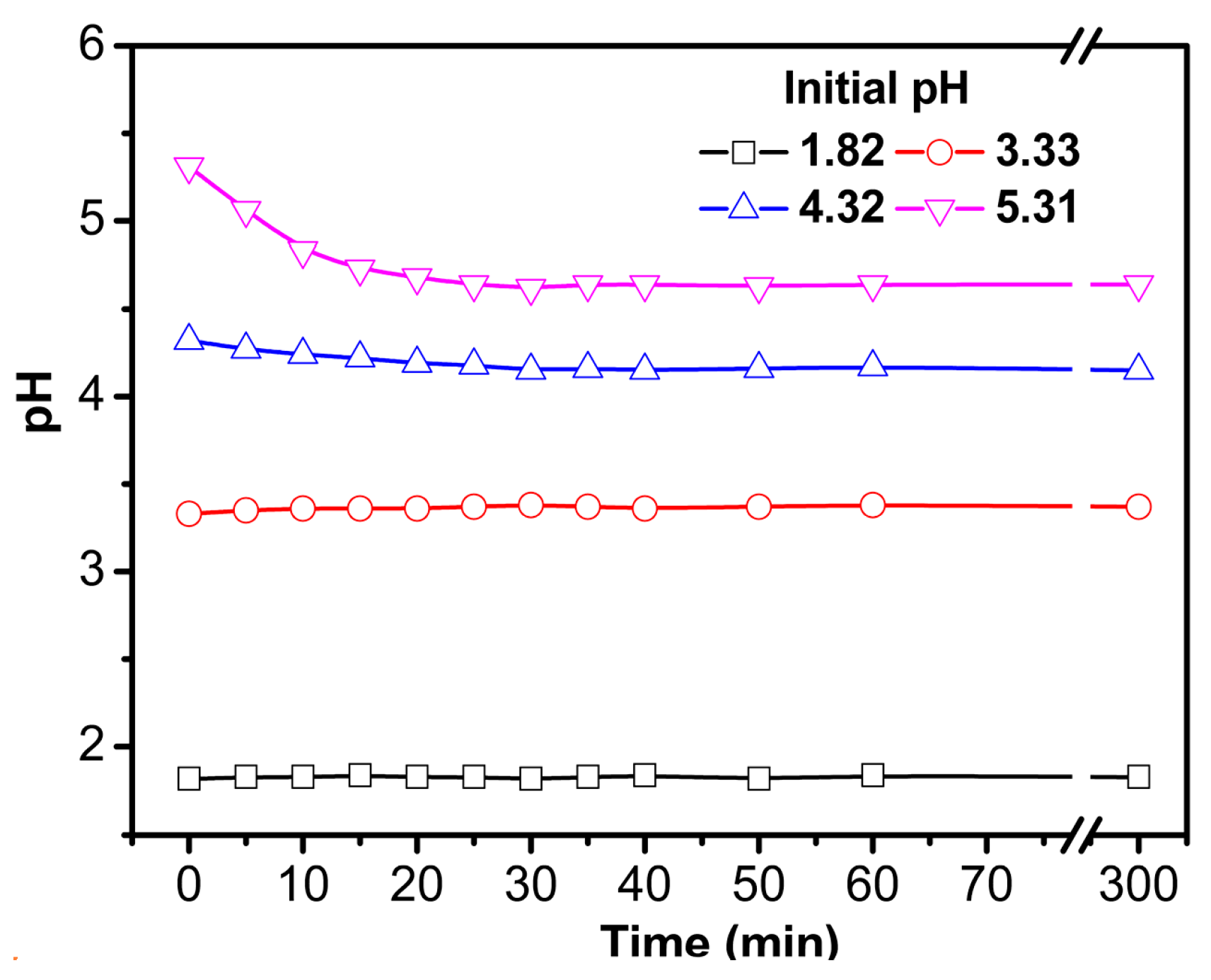
3.2.2. Effect of pH
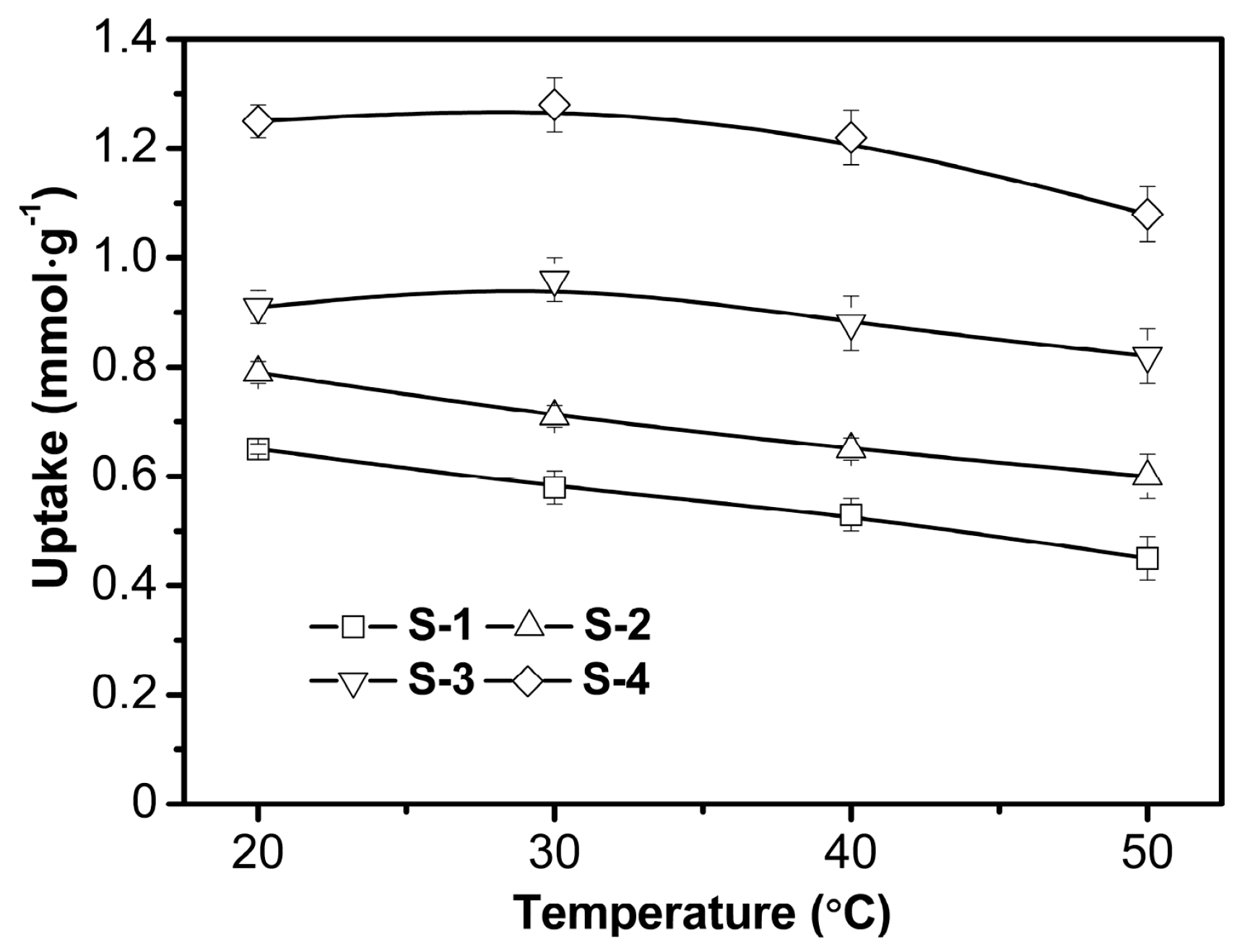
3.2.3. Effect of Temperature
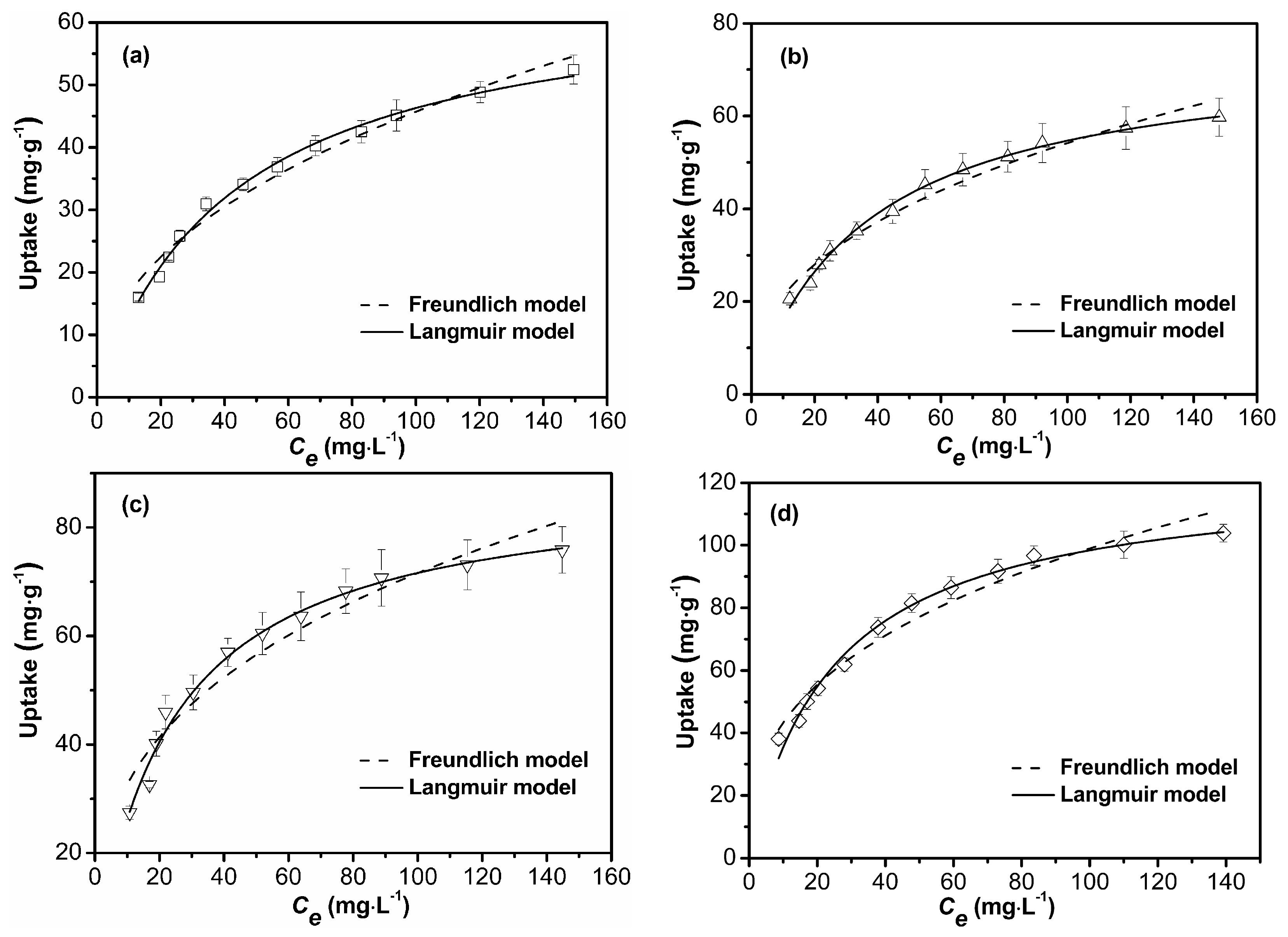
3.2.4. Adsorption Isotherm
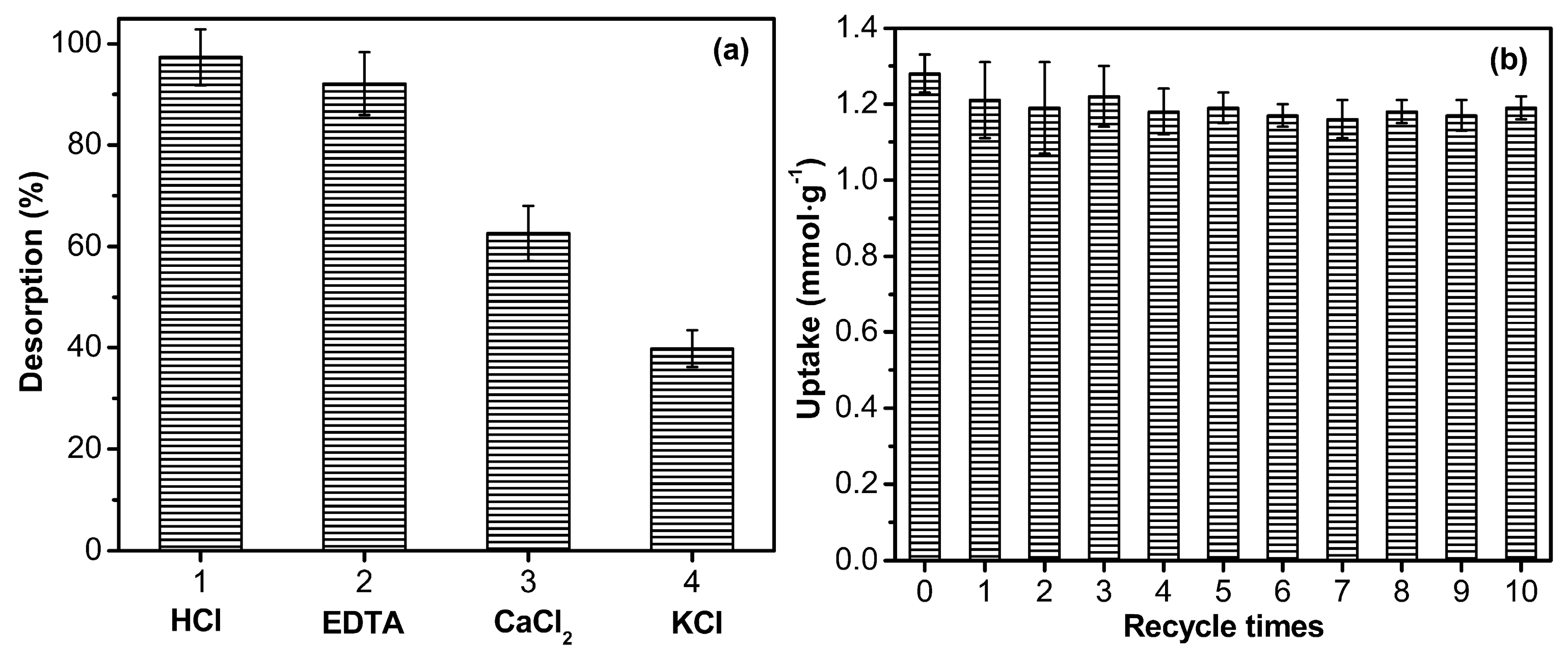
3.3. Desorption Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Phetphaisit, C.W.; Yuanyang, S.; Chaiyasith, W.C. Polyacrylamido-2-methyl-1-propane sulfonic acid-grafted-natural rubber as bio-adsorbent for heavy metal removal from aqueous standard solution and industrial wastewater. J. Hazard. Mater. 2016, 301, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tan, Y.; Che, Y.; Xin, H. Novel complex gel beads composed of hydrolyzed polyacrylamide and chitosan: An effective adsorbent for the removal of heavy metal from aqueous solution. Bioresour. Technol. 2010, 101, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Fan, J.; Zhang, J.; Kang, Y.; Liu, H.; Jiang, L.; Zhang, C. Sorption heavy metal ions by activated carbons with well-developed microporosity and amino groups derived from Phragmites australis by ammonium phosphates activation. J. Taiwan Inst. Chem. Eng. 2016, 58, 290–296. [Google Scholar] [CrossRef]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Chae, M.Y. Novel type of alginate gel-based adsorbents for heavy metal removal. J. Chem. Technol. Biotechnol. 2004, 79, 1080–1083. [Google Scholar] [CrossRef]
- Kuang, W.; Tan, Y.B.; Fu, L.H. Adsorption kinetics and adsorption isotherm studies of chromium from aqueous solutions by HPAM-chitosan gel beads. Desalin. Water Treat. 2012, 45, 222–228. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yue, Q.; Gao, B. Adsorption kinetics and desorption of Cu(II) and Zn(II) from aqueous solution onto humic acid. J. Hazard. Mater. 2010, 178, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Ying, D.; Li, K.; Wang, Y.; Jia, J. Fast removal of copper ions from aqueous solution using an eco–friendly fibrous adsorbent. Chemosphere 2016, 161, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.-H.; Kim, J.-O.; Cho, D.-W.; Kumar, R.; Baek, S.H.; Kurade, M.B.; Jeon, B.-H. Adsorption of As(III), As(V) and Cu(II) on zirconium oxide immobilized alginate beads in aqueous phase. Chemosphere 2016, 160, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.F.; Lebrun, L. Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. J. Membr. Sci. 2008, 307, 249–259. [Google Scholar] [CrossRef]
- Sakai, H.; Matsuoka, S.; Zinchenko, A.A.; Murata, S. Removal of heavy metal ions from aqueous solutions by complexation with DNA and precipitation with cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 210–214. [Google Scholar] [CrossRef]
- Zahri, N.A.M.; Jamil, S.; Abdullah, L.C.; Yaw, T.C.S.; Mobarekeh, M.N.; Huey, S.J.; Rapeia, N.S.M. Improved method for preparation of amidoxime modified poly(acrylonitrile-co-acrylic acid): Characterizations and adsorption case study. Polymers 2015, 7, 1205–1220. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Papelis, C.; Cath, T.Y.; Xu, P. Sorption of metals and metalloids from reverse osmosis concentrate on drinking water treatment solids. Sep. Purif. Technol. 2014, 134, 37–45. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.W.; Teong, L.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Mi, H.; Jiang, Z.G.; Kong, J. Hydrophobic poly(ionic liquid) for highly effective separation of methyl blue and chromium ions from water. Polymers 2013, 5, 1203–1214. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, W.J.D.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodex-trin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Fang, Z.; Zhang, X.; Zhang, B. Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan (chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. Aiche J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Uzunoğlu, D.; Özer, A. Adsorption of hazardous heavy metal copper(II) from aqueous effluents onto waste material fish (Dicentrarchus labrax) scales: Optimization, equilibrium, kinetics, thermodynamic, and characterization studies. Desalin. Water Treat. 2015, 57, 22794–22798. [Google Scholar] [CrossRef]
- Kim, N.; Park, M.; Park, D. A new efficient forest biowaste as biosorbent for removal of cationic heavy metals. Bioresour. Technol. 2015, 175, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.; Sarkar, A.K.; Pal, S. Rapid adsorptive removal of toxic Pb2+ ion from aqueous solution using recyclable, biodegradable nanocomposite derived from templated partially hydrolyzed xanthan gum and nanosilica. Bioresour. Technol. 2014, 170, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid and high capacity adsorption of heavy metals by Fe3O4/montmorillonite nanocomposite using response surface methodology: Preparation, characterization, optimization, equilibrium isotherms, and adsorption kinetics study. J. Taiwan Inst. Chem. Eng. 2015, 49, 192–198. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Pinto, M.D.E.; Goncalves, R.G.L.; dos Santos, R.M.M.; Araujo, E.A.; Perotti, G.F.; Macedo, R.D.; Bizeto, M.A.; Constantino, V.R.L.; Pinto, F.G.; Tronto, J. Mesoporous carbon derived from a biopolymer and a clay: Preparation, characterization and application for an organochlorine pesticide adsorption. Microporous Mesoporous Mater. 2016, 225, 342–354. [Google Scholar] [CrossRef]
- Bandeira, L.C.; Calefi, P.S.; Ciuffi, K.J.; de Faria, E.H.; Nassar, E.J.; Vicente, M.A.; Trujillano, R. Preparation of composites of laponite with alginate and alginic acid polysaccharides. Polym. Int. 2012, 61, 1170–1176. [Google Scholar] [CrossRef]
- Hill, E.H.; Zhang, Y.; Whitten, D.G. Aggregation of cationic p-phenylene ethynylenes on Laponite clay in aqueous dispersions and solid films. J. Colloid Interface Sci. 2015, 449, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Manilo, M.; Lebovka, N.; Barany, S. Characterization of the electric double layers of multi-walled carbon nanotubes, laponite and nanotube + laponite hybrids in aqueous suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 211–216. [Google Scholar] [CrossRef]
- Chen, P.; Xu, S.; Wu, R.; Wang, J.; Gu, R.; Du, J. A transparent Laponite polymer nanocomposite hydrogel synthesis via in-situ copolymerization of two ionic monomers. Appl. Clay Sci. 2013, 72, 196–200. [Google Scholar] [CrossRef]
- Zhou, H.S.; Yang, L.Q.; Li, H.J.; Gong, H.J.; Cheng, L.Z.; Zheng, H.S.; Zhang, L.M.; Lan, Y.Q. Downregulation of VEGF mRNA expression by triamcinolone acetonide acetate-loaded chitosan derivative nanoparticles in human retinal pigment epithelial cells. Int. J. Nanomed. 2012, 7, 4649–4660. [Google Scholar]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, E.O.; Alayande, O.S.; Msagati, T.A.M. Synthesis and characterization of biopolymeric chitosan derived from land snail shells and its potential for Pb2+ removal from aqueous solution. Materials 2015, 8, 8630–8640. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Che, Y.; Ma, Q. Fabrication and properties of superabsorbent complex gel beads composed of hydrolyzed polyacrylamide and chitosan. J. Appl. Polym. Sci. 2010, 116, 3338–3345. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Y.F.; Nie, W.Y.; Song, L.Y.; Zhang, T. Preparation of uniform magnetic chitosan microcapsules and their application in adsorbing copper ion(II) and chromium ion(III). Ind. Eng. Chem. Res. 2012, 51, 14099–14106. [Google Scholar] [CrossRef]
- Tao, X.; Li, K.; Yan, H.; Yang, H.; Li, A.M. Simultaneous removal of acid green 25 and mercury ions from aqueous solutions using glutamine modified chitosan magnetic composite microspheres. Environ. Pollut. 2016, 209, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Maity, A.; Ray, S.S. Synthesis of co-polymer-grafted gum karaya and silica hybrid organic-inorganic hydrogel nanocomposite for the highly effective removal of methylene blue. Chem. Eng. J. 2015, 279, 166–179. [Google Scholar] [CrossRef]
- Pal, S.; Patra, A.S.; Ghorai, S.; Sarkar, A.K.; Mahato, V.; Sarkar, S.; Singh, R.P. Efficient and rapid adsorption characteristics of templating modified guar gum and silica nanocomposite toward removal of toxic reactive blue and Congo red dyes. Bioresour. Technol. 2015, 191, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Podder, M.S.; Majumder, C.B. Corynebacterium glutamicum MTCC 2745 immobilized on granular activated carbon/MnFe2O4 composite: A novel biosorbent for removal of As(III) and As(V) ions. Spectroc. Acta Pt. A Mol. Biomol. Spectrosc. 2016, 168, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Nouri, L.; Ghodbane, I.; Hamdaoui, O.; Chiha, M. Batch sorption dynamics and equilibrium for the removal of cadmium ions from aqueous phase using wheat bran. J. Hazard. Mater. 2007, 149, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Zhu, G.Y.; Yu, W.B.; Wu, H.K.; Ji, H.B.; Shi, H.X.; She, Y.B.; Zheng, Y.F. Fast adsorption of p-nitrophenol from aqueous solution using β-cyclodextrin grafted silica gel. Appl. Surf. Sci. 2015, 356, 1155–1167. [Google Scholar] [CrossRef]
- Lin, S.-H.; Juang, R.-S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]

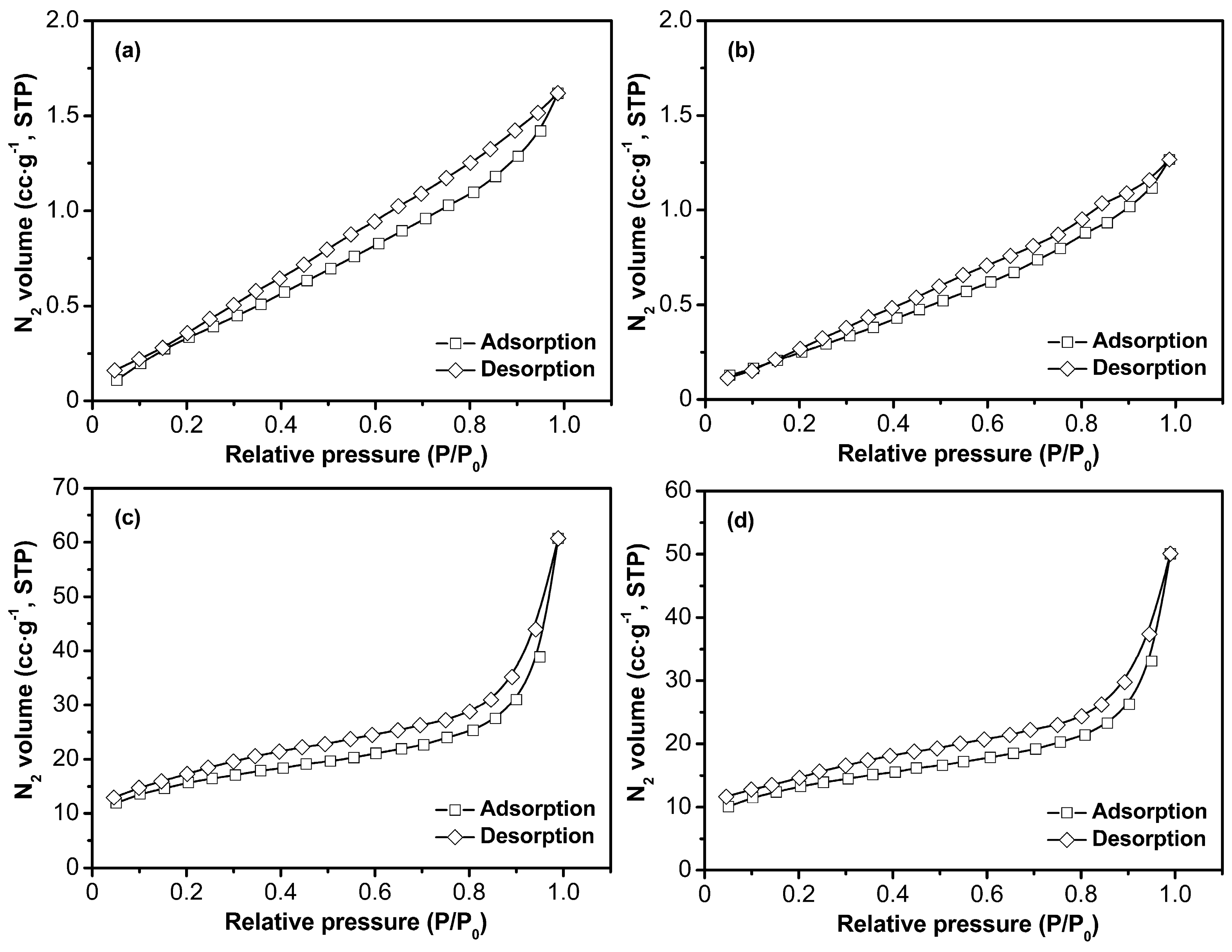
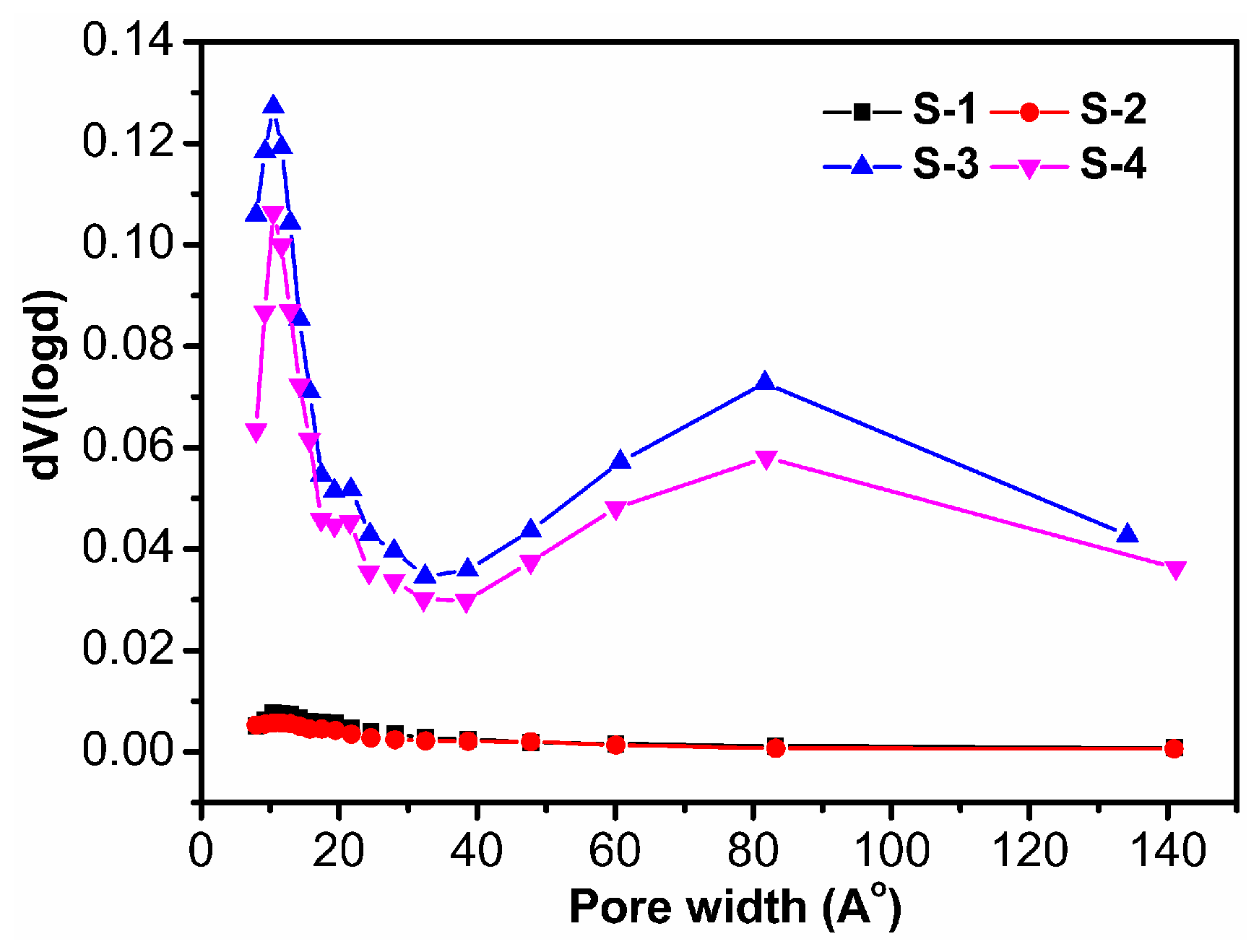
| Sample | AM (g) | AA (g) | AMPS (g) | MBA (g) | H2O (g) | Laponite (g) | Chitosan (g) | KPS (g) | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| S-1 | 5.0 | 2.5 | 2.5 | 0.03 | 90.0 | 0 | 0 | 0.05 | 96.9 |
| S-2 | 5.0 | 2.5 | 2.5 | 0.03 | 90.0 | 0 | 0.5 | 0.05 | 96.3 |
| S-3 | 5.0 | 2.5 | 2.5 | 0.03 | 90.0 | 0.5 | 0 | 0.05 | 98.1 |
| S-4 | 5.0 | 2.5 | 2.5 | 0.03 | 90.0 | 0.5 | 0.5 | 0.05 | 98.6 |
| Element | Atomic % | Atomic % |
|---|---|---|
| C | 55.30 | 56.04 |
| N | 11.63 | 11.55 |
| O | 29.91 | 28.42 |
| S | 1.72 | 1.98 |
| Mg | 0.61 | 0.85 |
| Si | 0.83 | 1.16 |
| Total | 100.00 | 100.00 |
| Sample | ABET (m2·g−1) | Vtotal (cm3·g−1) | D (nm) |
|---|---|---|---|
| S-1 | 1.89 | 0.00249 | 5.27 |
| S-2 | 1.19 | 0.00195 | 6.55 |
| S-3 | 52.86 | 0.09350 | 7.08 |
| S-4 | 44.69 | 0.07715 | 6.90 |
| Model | Coefficient | S-1 | S-2 | S-3 | S-4 |
|---|---|---|---|---|---|
| Pseudo-first | K1 | 0.040 | 0.028 | 0.113 | 0.057 |
| qe | 0.57 | 0.72 | 0.97 | 1.31 | |
| R2 | 0.9985 | 0.9967 | 0.9970 | 0.9886 | |
| Pseudo-second | K2 | 0.087 | 0.042 | 0.523 | 0.121 |
| qe | 0.62 | 0.81 | 0.98 | 1.34 | |
| R2 | 0.9970 | 0.9886 | 0.9991 | 0.9953 |
| Model | Coefficient | S-1 | S-2 | S-3 | S-4 |
|---|---|---|---|---|---|
| Langmuir | qmax | 66.16 | 74.82 | 88.85 | 122.76 |
| b | 0.023 | 0.027 | 0.042 | 0.040 | |
| R2 | 0.9936 | 0.9947 | 0.9874 | 0.9892 | |
| Freundlich | Kf | 5.99 | 8.22 | 14.91 | 18.88 |
| n | 0.44 | 0.41 | 0.34 | 0.36 | |
| R2 | 0.9741 | 0.9709 | 0.9359 | 0.9614 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Cao, H.; Zhu, Y.; Wang, S.; Qian, D.; Chen, G.; Sun, M.; Huang, W. Rapid and Effective Removal of Cu2+ from Aqueous Solution Using Novel Chitosan and Laponite-Based Nanocomposite as Adsorbent. Polymers 2017, 9, 5. https://doi.org/10.3390/polym9010005
Cao J, Cao H, Zhu Y, Wang S, Qian D, Chen G, Sun M, Huang W. Rapid and Effective Removal of Cu2+ from Aqueous Solution Using Novel Chitosan and Laponite-Based Nanocomposite as Adsorbent. Polymers. 2017; 9(1):5. https://doi.org/10.3390/polym9010005
Chicago/Turabian StyleCao, Jie, Han Cao, Yuejun Zhu, Shanshan Wang, Dingwei Qian, Guodong Chen, Mingbo Sun, and Weian Huang. 2017. "Rapid and Effective Removal of Cu2+ from Aqueous Solution Using Novel Chitosan and Laponite-Based Nanocomposite as Adsorbent" Polymers 9, no. 1: 5. https://doi.org/10.3390/polym9010005