Enhanced Anti-Ultraviolet and Thermal Stability of a Pesticide via Modification of a Volatile Organic Compound (VOC)-Free Vinyl-Silsesquioxane in Desert Areas
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Vinyl-SSO
2.3. Free Radical Polymerization of VS in Citral Oil in Water Emulsion
2.4. Characterization and Measurements
2.5. Mosquito Repellent Bioassays
2.6. Statistical Analysis
3. Results and Discussion
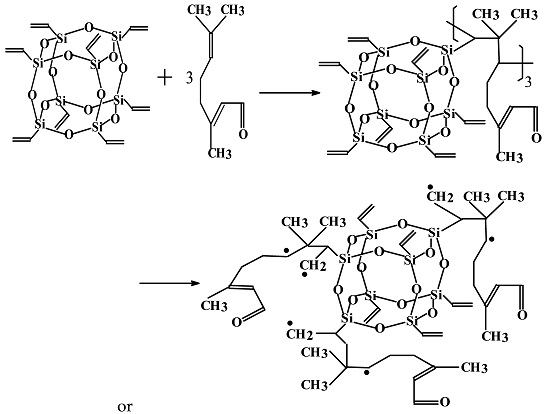
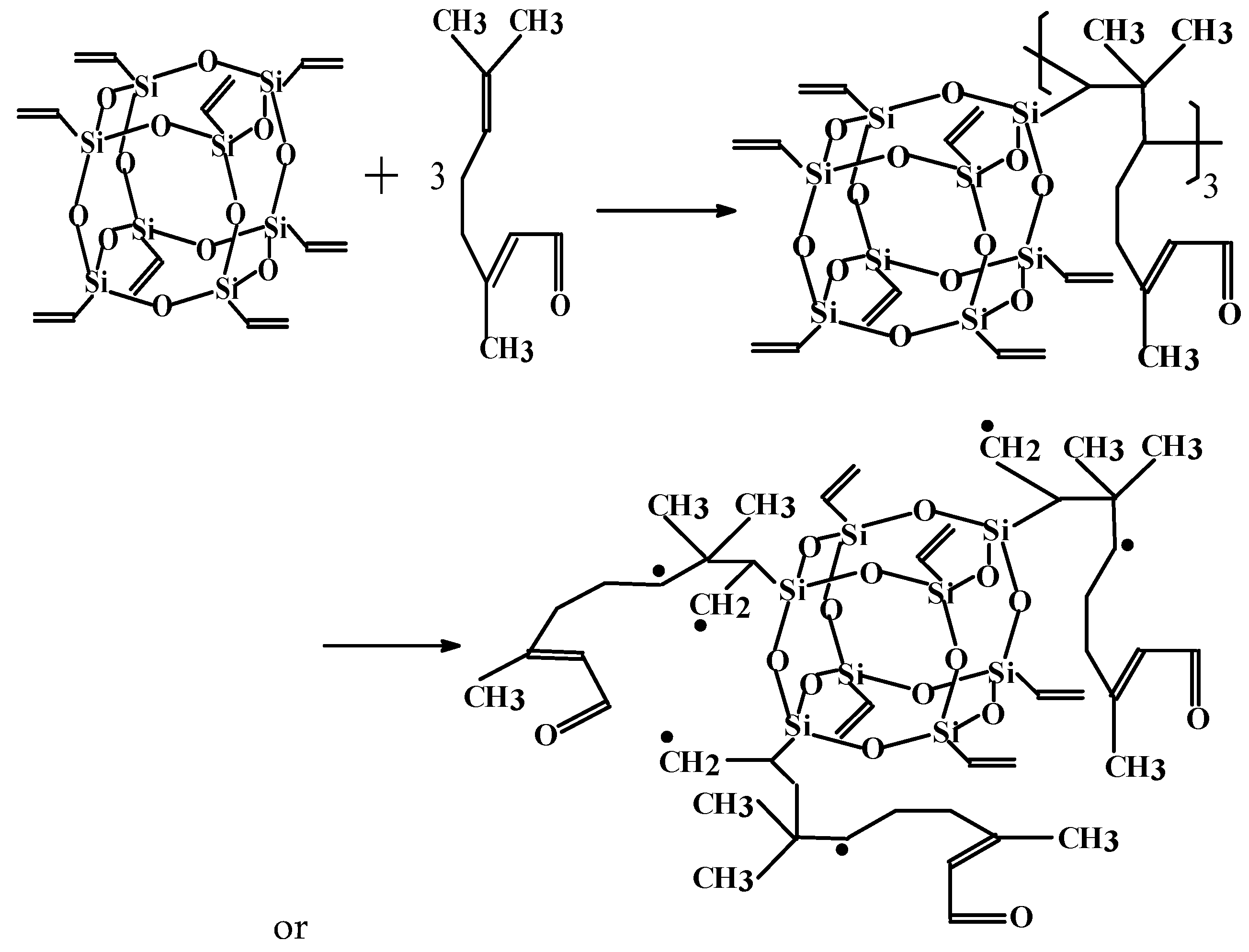
3.1. Determination and Validation of the Main Reaction
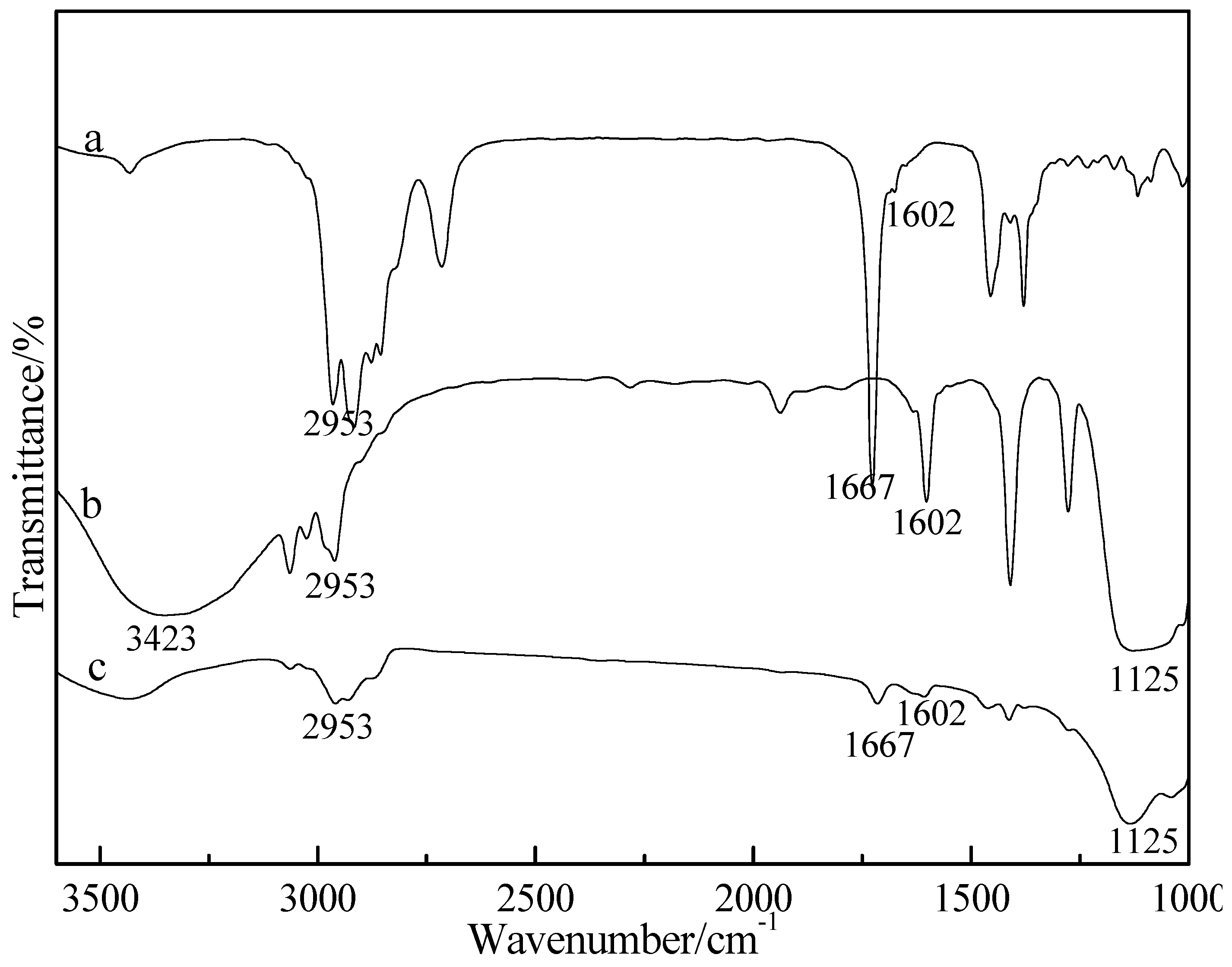
3.2. Characterization of FTIR Spectrum
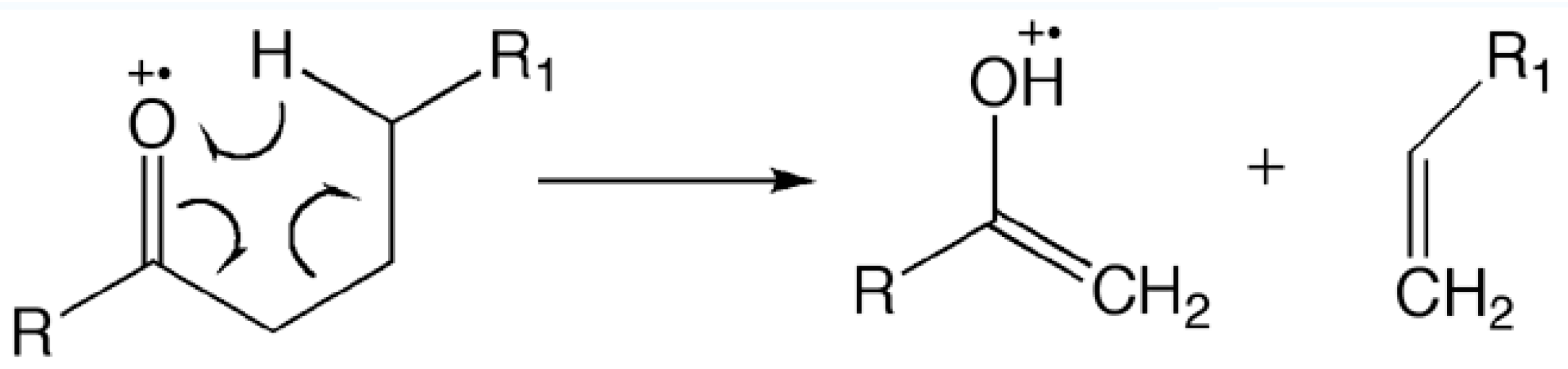
3.3. Characterization of Mass Spectrometry
3.4. Transparency in the UV-B Region
3.5. Thermogravimetric Analysis of VS-Citral
3.6. Repellent Effect for Citral and VS-Citral.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naters, W.V.; Carlson, J.R. Insects as chemosensors of humans and crops. Nature 2006, 444, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Trongtokit, Y.; Rongsriyam, Y.; Komalamisra, N.; Apiwathnasorn, C. Comparative repellency of thirty-eight essential oils against mosquito bites. Phytother. Res. 2005, 19, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Memon, G.Z.; Bhanger, M.I.; Akhtar, M. Adsorption of methyl parathion pesticide from water using watermelon peels as a low cost adsorbent. Chem. Eng. J. 2008, 138, 616–621. [Google Scholar] [CrossRef]
- Mitchell, D.L.; Greinert, R.; de Gruijl, F.R. Effects of chronic low-dose ultraviolet B radiation on DNA damage and repair in mouse skin. Cancer. Res. 1999, 59, 2875–2884. [Google Scholar] [PubMed]
- Lu, Y.; Lou, Y.; Yen, P.; Mitchell, D.; Huang, M.; Conney, A.H. Time course for early adaptive responses to ultraviolet B light in the epidermis of SKH-1 mice. Cancer. Res. 1999, 59, 4591–4602. [Google Scholar] [PubMed]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole). Parasitol. Res. 2013, 112, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Schneidmiller, R.G.; Hoover, D.R. Essential oils and their compositions as spatial repellents for pestiferous social wasps. Pest. Manag. Sci. 2013, 69, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakiset, A. Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Juntarajumnong, W.; Chandrapatya, A. Insecticidal activities of essential oils from fruits of litsea salicifolia roxb. ex Wall. Against sitophilus zeamais motschulsky and tribolium castaneum (Herbst). Pak. J. Zool. 2010, 42, 551–557. [Google Scholar]
- Plarre, R.; Poschko, M.; Prozell, S.; Frank, A.; Wohlgemuth, R.; Phillips, J.K. Effects of oil of cloves and citronellol, two commercially available repellents, against the webbing clothes moth Tineola bisselliella Hum (Lepidoptera: Tineidae). Anz. Schadlingskd. Pfl. 1997, 70, 45–50. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; You, H.; Tolbert, S.H.; Loy, D.A. Comparison of new periodic, mesoporous, hexylene-bridged polysilsesquioxanes with Pm3n symmetry versus sol-gel polymerized, hexylene-bridged gels. J. Non-Cryst. Solids 2014, 406, 139–143. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; Tolbert, S.H.; Li, Z.; Loy, D.A. Controlling nanostructure in periodic mesoporous hexylene-bridged polysilsesquioxanes. J. Non-Cryst. Solids 2015, 419, 6–11. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; Li, Z.; Loy, D.A. Influence of alkylene-bridging group length on mesostructure and porosity in cubic (Pm3n) periodic mesoporous bridged polysilsesquioxanes. J. Porous Mater. 2014, 21, 39–44. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; You, H.; Williams, R.J.J. Synthesis and characterization of a nanostructured photoluminescent silsesquioxane containing urea and dodecyl groups that can be patterned on carbon films. Eur. Polym. J. 2011, 47, 1526–1533. [Google Scholar] [CrossRef]
- Lin, D.R.; Zhao, Q.; Hu, L.J.; Xing, B.S. Synthesis and characterization of cubic mesoporous bridged polysilsesquioxane for removing organic pollutants from water. Chemosphere 2014, 103, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.C.; Shea, K.J. Organo-silica hybrid functional nanomaterials: How do organic bridging groups and silsesquioxane moieties work hand-in-hand? Chem. Soc. Rev. 2011, 40, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.J.; Moreau, J.; Loy, D.A.; Corriu, R.J.P.; Boury, B. Bridged Polysilsesquioxanes. Molecular-engineering nanostructured hybrid organic-inorganic materials. In Functional Hybrid Materials; Gómez-Romero, P., Sanchez, C., Eds.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Loy, D.A. Sol-gel processing of hybrid organic-inorganic materials based on polysilsesquioxanes. In Hybrid Materials: Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Lin, D.R.; Hu, L.J.; Xing, B.S.; You, H.; Loy, D.A. Mechanisms of competitive adsorption organic pollutants on hexylene-bridged polysilsesquioxane. Materials 2015, 8, 5806–5817. [Google Scholar] [CrossRef]
- Hergenrother, W.L.; Lin, C.J.; Hilton, A.S.; Hogan, T.E.; Brumbaugh, D.R. Reduction of volatile organic compound emission. I. Synthesis and characterization of alkoxy-modified silsesquioxane. J. Appl. Polym. Sci. 2010, 115, 79–90. [Google Scholar] [CrossRef]
- Wang, H.; Lin, D.; Wang, D.; Hu, L.; Huang, Y.; Liu, L.; Loy, D.A. Computational and experimental determinations of the UV adsorption of polyvinyl-silsesquioxane-silica and titanium dioxide hybrids. Bio-Med. Mater. Eng. 2014, 24, 651–657. [Google Scholar]
- Wang, H.; Liu, L.; Huang, Y.; Wang, D.; Hu, L.; Loy, D.A. Enhancement corrosion resistance of (γ-glycidyloxypropyl)-silsesquioxane-titanium dioxide films and its validation by gas molecule diffusion coefficients using molecular dynamics (MD) simulation. Polymers 2014, 6, 300–310. [Google Scholar] [CrossRef]
- Lichtenhan, J.D. Organic/Inorganic Hybrid Materials; Blum, F.D., Laine, R.M., Eds.; ACS, Division of Polymer Chemistry: Washington, DC, USA, 2003. [Google Scholar]
- Yang, B.; Li, J.; Wang, J.; Xu, H.; Guang, S.; Li, C. Poly(vinyl pyrrolidone-co-octavinyl polyhedral oligomeric silsesquioxane) hybrid nanocomposites: Preparation, thermal properties, and Tg improvement mechanism. J. Appl. Polym. Sci. 2009, 111, 2963–2969. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, C.; Liu, B.; Jiang, C.; Mu, J. Synthesis and thermal stability of hybrid polymers using UV photopolymerization based on polyhedral oligomeric silsesquioxanes. High Perform. Polym. 2012, 24, 274–281. [Google Scholar] [CrossRef]
- Seno, M.; Hasegawa, M.; Hirano, T.; Sato, T. Radical polymerization behavior of trimethoxy- vinylsilane. J. Polym. Sci. Pol. Chem. 2005, 43, 5864–5871. [Google Scholar] [CrossRef]
- Rzaev, Z.M.; Bryksina, L.V.; Kyazimov, S.K.; Sadykh-Zade, S.I. Radical copolymerization of maleic anhydride, styrene, and vinyltriethoxysilane. Vysokomol. Soedin. 1972, 14, 259–267. [Google Scholar] [CrossRef]
- Zhang, X.W.; Hu, L.J.; Sun, D.Z.; Zhao, W. Study of three-dimensional configurations of organic/inorganic hybrid nanostructural blocks: A quantum chemical investigation for cage structure of (γ-glycidoxypropyl)-silsesquioxanes. J. Mol. Struct. 2008, 872, 197–204. [Google Scholar] [CrossRef]
- Chen, X.D.; Wang, D.; Lin, D.R.; Zhang, Q.; Hu, L.J. Transparency of modified vinyl-silsesquioxane films and its validation by computing energy-band structure. J. Mater. Res. 2010, 25, 76–81. [Google Scholar] [CrossRef]
- Logan, J.G.; Stanczyk, N.M.; Hassanali, A.; Kemei, J.; Santana, A.E.G.; Ribeiro, K.A.L.; Pickett, J.A.; Mordue, A.J. Arm-in-cage testing of natural human-derived mosquito repellents. Malar. J. 2010. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.L.; Li, J.T.; Bernstein, D.I.; Hamilton, R.; Spector, S.L.; Tan, R.; Sicherer, S.; Golden, D.B.K.; Khan, D.A.; Nicklas, R.A.; et al. Allergy diagnostic testing: an updated practice parameter. Ann. Allerg. Asthma. Im. 2008, 100, S1–S148. [Google Scholar] [CrossRef]
- World Health Organization Pesticide Evaluation Scheme (WHOPES). Guidelines for Efficacy Testing of Mosquito Repellents for Human Skin; World Health Organization: Geneva, Switzerland, 2006; Available online: http://apps.who.int/iris/bitstream/10665/70072/1/WHO_HTM_NTD_WHOPES_2009.4_eng.pdf (accessed on 1 August 2016).
- Schreck, C.E. Techniques for the evaluation of insect repellents: a critical review. Annu. Rev. Entomol. 1977, 22, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Borzacchiello, A.; Netti, P.A. Isothermal and non-isothermal polymerization of methyl methacrylate in presence of multiple initiators. Chem. Eng. J. 2010, 162, 776–786. [Google Scholar] [CrossRef]
- Wang, B.; Ramirez, A.P.; Slade, J.J.; Morken, J.P. Enantioselective synthesis of (−)-sclerophytin A by a stereoconvergent epoxide hydrolysis. J. Am. Chem. Soc. 2010, 132, 16380–16382. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Masuda, H.; Ho, C.T. Formation mechanism of p-methylacetophenone from citral via a tert-alkoxy radical intermediate. J. Agric. Food. Chem. 2004, 52, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Vanbrugg, E. Free radical additions 2. Free radical cyclization of citronellol. Recl. Trav. Chim. Pay-B. 1968, 87, 1134–1136. [Google Scholar] [CrossRef]
- Erba, I.E.; Fasce, D.P.; Williams, R.J.J.; Erra-Balsells, R.; Fukuyama, Y.; Nonami, H. Poly-(silsesquioxanes) derived from the hydrolytic condensation of organotrialkoxysilanes containing hydroxyl groups. J. Organomet. Chem. 2003, 686, 42–51. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Sirtori, C. Decontamination of industrial wastewater containing pesticides by combining large-scale homogeneous solar photocatalysis and biological treatment. Chem. Eng. J. 2010, 160, 447–456. [Google Scholar] [CrossRef]
- Fasce, D.P.; Williams, R.J.J.; Erra-Balsells, R.; Ishikawa, Y.; Nonami, H. One-Step synthesis of polyhedral silsesquioxanes bearing bulky substituents: UV-MALDI-TOF and ESI-TOF mass spectrometry characterization of reaction products. Macromolecules 2001, 34, 3534–3539. [Google Scholar] [CrossRef]
- Nibbering, N.M. The McLafferty rearrangement: A personal recollection. J. Am. Soc. Mass Spectrom. 2004, 15, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.L. Focus in honor of Fred McLafferty, 2003 Distinguished Contribution awardee, for the discovery of the "McLafferty Rearrangement”. J. Am. Soc. Mass Spectrom. 2004, 15, 951–955. [Google Scholar] [CrossRef] [PubMed]

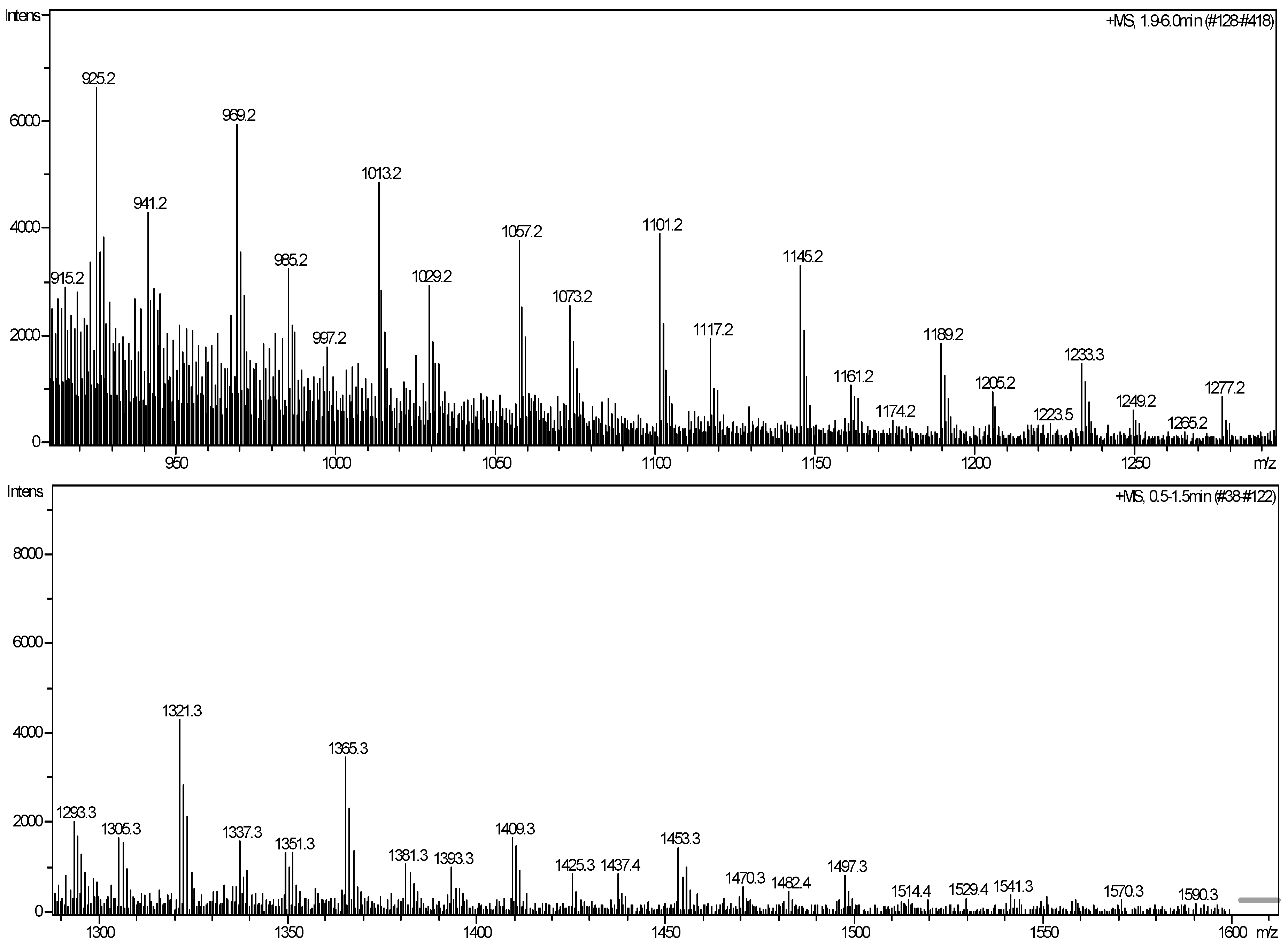


| m/z (exp.) | Assigned structure (+Na+、H+、K+) | m/z (pre.) |
|---|---|---|
| 925.2 | T5(OH)3(OCH3)2(C10H16O)3H+ | 926.4 |
| 941.2 | T5(OH)2(OCH3)3(C10H16O)3H+ | 940.5 |
| 969.2 | T6(C10H16O)3K+ | 970.5 |
| T6(OH)2(C10H16O)3Na+ | 972.5 | |
| T6(OH)4(C10H16O)3H+ | 968.5 | |
| 985.2 | T6(OH)4(C10H16O)3Na+ | 990.5 |
| T6(OH)6(C10H16O)3H+ | 986.5 | |
| 1,013.2 | [1057.2-C2H4O] | 1,014.7 |
| 1,029.2 | [1073.2-C2H4O] | 1,032.7 |
| 1,057.2 | T7(OH)(C10H16O)3K+ | 1,058.7 |
| T7(OH)3(C10H16O)3Na+ | 1,060.6 | |
| T7(OH)5(C10H16O)3H+ | 1,056.7 | |
| 1,073.2 | T7(OH)3(C10H16O)3K+ | 1,076.7 |
| 1,101.2 | [1145.2-C2H4O] | 1,102.8 |
| 1,117.2 | [1161.2-C2H4O] | 1,120.9 |
| 1,145.2 | T8(OH)2(C10H16O)3K+ | 1,146.8 |
| T8(OH)4(C10H16O)3Na+ | 1,148.8 | |
| T8(OH)6(C10H16O)3H+ | 1,144.8 | |
| 1,161.2 | T8(OH)4(C10H16O)3K+ | 1164.9 |
| 1,189.2 | [1233.2-C2H4O] | 1,191.0 |
| 1,205.2 | [1249.2-C2H4O] | 1,209.0 |
| 1,233.2 | T9(OH)3(C10H16O)3K+ | 1,235.0 |
| T9(OH)5(C10H16O)3Na+ | 1,236.9 | |
| T9(OH)7(C10H16O)3H+ | 1,232.9 | |
| 1,249.2 | T9(OH)5(C10H16O)3K+ | 1,253.0 |
| 1,277.2 | [1321.2-C2H4O] | 1,279.1 |
| 1,321.3 | T10(OH)4(C10H16O)3K+ | 1,323.1 |
| 1,365.3 | [1409.2-C2H4O] | 1,367.2 |
| 1,409.3 | T11(OH)5(C10H16O)3K+ | 1,411.2 |
| 1,453.3 | [1497.2-C2H4O] | 1,455.4 |
| 1,497.3 | T12(OH)6(C10H16O)3K+ | 1,499.4 |
| 1,541.3 | [1583.2-C2H4O] | 1,543.5 |
| 1,590.3 | T13(OH)7(C10H16O)3K+ | 1,587.5 |
| Volunteers | Untreated | Repellency (%) (±SE) at hours after treatment | |||||
|---|---|---|---|---|---|---|---|
| N | 0 h | 1 h | 2 h | ||||
| N | R (%) | N | R (%) | N | R (%) | ||
| V1 | 43 | 0 | 100 | 7 | 83.7 | 13 | 69.8 |
| V2 | 27 | 0 | 100 | 5 | 81.5 | 14 | 48.1 |
| V3 | 23 | 1 | 95.7 | 11 | 52.2 | 14 | 39.1 |
| V4 | 68 | 0 | 100 | 13 | 80.9 | 16 | 76.5 |
| V5 | 38 | 2 | 94.7 | 7 | 81.6 | 11 | 71.1 |
| V6 | 42 | 0 | 100 | 11 | 73.8 | 14 | 66.7 |
| V7 | 34 | 0 | 100 | 11 | 67.6 | 13 | 61.8 |
| V8 | 32 | 3 | 90.6 | 12 | 62.5 | 17 | 46.9 |
| AVG | 38.38 ± 2.96 | 0.75 ± 0.21 | 97.63 ± 0.72 | 9.63 ± 0.77 | 72.98 ± 3.11 | 12.75 ± 0.71 | 60 ± 4.23 |
| Volunteers | Untreated | Repellency (%) (±SE) at hours after treatment | |||||
|---|---|---|---|---|---|---|---|
| N | 0 h | 1 h | 2 h | ||||
| N | R (%) | N | R (%) | N | R (%) | ||
| V1 | 23 | 5 | 78.3 | 11 | 52.2 | 12 | 47.8 |
| V2 | 25 | 4 | 84 | 10 | 60.0 | 6 | 76.0 |
| V3 | 23 | 3 | 87.0 | 8 | 65.2 | 10 | 56.5 |
| V4 | 24 | 0 | 100 | 7 | 70.8 | 12 | 50 |
| V5 | 26 | 4 | 84.6 | 11 | 57.7 | 19 | 26.9 |
| V6 | 21 | 0 | 100 | 7 | 66.7 | 10 | 52.4 |
| V7 | 22 | 0 | 100 | 6 | 72.7 | 10 | 54.5 |
| V8 | 23 | 4 | 82.6 | 10 | 56.5 | 13 | 43.5 |
| AVG | 23.38 ± 0.66 | 2.50 ± 0.71 | 89.56 ± 3.02 | 8.75 ± 0.83 | 62.73 ± 5.31 | 11.5 ± 1.19 | 50.95 ± 6.10 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Kong, M.; Li, L.; Li, X.; Zhang, X. Enhanced Anti-Ultraviolet and Thermal Stability of a Pesticide via Modification of a Volatile Organic Compound (VOC)-Free Vinyl-Silsesquioxane in Desert Areas. Polymers 2016, 8, 282. https://doi.org/10.3390/polym8080282
Lin D, Kong M, Li L, Li X, Zhang X. Enhanced Anti-Ultraviolet and Thermal Stability of a Pesticide via Modification of a Volatile Organic Compound (VOC)-Free Vinyl-Silsesquioxane in Desert Areas. Polymers. 2016; 8(8):282. https://doi.org/10.3390/polym8080282
Chicago/Turabian StyleLin, Derong, Maozhu Kong, Liangyu Li, Xindan Li, and Xingwen Zhang. 2016. "Enhanced Anti-Ultraviolet and Thermal Stability of a Pesticide via Modification of a Volatile Organic Compound (VOC)-Free Vinyl-Silsesquioxane in Desert Areas" Polymers 8, no. 8: 282. https://doi.org/10.3390/polym8080282