The Regulation of Osteogenesis Using Electroactive Polypyrrole Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation of PPy Films
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
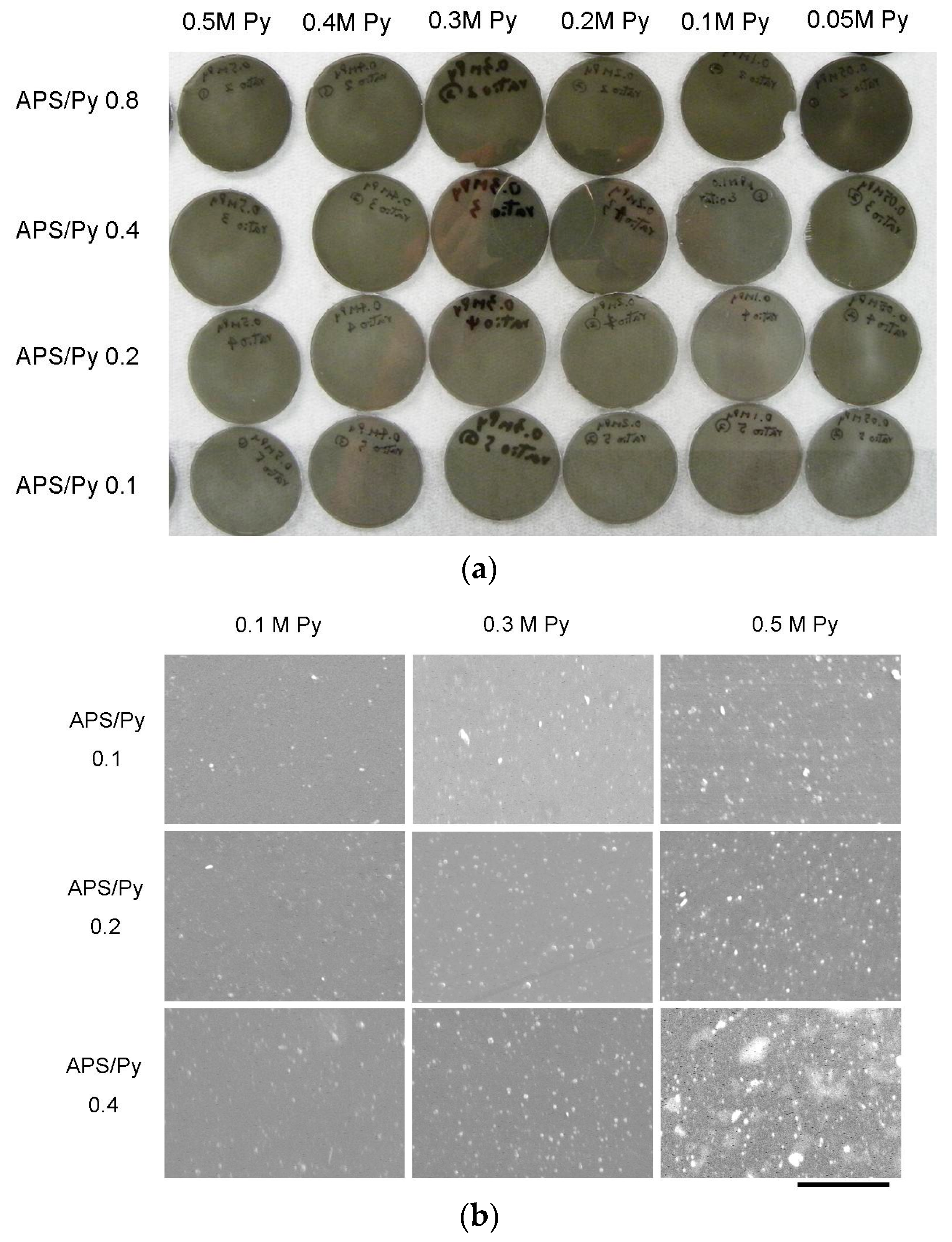
2.4. Scanning Electron Microscopy (SEM)
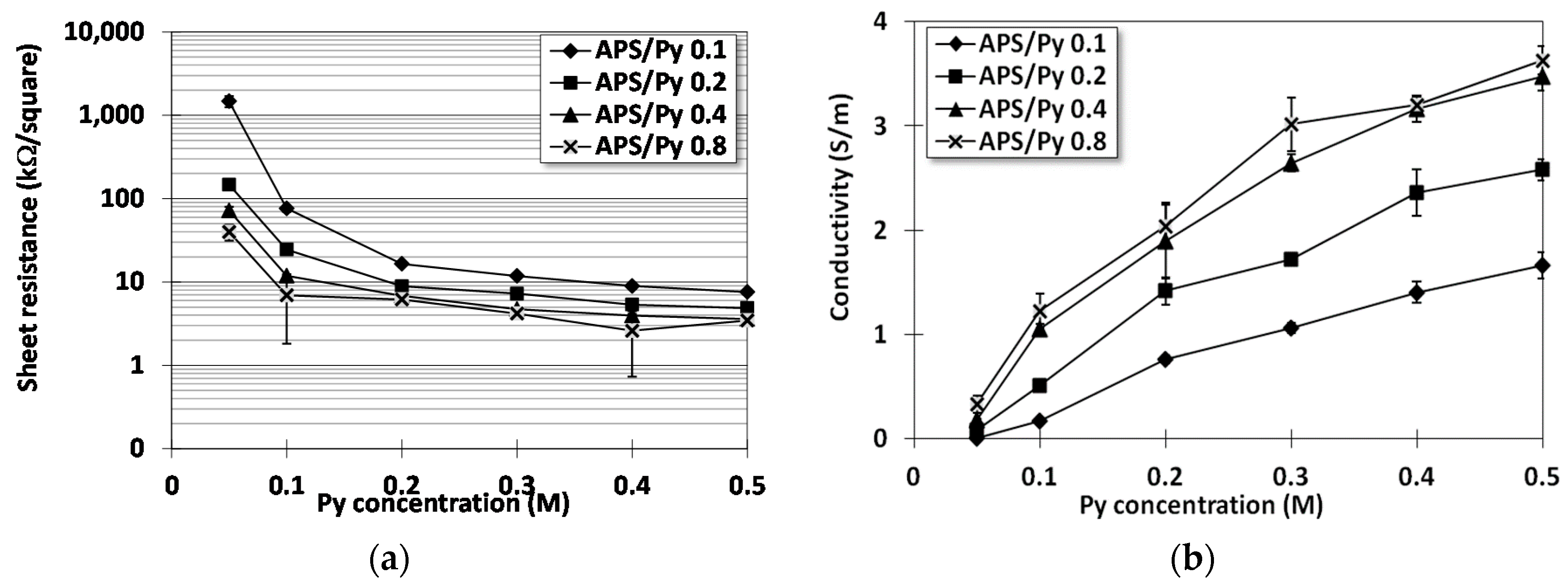
2.5. Four Point Probe Analysis
2.6. X-Ray Photon-Electron Spectrometry (XPS)
2.7. In Vitro Cell Culture of BMSCs
2.8. Biocompatibility of PPy Films
2.9. Quantification of Calcium Deposition in Extracellular Matrix
3. Results and Discussion
3.1. Characteristics of Microstructures and Chemical Composition
3.2. Electrical Resistance
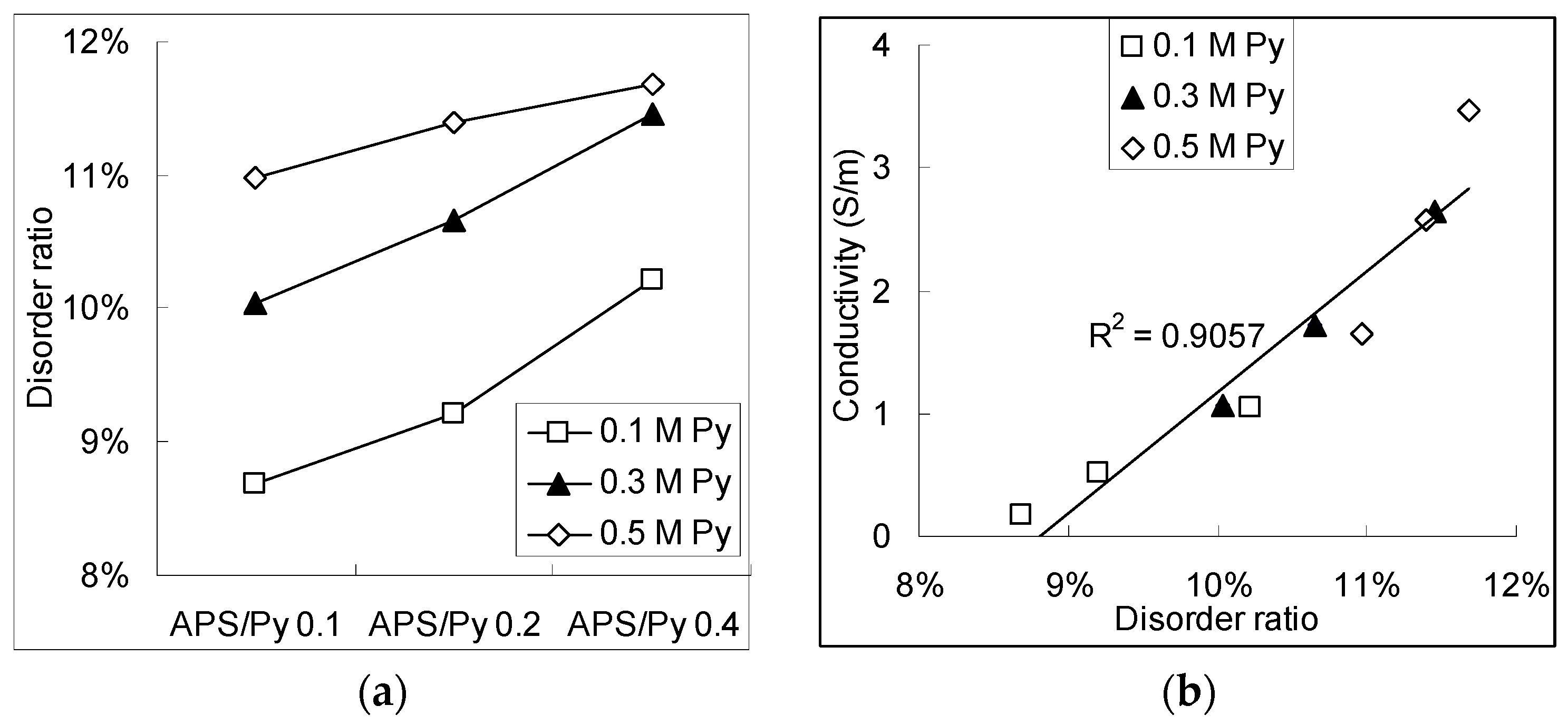
3.3. XPS Analysis and Molecular Structures
3.4. MTT Assay for the Biocompatibilities of PPy Films
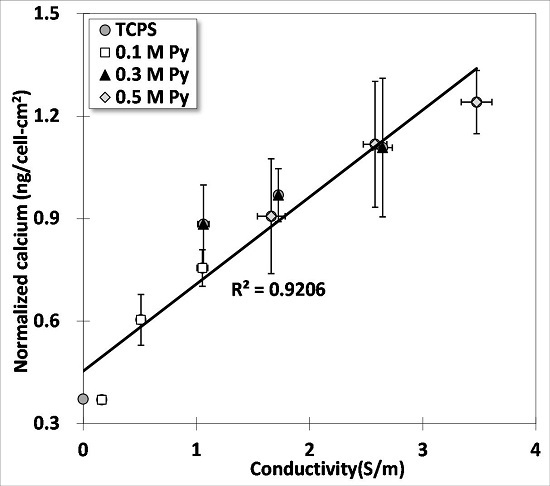
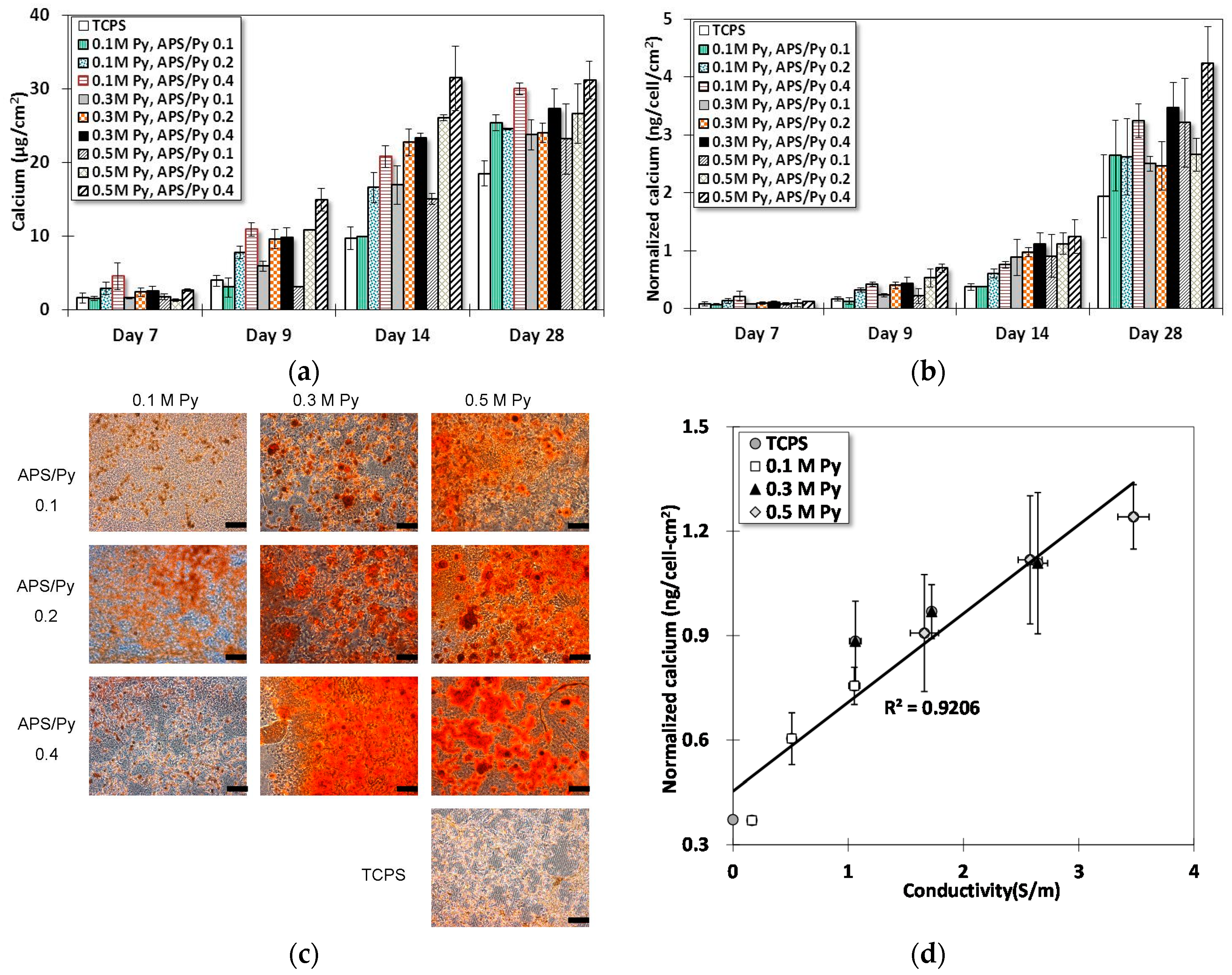
3.5. Osteogenesis of BMSCs on PPy Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Ahn, A.C.; Grodzinsky, A.J. Relevance of collagen piezoelectricity to “Wolff’s law”: A critical review. Med. Eng. Phys. 2009, 31, 733–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.W.; Hsu, Y.T.; Cheng, Y.C.; Li, C.; Ruaan, R.C.; Chien, C.C.; Chung, C.A.; Tsao, C.W. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C 2014, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bodhak, S.; Bose, S.; Kinsel, W.C.; Bandyopadhyay, A. Investigation of in vitro bone cell adhesion and proliferation on ti using direct current stimulation. Mater. Sci. Eng. C 2012, 32, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, B.M.; Brunker, L.B.; Brown, A.A.; Beck, J.P.; Burns, G.L.; Bloebaum, R.D. An evaluation of electrical stimulation for improving periprosthetic attachment. J. Biomed. Mater. Res. Part B 2011, 97B, 190–200. [Google Scholar] [CrossRef] [PubMed]
- McCullen, S.D.; McQuilling, J.P.; Grossfeld, R.M.; Lubischer, J.L.; Clarke, L.I.; Loboa, E.G. Application of low-frequency alternating current electric fields via interdigitated electrodes: Effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng. Part C 2010, 16, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.J.; Zhou, S.B.; Li, L.; Li, J.R.; Luo, C.; Wang, J.X.; Li, X.H.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cedillo, M.L.; Alvarado-Estrada, K.N.; Pozos-Guillen, A.J.; Murguia-Ibarra, J.S.; Vidal, M.A.; Cervantes-Uc, J.M.; Rosales-Ibanez, R.; Cauich-Rodriguez, J.V. Multiwall carbon nanotubes/polycaprolactone scaffolds seeded with human dental pulp stem cells for bone tissue regeneration. J. Mater. Sci. 2016, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, Z.; Rouabhia, M. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J. Bone Miner. Metab. 2011, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neoh, K.G.; Hu, X.; Kang, E.T.; Wang, W. Combined effects of direct current stimulation and immobilized BMP-2 for enhancement of osteogenesis. Biotechnol. Bioeng. 2013, 110, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Yow, S.Z.; Lim, T.H.; Yim, E.K.F.; Lim, C.T.; Leong, K.W. A 3D electroactive polypyrrole-collagen fibrous scaffold for tissue engineering. Polymers 2011, 3, 527–544. [Google Scholar] [CrossRef]
- Bidez, P.R.; Li, S.X.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci.-Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Onoda, M.; Abe, Y.; Tada, K. Experimental study of culture for mouse fibroblast used conductive polymer films. Thin Solid Films 2010, 519, 1230–1234. [Google Scholar] [CrossRef]
- Lundin, V.; Herland, A.; Berggren, M.; Jager, E.W.; Teixeira, A.I. Control of neural stem cell survival by electroactive polymer substrates. PLoS ONE 2011, 6, e18624. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Ji, L.W.; Li, D.F.; Wang, J.Y. Characterization of nanostructure and cell compatibility of polyaniline films with different dopant acids. J. Phys. Chem. B 2008, 112, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; Stein, J.L.; Van Wijnen, A.J.; Montecino, M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996, 76, 593–629. [Google Scholar] [PubMed]
- Castano, H.; O’Rear, E.A.; McFetridge, P.S.; Sikavitsas, V.I. Polypyrrole thin films formed by admicellar polymerization support the osteogenic differentiation of mesenchymal stem cells. Macromol. Biosci. 2004, 4, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.K.; Samanta, S.B.; Malhotra, B.D.; Chand, S. Application of electrochemically prepared polypyrrole-polyvinyl sulphonate films to DNA biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and characterization of polypyrrole (PPy) thin films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Kharat, H.J.; Kakde, K.P.; Savale, P.A.; Datta, K.; Ghosh, P.; Shirsat, M.D. Synthesis of polypyrrole films for the development of ammonia sensor. Polym. Adv. Technol. 2007, 18, 397–402. [Google Scholar] [CrossRef]
- Shaktawat, V.; Sharma, K.; Saxena, N.S. Structural and electrical characterization of protonic acid doped polypyrrole. J. Ovonic Res. 2010, 6, 239–245. [Google Scholar]
- Blinova, N.V.; Stejskal, J.; Trchova, M.; Prokes, J.; Omastova, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Aleman, C.; Casanovas, J.; Torras, J.; Bertran, O.; Armelin, E.; Oliver, R.; Estrany, F. Cross-linking in polypyrrole and poly (N-methylpyrrole): Comparative experimental and theoretical studies. Polymer 2008, 49, 1066–1075. [Google Scholar] [CrossRef]
- Pfluger, P.; Street, G.B. Chemical, electronic, and structural properties of conducting heterocyclic polymers: A view by xps. J. Chem. Phys. 1984, 80, 544–553. [Google Scholar] [CrossRef]
- Street, G.B.; Clarke, T.C.; Geiss, R.H.; Lee, V.Y.; Nazzal, A.; Pfluger, P.; Scott, J.C. Characterization of polypyrrole. J. Phys. 1983, 44, 599–606. [Google Scholar] [CrossRef]
- Brezoi, D.-V. Polypyrrole films prepared by chemical oxidation of pyrrole in aqueous FeCl3 solution. J. Sci. Arts 2010, 1, 53–58. [Google Scholar]
- Joo, J.; Lee, J.K.; Baeck, J.S.; Kim, K.H.; Oh, E.J.; Epstein, J. Electrical, magnetic, and structural properties of chemically and electrochemically synthesized polypyrroles. Synth. Metals 2001, 117, 45–51. [Google Scholar] [CrossRef]
- Prigodin, V.N.; Efetov, K.B. Localization transition in a random network of metallic wires—A model for highly conducting polymers. Phys. Rev. Lett. 1993, 70, 2932–2935. [Google Scholar] [CrossRef] [PubMed]
- Samukhin, A.N.; Prigodin, V.N.; Jastrabik, L.; Epstein, A.J. Hopping conductivity of a nearly-1D fractal: A model for conducting polymers. Phys. Rev. B 1998, 58, 11354–11370. [Google Scholar] [CrossRef]
- Gomez, N.; Schmidt, C.E. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. J. Biomed. Mater. Res. Part A 2007, 81A, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Schmidt, C.E. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials 2001, 22, 1055–1064. [Google Scholar] [CrossRef]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Hsu, Y.-T.; Hu, W.-W. The Regulation of Osteogenesis Using Electroactive Polypyrrole Films. Polymers 2016, 8, 258. https://doi.org/10.3390/polym8070258
Li C, Hsu Y-T, Hu W-W. The Regulation of Osteogenesis Using Electroactive Polypyrrole Films. Polymers. 2016; 8(7):258. https://doi.org/10.3390/polym8070258
Chicago/Turabian StyleLi, Chuan, Yi-Ting Hsu, and Wei-Wen Hu. 2016. "The Regulation of Osteogenesis Using Electroactive Polypyrrole Films" Polymers 8, no. 7: 258. https://doi.org/10.3390/polym8070258