A Facile Route to Synthesize Nanographene Reinforced PBO Composites Fiber via in Situ Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
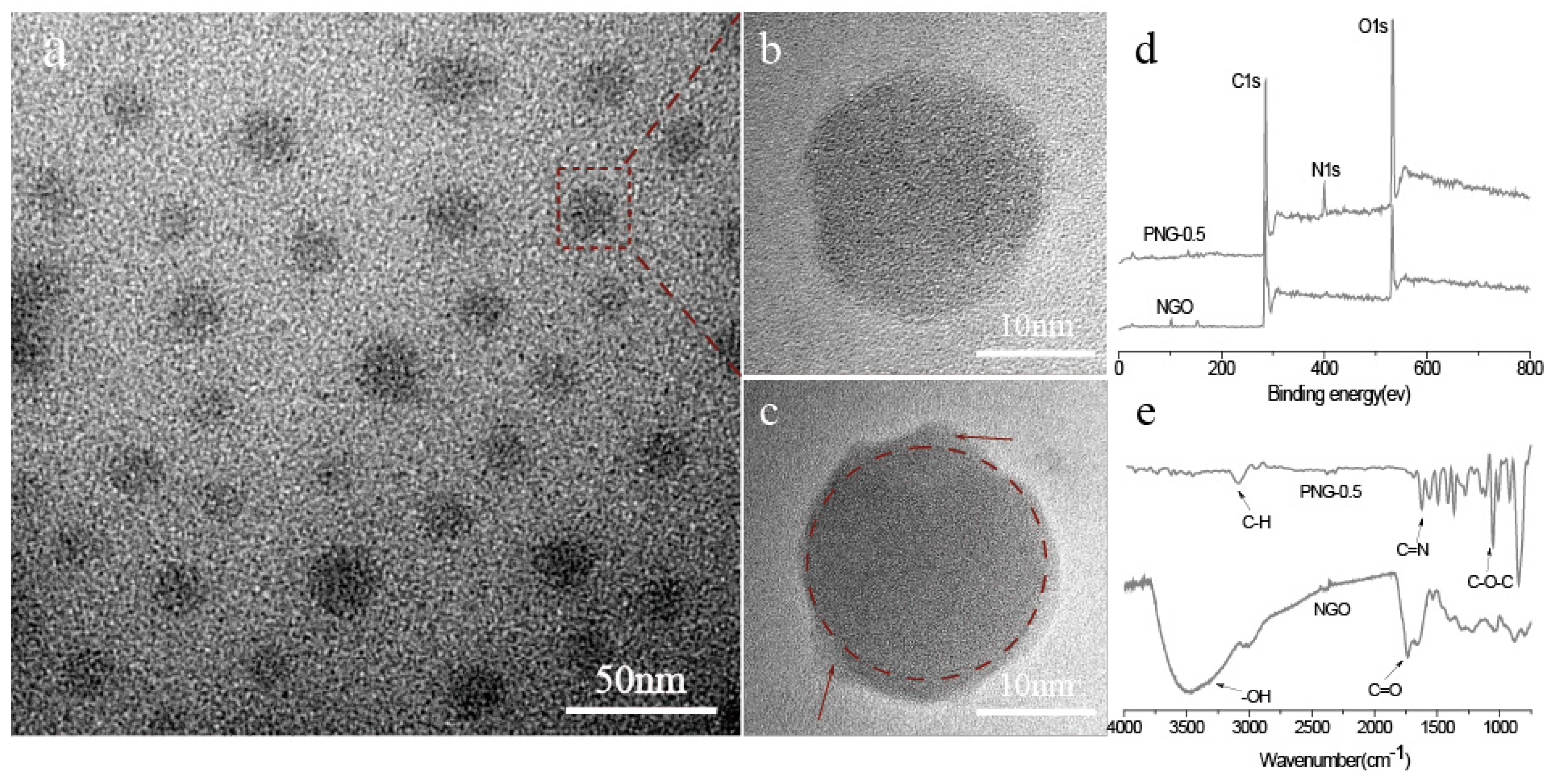
2.2. The Synthesis and Purification of NGO
2.3. Preparation of PNG Composites
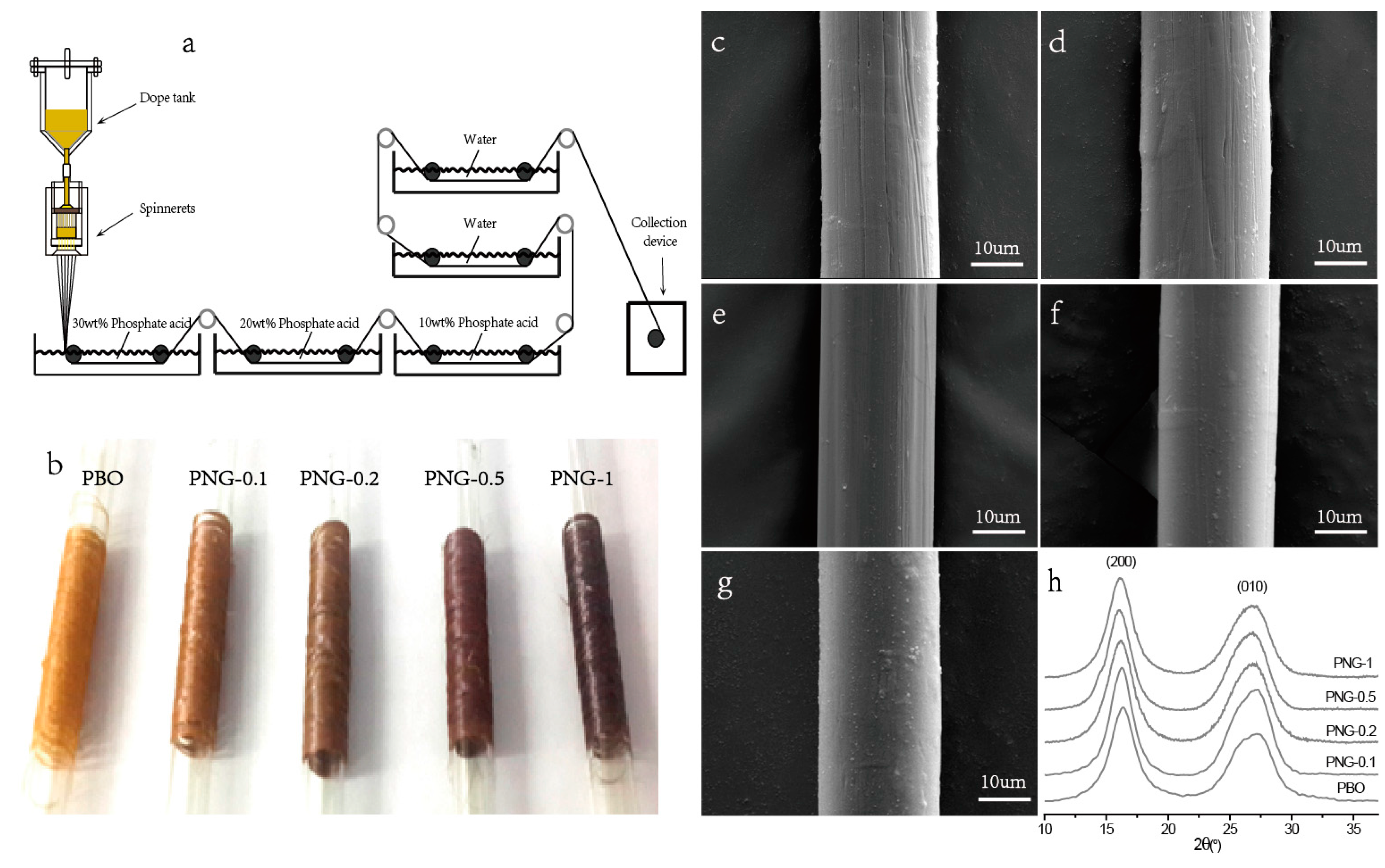
2.4. Fabrication of PNG Composites Fibers
2.5. Characterization
3. Result and Discussion
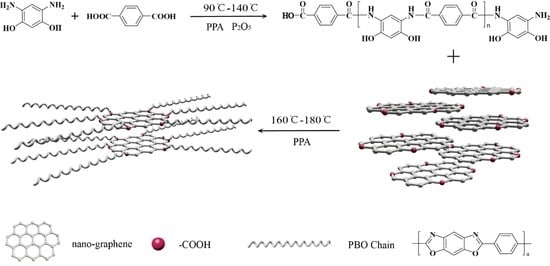
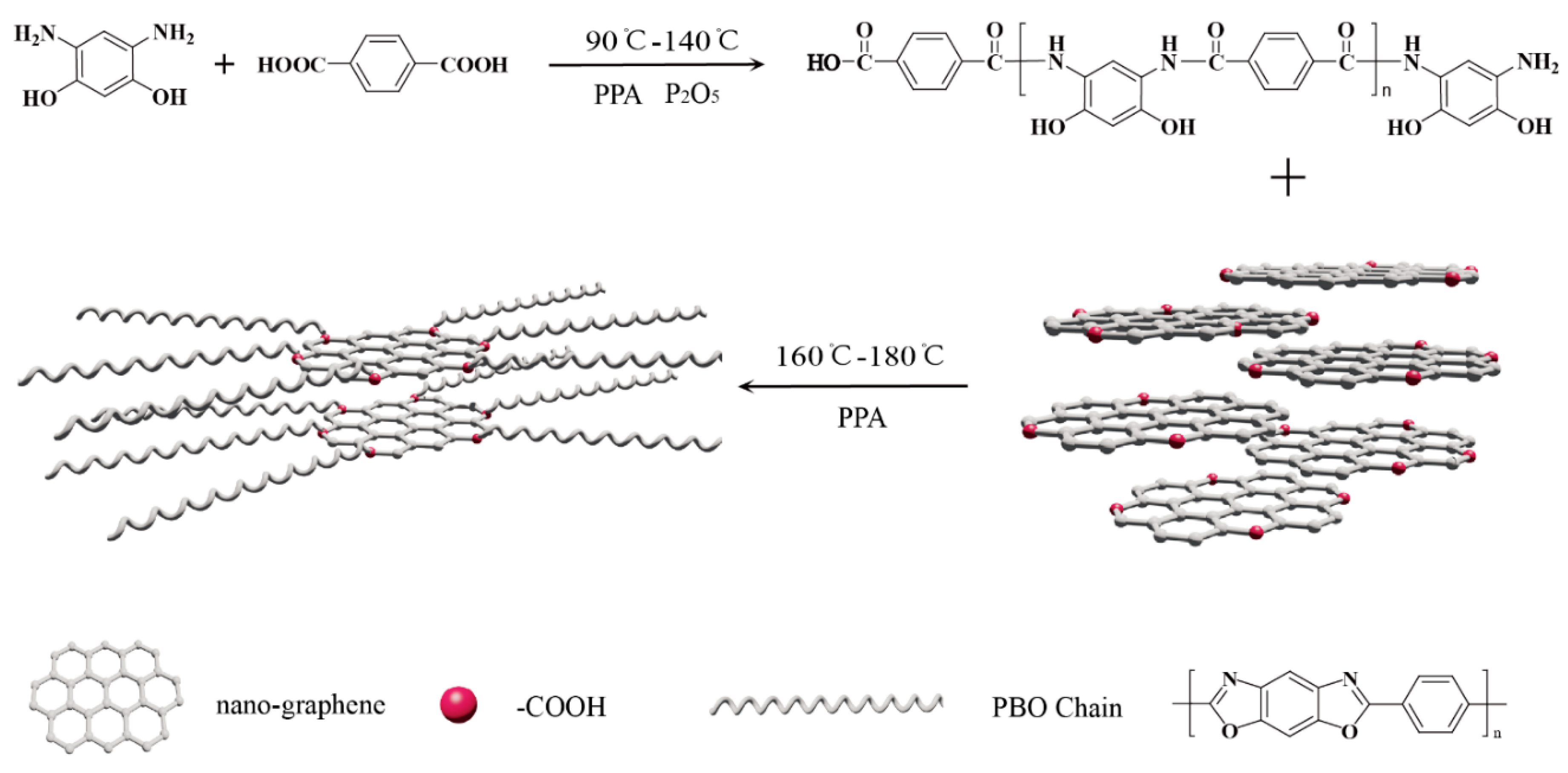
3.1. Synthesis of PBO/Nanographene Composites
3.2. Structure and Properties of NGO and PNG Composites
3.3. Properties of PNG Composites Fibers
3.4. Mechanical Properties
3.5. Thermal Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics. 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; James, D.K.; Tour, J.M. New routes to graphene, graphene oxide and their related applications. Adv. Mater. 2012, 24, 4924–4955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized grapheme. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Chen, L.; Hernandez, Y.; Feng, X.L.; Mullen, K. From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dang, T.D.; Arnold, F.E.; Bhattacharyya, A.R.; Min, B.G.; Zhang, X.F.; Vaia, R.A.; Park, C.; Adams, W.W.; Hauge, R.H.; et al. Synthesis, structure, and properties of PBO/SWNT composites. Macromolecules 2002, 35, 9039–9043. [Google Scholar] [CrossRef]
- Hu, X.D.; Jenkins, S.E.; Min, B.G.; Polk, M.B.; Kumar, S. Rigid-rod polymers: synthesis, processing, simulation, structure, and properties. Macromol. Mater. Eng. 2003, 288, 823–843. [Google Scholar] [CrossRef]
- Kitagawa, T.; Yabuki, K.; Young, R.J. An investigation into the relationship between processing, structure and properties for high-modulus PBO fibers. Part 1. Raman band shifts and broadening in tension and compression. Polymer 2001, 42, 2101–2112. [Google Scholar] [CrossRef]
- Chae, H.G.; Kumar, S. Rigid-rod polymeric fibers. J. Appl. Polym. Sci. 2006, 100, 791–802. [Google Scholar] [CrossRef]
- Chae, H.G.; Kumar, S. Making strong fibers. Science 2008, 319, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Song, K.N.; Zhang, Y.Y.; Meng, J.S.; Emily, C.G.; Navid, T.; Li, H.; Marilyn, L.M. Structural polymer-based carbon nanotube composite fibers: Understanding the processing-structure-performance relationship. Materials 2013, 6, 2543–2577. [Google Scholar] [CrossRef]
- Cai, D.Y.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Mrinal, B. Polymer nanocomposites-A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262–297. [Google Scholar]
- Li, Y.W.; Li, J.; Song, Y.J.; Hu, Z.; Zhao, F.; Huang, Y.D. In situ polymerization and characterization of graphene oxide-co-poly(phenylene benzobisoxazole) copolymer fibers derived from composite inner salts. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1831–1842. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Baik, D.H.; Jang, J.W.; Min, B.G.; Yoon, K.H. Preparation, structure and properties of poly(p-phenylene benzobisoxazole) composite fibers reinforced with grapheme. Macromol. Res. 2014, 22, 279–286. [Google Scholar] [CrossRef]
- Kobashi, K.; Chen, Z.Y.; Lomeda, J.; Rauwald, U.; Hwang, W.F.; Tour, J.M. Copolymer of single-walled carbon nanotubes and poly(p-phenylene benzobisoxazole). Chem. Mater. 2007, 19, 291–300. [Google Scholar] [CrossRef]
- Zhou, C.J.; Wang, S.F.; Zhang, Y.; Zhuang, Q.X.; Han, Z.W. In situ preparation and continuous fiber spinning of poly(p-phenylene benzobisoxazole) composites with oligo-hydroxyamide-functionalized multi-walled carbon nanotubes. Polymer 2008, 49, 2520–2530. [Google Scholar] [CrossRef]
- Xie, Z.; Zhuang, Q.X.; Wang, Q.; Liu, X.Y.; Chen, Y.; Han, Z.W. In situ synthesis and characterization of poly(2,5-benzoxazole)/multiwalled carbon nanotubes composites. Polymer 2011, 52, 5271–5276. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Tang, P.Y.; Li, D.L.; Song, Y.J.; Li, Y.W.; Zhao, L.; Li, C.Y.; Huang, Y.D. One-pot preparation and continuous spinning of carbon nanotube/poly(p-phenylene benzobisoxazole) copolymer fibers. J. Mater. Chem. 2012, 22, 19863–19871. [Google Scholar] [CrossRef]
- Yang, W.; He, C.L.; Zhang, L.C.; Wang, Y.; Shi, Z.W.; Cheng, M.; Xie, G.B.; Wang, D.M.; Yang, R.; Shi, D.X.; et al. Growth, characterization, and properties of nanographene. Small 2012, 8, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.L.; Shi, D.X.; Zhang, G.Y. A review of nanographene: Growth and applications. Mod. Phy. Lett. B 2014, 28, 1430009–1430021. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H.J. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Wu, P.P.; Han, Z.W. Electron paramagnetic resonance of poly(benzazole)s and conducting properties of N1-implanted poly(benzazole)s. Polymer 2001, 42, 217–226. [Google Scholar] [CrossRef]
- Wang, S.F.; Wu, P.P.; Han, Z.W. Photophysical properties of lyotropic liquid crystalline polybenzazoles and evidence of aggregate formation for polybenzazoles in methanesulfonic acid. Macromolecules 2003, 36, 4567–4576. [Google Scholar] [CrossRef]
- Freeman, S.M.; Weissenberg, K. Some new rheological phenomena and their significance for the constitution of materials. Nature 1948, 162, 320–323. [Google Scholar] [CrossRef]

| Sample | Fiber diameter (μm) | Tensile modulus (GPa) | Strain to failure (%) | Tensile strength (GPa) | Decomposition temperature (°C) |
|---|---|---|---|---|---|
| PBO | 20 ± 3 | 113 ± 6.6 | 2.83 ± 0.03 | 3.20 ± 0.32 | 633 |
| PNG (0.1 wt %) | 20 ± 2 | 128 ± 5.2 | 2.77 ± 0.03 | 3.54 ± 0.26 | 643 |
| PNG (0.25 wt %) | 20 ± 1 | 137 ± 4.3 | 2.74 ± 0.02 | 3.78 ± 0.18 | 646 |
| PNG (0.5 wt %) | 20 ± 2 | 151 ± 6.2 | 2.68 ± 0.02 | 4.05 ± 0.33 | 652 |
| PNG (1 wt %) | 20 ± 2 | 144 ± 5.6 | 2.63 ± 0.03 | 3.8 ± 0.19 | 644 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, S.; Dong, J.; Song, Y.; Mao, J.; Xie, H.; Qian, Y.; Huang, Y.; Jiang, Z. A Facile Route to Synthesize Nanographene Reinforced PBO Composites Fiber via in Situ Polymerization. Polymers 2016, 8, 251. https://doi.org/10.3390/polym8070251
Wang M, Zhang S, Dong J, Song Y, Mao J, Xie H, Qian Y, Huang Y, Jiang Z. A Facile Route to Synthesize Nanographene Reinforced PBO Composites Fiber via in Situ Polymerization. Polymers. 2016; 8(7):251. https://doi.org/10.3390/polym8070251
Chicago/Turabian StyleWang, Mingqiang, Shuai Zhang, Jidong Dong, Yuanjun Song, Jiao Mao, Huaquan Xie, Yue Qian, Yudong Huang, and Zaixing Jiang. 2016. "A Facile Route to Synthesize Nanographene Reinforced PBO Composites Fiber via in Situ Polymerization" Polymers 8, no. 7: 251. https://doi.org/10.3390/polym8070251