Synthesis and Characterization of PEDOT:P(SS-co-VTMS) with Hydrophobic Properties and Excellent Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of P(SS-co-VTMS) Copolymer and PEDOT:P(SS-co-VTMS)
2.3. Characterization of P(SS-co-VTMS) and PEDOT:P(SS-co-VTMS)
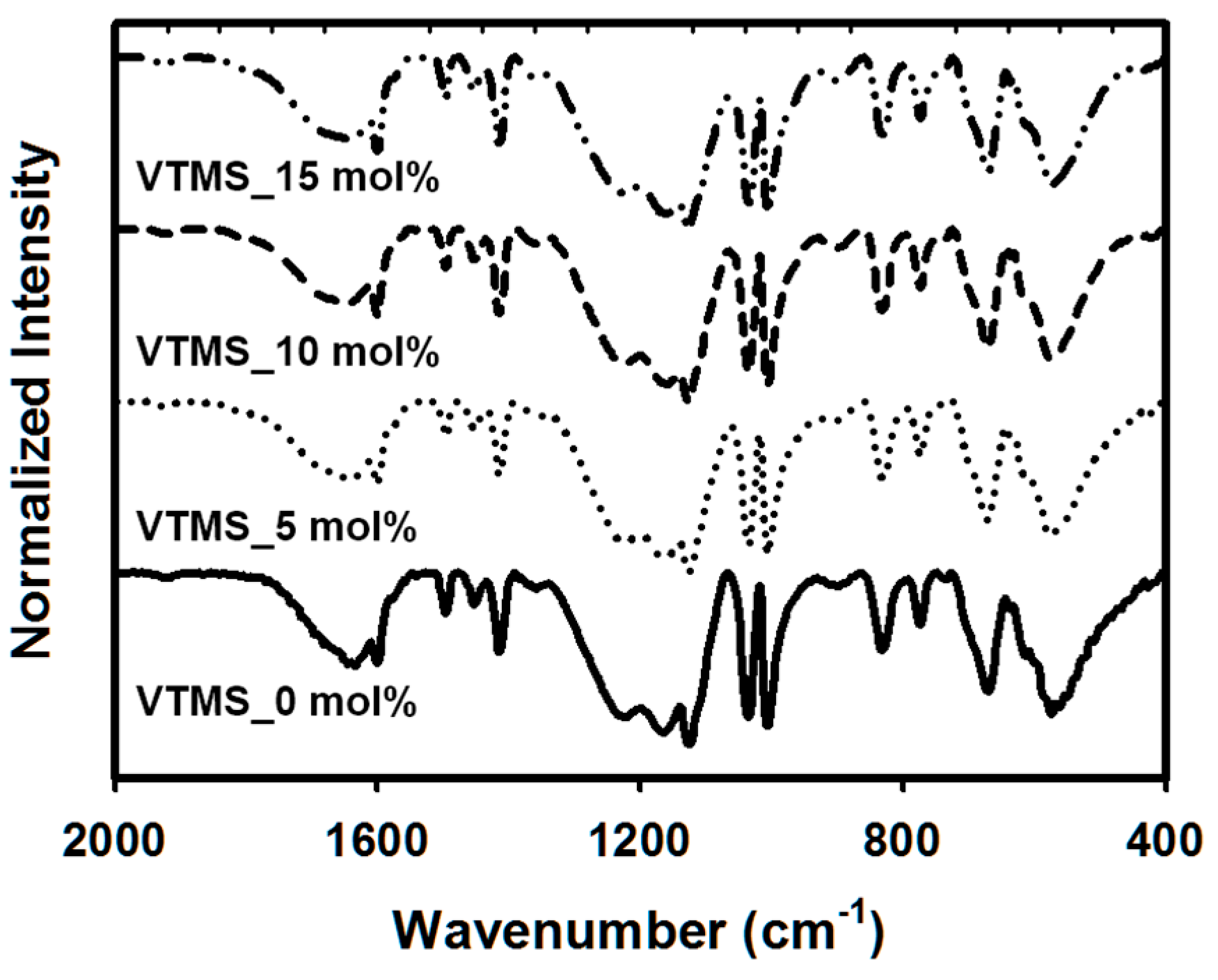
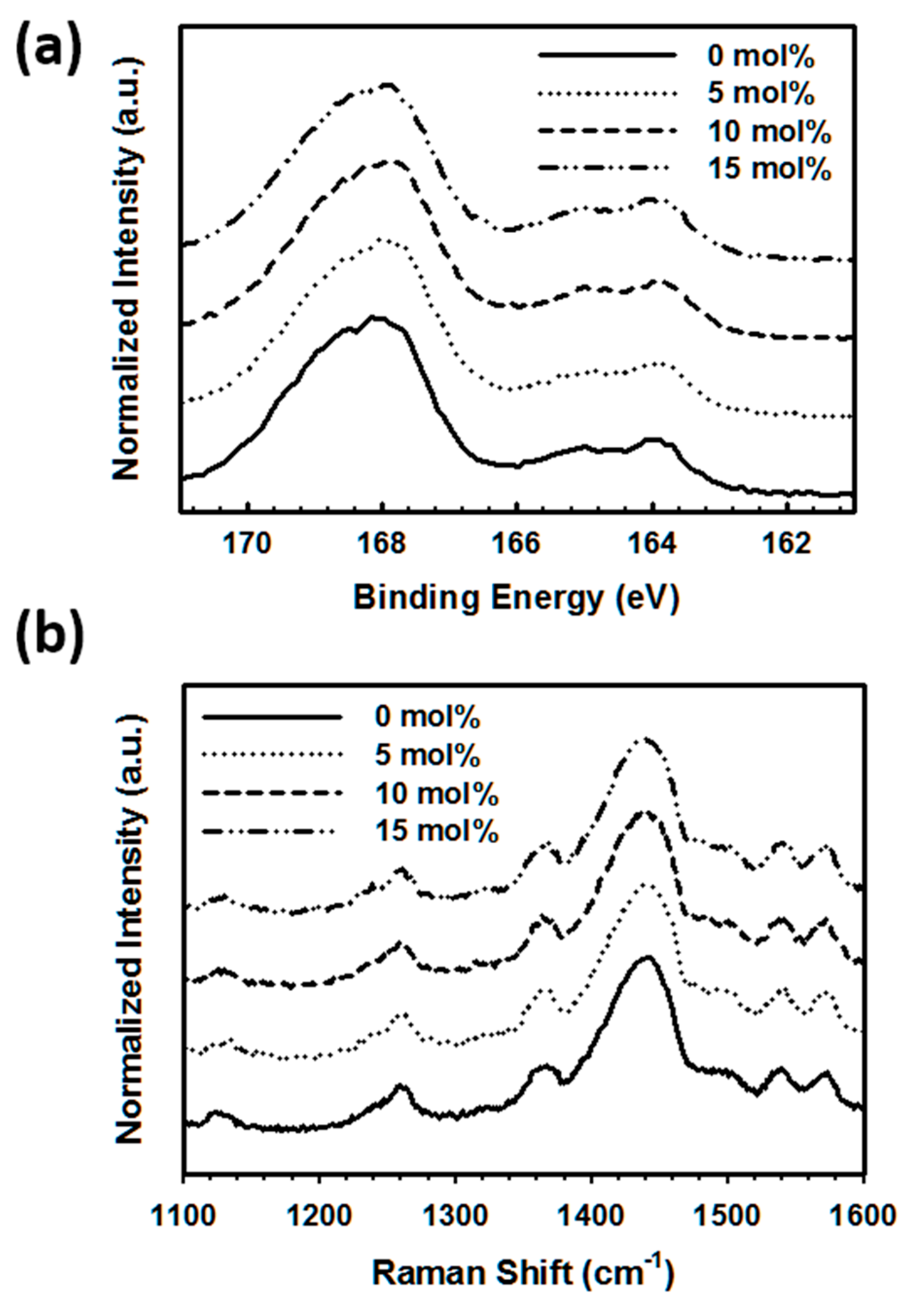
3. Results and Discussions
3.1. The Synthesis and Characterization of P(SS-co-VTMS) Copolymers
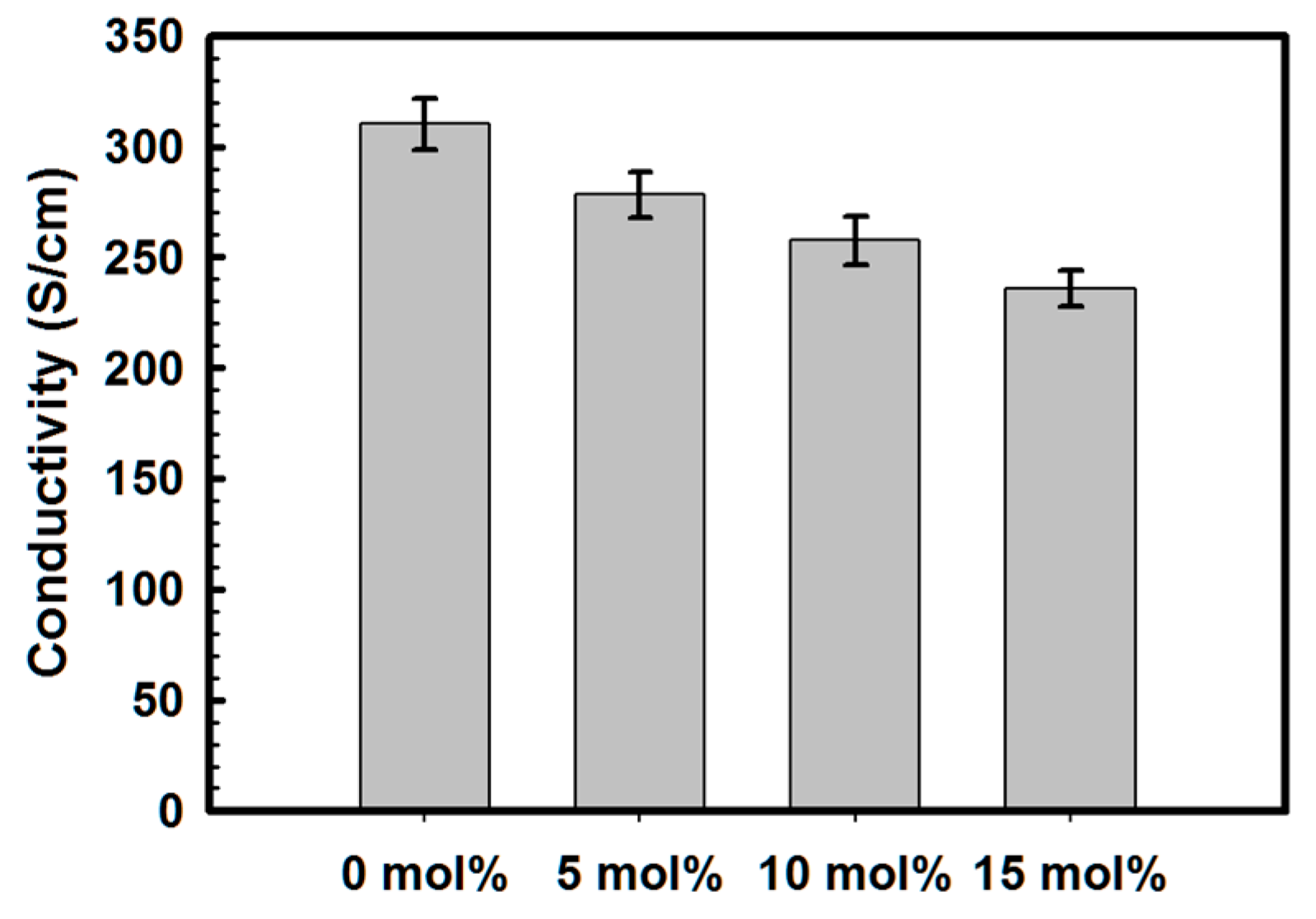
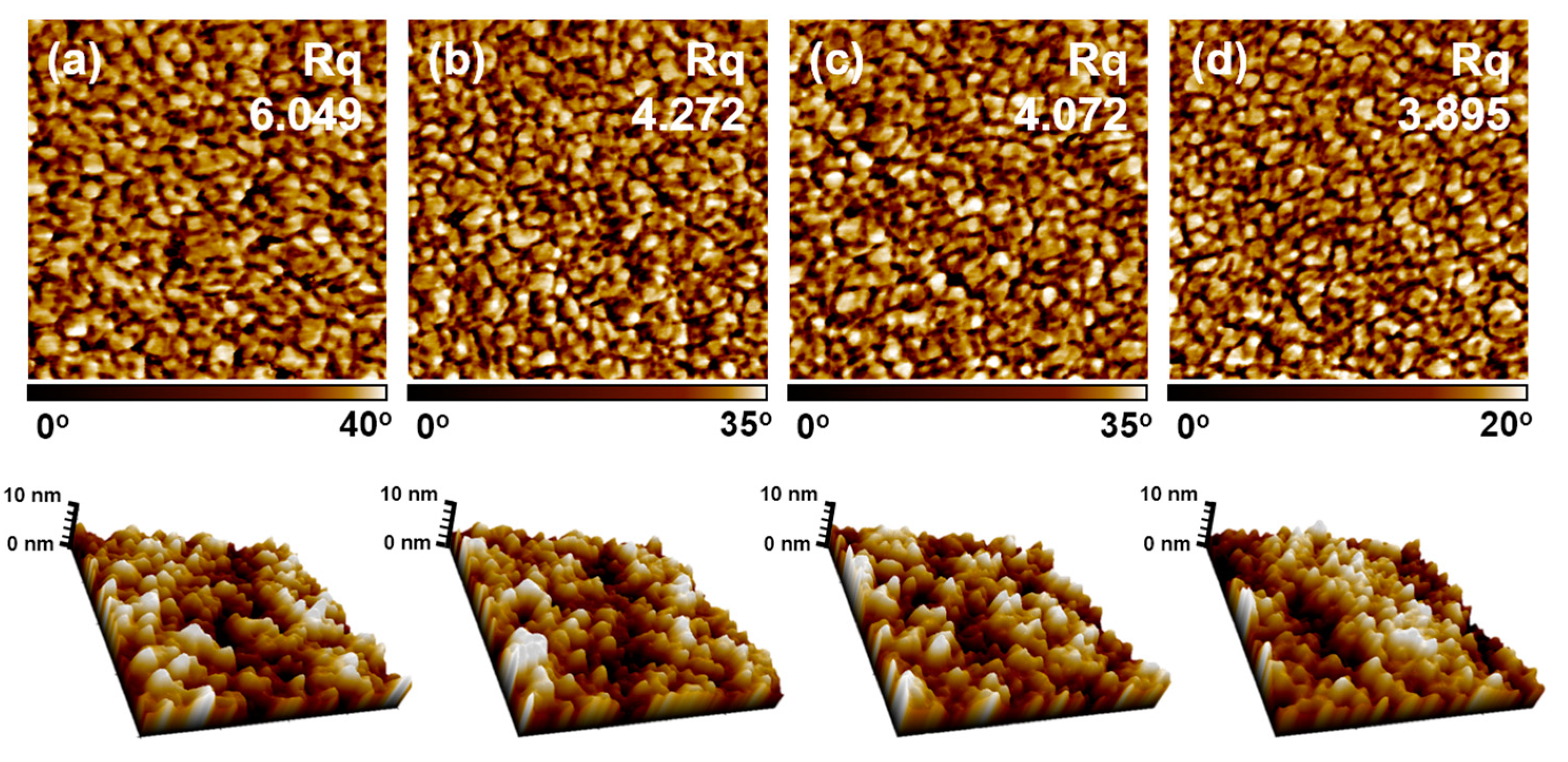
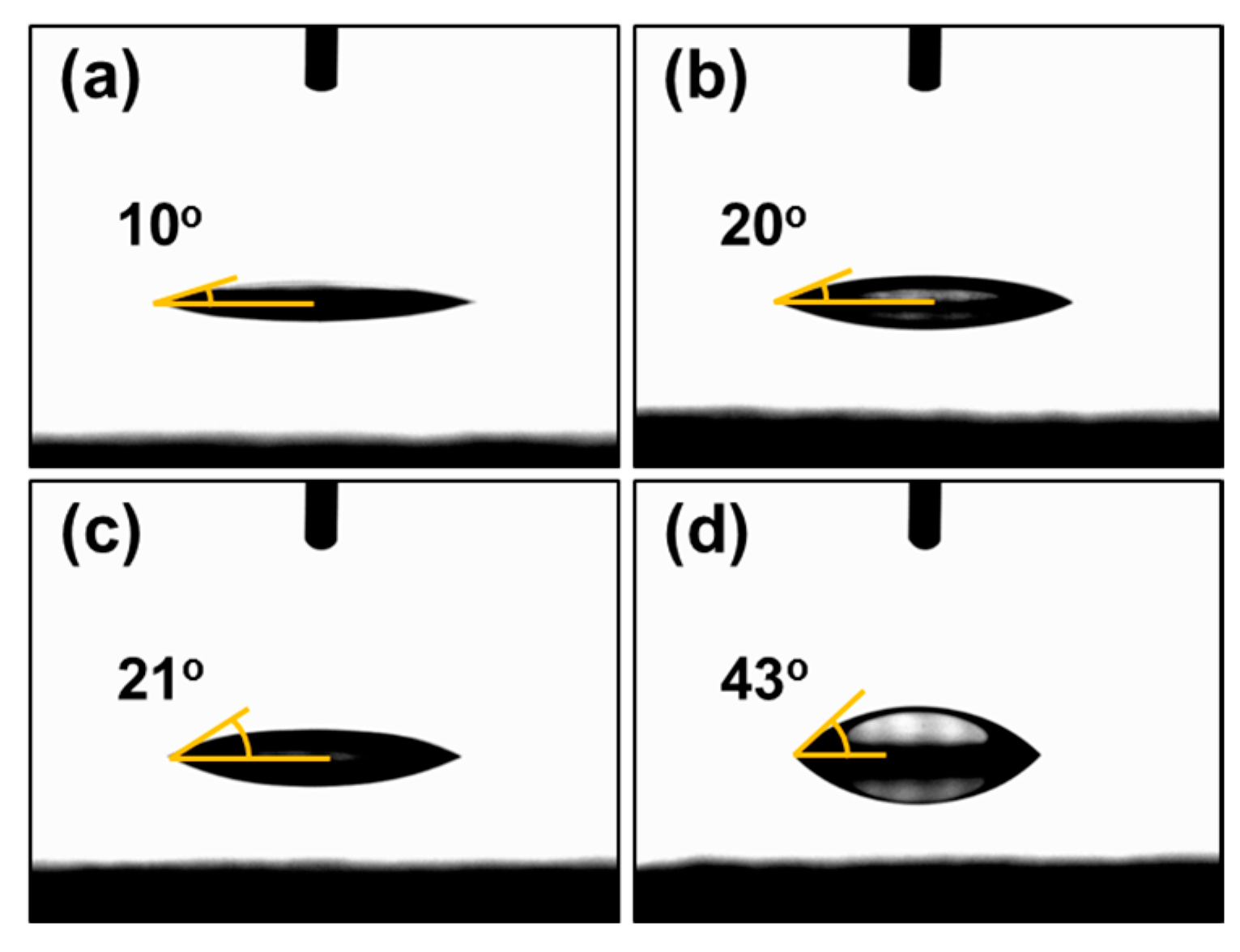
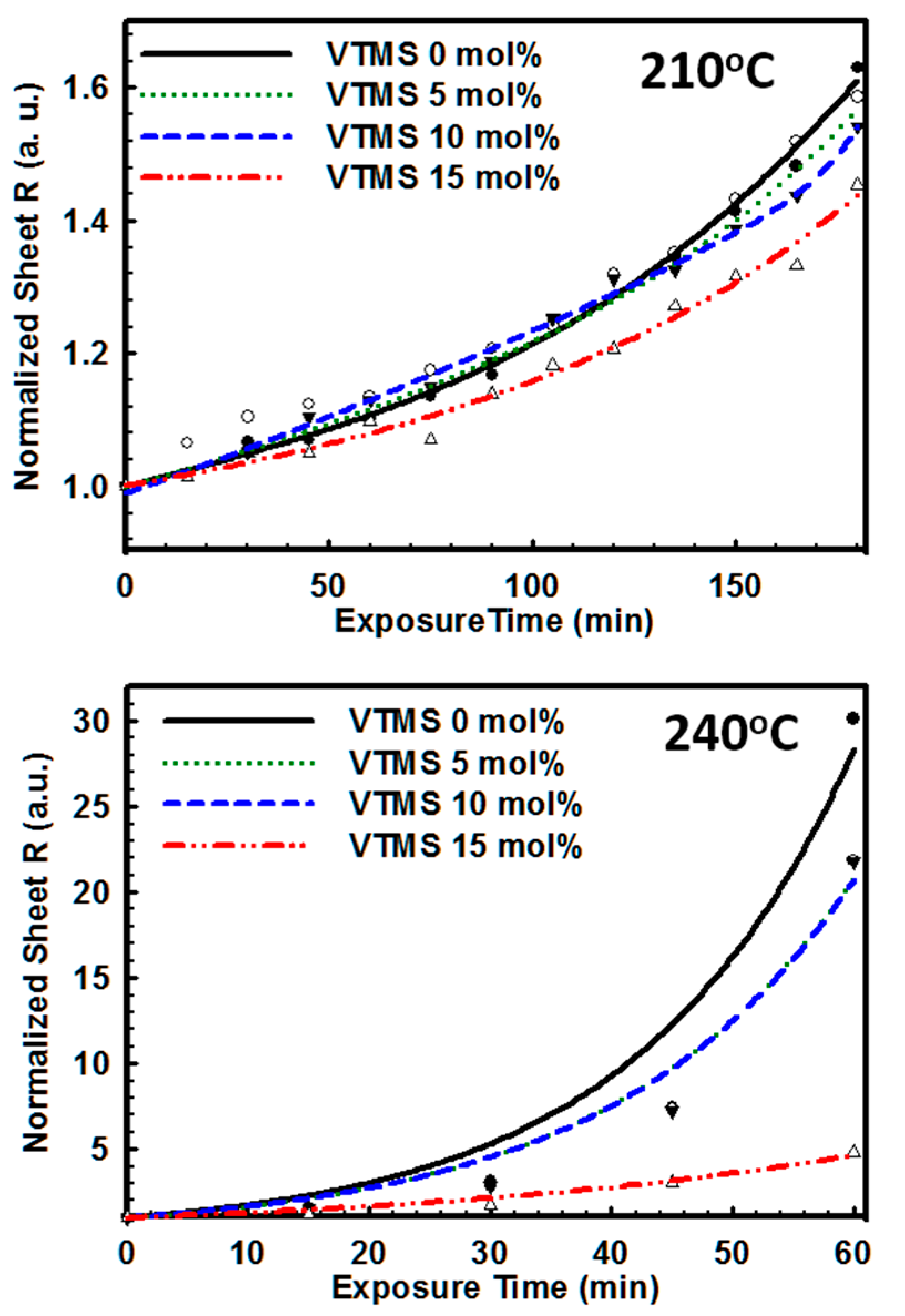
3.2. Effect of Introduction of VTMS into the PSS Main Chain on the Physical and Electrical Properties of PEDOT:P(SS-co-VTMS) Conductive Films
3.3. Practical Implications of the High Hydrophobicity and Thermal Stability of PEDOT:P(SS-co-VTMS)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ito-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Na, S.-I.; Wang, G.; Kim, S.-S.; Kim, T.-W.; Oh, S.-H.; Yu, B.-K.; Lee, T.; Kim, D.-Y. Evolution of nanomorphology and anisotropic conductivity in solvent-modified PEDOT:PSS films for polymeric anodes of polymer solar cells. J. Mater. Chem. 2009, 19, 9045–9053. [Google Scholar] [CrossRef]
- Takano, T.; Masunaga, H.; Fujiwara, A.; Okuzaki, H.; Sasaki, T. Pedot nanocrystal in highly conductive PEDOT:PSS polymer films. Macromolecules 2012, 45, 3859–3865. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, H.; Kim, S.; Son, W.; Cheong, I.W.; Kim, J.H. Transparent and flexible organic semiconductor nanofilms with enhanced thermoelectric efficiency. J. Mater. Chem. A 2014, 2, 7288–7294. [Google Scholar] [CrossRef]
- Massonnet, N.; Carella, A.; Jaudouin, O.; Rannou, P.; Laval, G.; Celle, C.; Simonato, J.-P. Improvement of the seebeck coefficient of PEDOT:PSS by chemical reduction combined with a novel method for its transfer using free-standing thin films. J. Mater. Chem. C 2014, 2, 1278–1283. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Highly conductive poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) films treated with an amphiphilic fluoro compound as the transparent electrode of polymer solar cells. Energy Environ. Sci. 2012, 5, 5325–5332. [Google Scholar] [CrossRef]
- Chou, T.-R.; Chen, S.-H.; Chiang, Y.-T.; Lin, Y.-T.; Chao, C.-Y. Highly conductive PEDOT:PSS films by post-treatment with dimethyl sulfoxide for ito-free liquid crystal display. J. Mater. Chem. C 2015, 3, 3760–3766. [Google Scholar] [CrossRef]
- Kim, N.; Kang, H.; Lee, J.-H.; Kee, S.; Lee, S.H.; Lee, K. Highly conductive all-plastic electrodes fabricated using a novel chemically controlled transfer-printing method. Adv. Mater. 2015, 27, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.-E.; Lee, C.-F.; Chiu, W.-Y. Preparation of thermally curable conductive films PEDOT:P(SS-NMA) and their performances on weather stability and water resistance. Polymer 2011, 52, 5065–5074. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of polymer solar cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ballantyne, A.M.; Nelson, J.; Bradley, D.D.C. Effects of thickness and thermal annealing of the PEDOT:PSS layer on the performance of polymer solar cells. Org. Electron. 2009, 10, 205–209. [Google Scholar] [CrossRef]
- Somboonsub, B.; Invernale, M.A.; Thongyai, S.; Praserthdam, P.; Scola, D.A.; Sotzing, G.A. Preparation of the thermally stable conducting polymer pedot—Sulfonated poly(imide). Polymer 2010, 51, 1231–1236. [Google Scholar] [CrossRef]
- Srisuwan, S.; Ding, Y.; Mamangun, D.; Thongyai, S.; Praserthdam, P.; Sotzing, G.A. Secondary dopants modified pedot-sulfonated poly(imide)s for high-temperature range application. J. Appl. Polym. Sci. 2013, 128, 3840–3845. [Google Scholar] [CrossRef]
- Sukchol, K.; Thongyai, S.; Praserthdam, P.; Sotzing, G.A. Effects of the addition of anionic surfactant during template polymerization of conducting polymers containing pedot with sulfonated poly(imide) and poly(styrene sulfonate) as templates for nano-thin film applications. Synth. Met. 2013, 179, 10–17. [Google Scholar] [CrossRef]
- Yin, H.-E.; Huang, F.-H.; Chiu, W.-Y. Hydrophobic and flexible conductive films consisting of PEDOT:PSS-PBA/fluorine-modified silica and their performance in weather stability. J. Mater. Chem. 2012, 22, 14042–14051. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Shen, H.-P.; Wu, C.-H.; Chiu, W.-Y.; Chen, W.-C.; Tai, H.-J. Core-shell composite latexes derived from PEDOT:PSS dispersion and the preparation of conductive, flexible and transparent films. J. Mater. Chem. C 2013, 1, 5351–5358. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, S.H.; Kim, F.S.; Choi, H.H.; Kim, J.H. Simultaneous enhancement of the efficiency and stability of organic solar cells using pedot:Pss grafted with a pegme buffer layer. Org. Electron. 2015, 26, 191–199. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies: Tables and Charts; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In situ spectroelectrochemical raman studies of poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807–6812. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. A highly thermostable ceramic-grafted microporous polyethylene separator for safer lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Lee, Y.-Y.; Kim, S.J.; Hong, S.-S.; Lee, G.-D.; Park, S.S. Synthesis and characteristics of hard coating solution using colloidal silica and organic silane through sol-gel process. Appl. Chem. Eng. 2011, 22, 691–696. [Google Scholar]
- Pingree, L.S.C.; MacLeod, B.A.; Ginger, D.S. The changing face of PEDOT:PSS films: Substrate, bias, and processing effects on vertical charge transport. J. Phys. Chem. C 2008, 112, 7922–7927. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The origin of the high conductivity of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT-PSS) plastic electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, E.; Sakkopoulos, S.; Dalas, E.; Paliatsas, N.; Karageorgopoulos, D.; Petraki, F.; Kennou, S.; Choulis, S.A. Thermal degradation mechanisms of PEDOT:PSS. Org. Electron. 2009, 10, 61–66. [Google Scholar] [CrossRef]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.; Im, S.; Kim, S.; Kim, S.; Kim, J.H. Synthesis and Characterization of PEDOT:P(SS-co-VTMS) with Hydrophobic Properties and Excellent Thermal Stability. Polymers 2016, 8, 189. https://doi.org/10.3390/polym8050189
Cho W, Im S, Kim S, Kim S, Kim JH. Synthesis and Characterization of PEDOT:P(SS-co-VTMS) with Hydrophobic Properties and Excellent Thermal Stability. Polymers. 2016; 8(5):189. https://doi.org/10.3390/polym8050189
Chicago/Turabian StyleCho, Wonseok, Soeun Im, Seyul Kim, Soyeon Kim, and Jung Hyun Kim. 2016. "Synthesis and Characterization of PEDOT:P(SS-co-VTMS) with Hydrophobic Properties and Excellent Thermal Stability" Polymers 8, no. 5: 189. https://doi.org/10.3390/polym8050189






