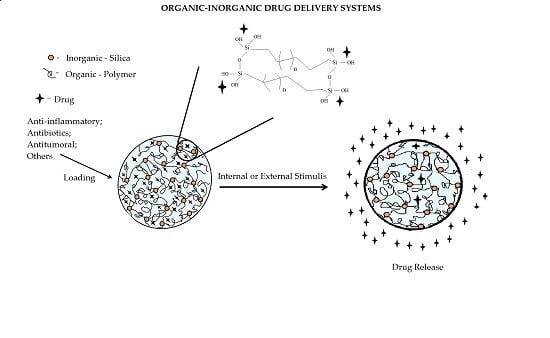
Drug Delivery Systems Obtained from Silica Based Organic-Inorganic Hybrids
Abstract
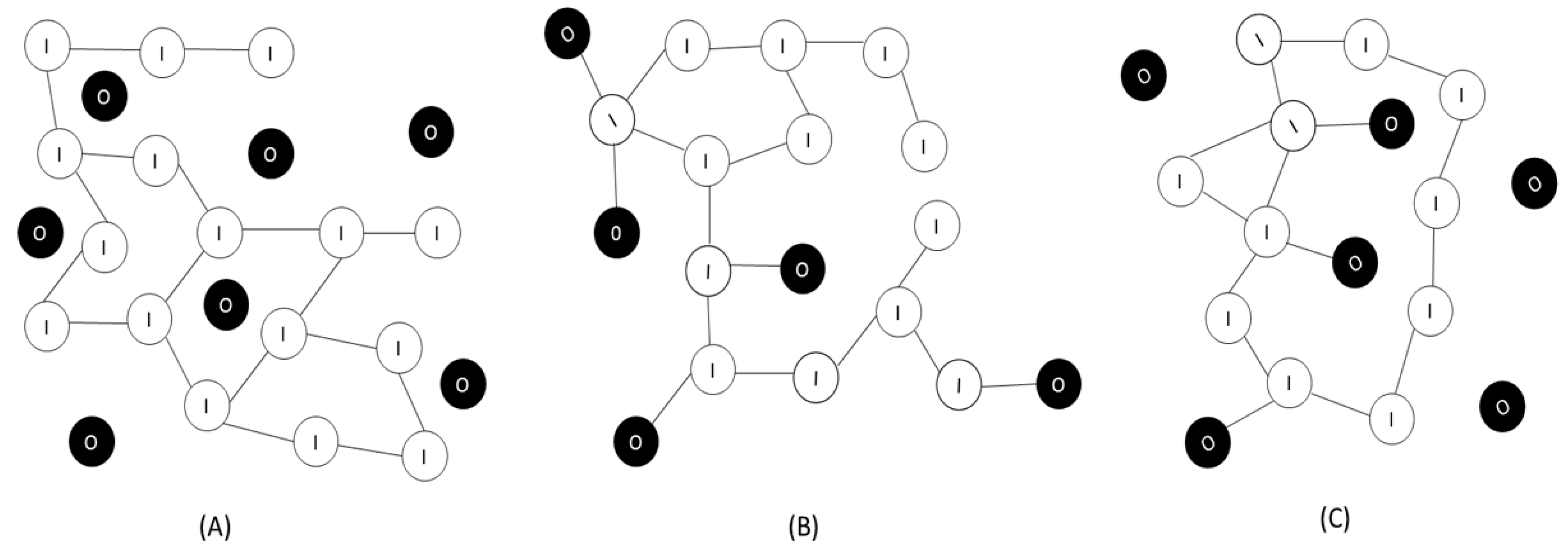
:1. Introduction: Hybrid Materials in Drug Delivery
2. Applications of Organic-Inorganic Hybrids Materials in Drug Delivery
2.1. Stimuli-Responsive Systems
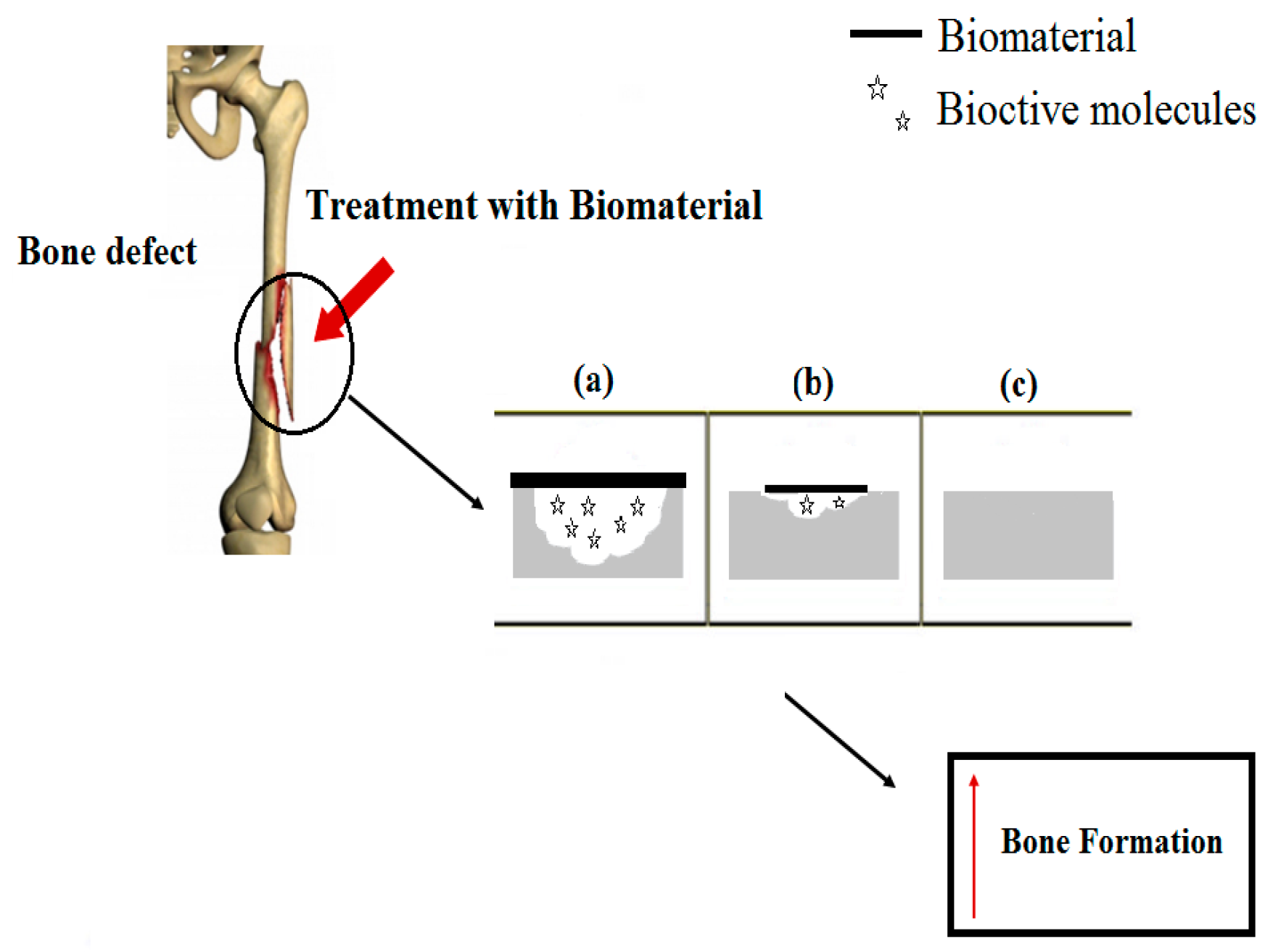
2.2. Implantable Biomaterials
2.3. Film-Forming Materials
2.4. New Approaches of DDS
3. Conclusions
Acknowledgments
Author Contributions
Conflict of Interests
References
- Belting, M.; Sandgren, S.; Wittrup, A. Nuclear delivery of macromolecules: Barriers and carriers. Adv. Drug Deliv. Rev. 2005, 57, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery strategies for sustained drug release in the lungs. Adv. Drug Deliv. Rev. 2014, 75, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006, 316, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, C.W.; Andreazza, I.F.; Ganter, J.L.M.S.; Ferrero, C.; Bresolin, T.M.B. Xanthan and galactomannan (from M. scabrella) matrix tablets for oral controlled delivery of theophylline. Int. J. Pharm. 2005, 296, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Nori, A.; Kopeček, J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv. Drug Deliv. Rev. 2005, 57, 609–636. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, L.-S.; Tan, D.C.-W.; Moochhala, S.M.; Yang, Y.-Y. Mathematical modeling and in vitro study of controlled drug release via a highly swellable and dissoluble polymer matrix: Polyethylene oxide with high molecular weights. J. Control Release 2005, 102, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 2012, 64, 237–245. [Google Scholar] [CrossRef]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Interface Sci. 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Bronich, T.K.; Nehls, A.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Novel drug delivery systems based on the complexes of block ionomers and surfactants of opposite charge. Colloids Surf. B 1999, 16, 243–251. [Google Scholar] [CrossRef]
- kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 72. [Google Scholar] [CrossRef]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic® block copolymers. J. Control Release 2004, 94, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Poloxamers in the Pharmaceutical Industry; Polymers for Controlled Drug Delivery: Boca Raton, FL, USA, 1991; pp. 189–214. [Google Scholar]
- Zhao, Y.; Moddaresi, M.; Jones, S.A.; Brown, M.B. A dynamic topical hydrofluoroalkane foam to induce nanoparticle modification and drug release in situ. Eur. J. Pharm. Biopharm. 2009, 72, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Dragan, U.; Magdalena, S. Poly(lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr. Nanosci. 2009, 5, 1–14. [Google Scholar]
- Patel, N.R.; Damann, K.; Leonardi, C.; Sabliov, C.M. Itraconazole-loaded poly(lactic-co-glycolic) acid nanoparticles for improved antifungal activity. Nanomedicine 2010, 5, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.C.C.; Barros-Timmons, A.; Trindade, T. Polymer based nanocomposites: Synthetic strategies for hybrid materials. Quim. Nova 2004, 27, 798–806. [Google Scholar] [CrossRef]
- José, N.M.; Prado, L.A.S.D. Hybrid organic-inorganic materials: Preparation and some applications. Quim. Nova 2005, 28, 281–288. [Google Scholar] [CrossRef]
- Ariga, K.; Vinu, A.; Hill, J.P.; Mori, T. Coordination chemistry and supramolecular chemistry in mesoporous nanospace. Coord. Chem. Rev. 2007, 251, 2562–2591. [Google Scholar] [CrossRef]
- Burkett, C.M.; Underwood, L.A.; Volzer, R.S.; Baughman, J.A.; Edmiston, P.L. Organic-Inorganic hybrid materials that rapidly swell in non-polar liquids: Nanoscale morphology and swelling mechanism. Chem. Mater. 2008, 20, 1312–1321. [Google Scholar] [CrossRef]
- Colilla, M.; Salinas, A.J.; Vallet-Regí, M. Amino-polysiloxane hybrid materials for bone reconstruction. Chem. Mater. 2006, 18, 5676–5683. [Google Scholar] [CrossRef]
- Ogoshi, T.; Chujo, Y. Organic-inorganic polymer hybrids prepared by the sol-gel method. Compos. Interfaces 2005, 11, 539–566. [Google Scholar] [CrossRef]
- Philipp, G.; Schmidt, H. New materials for contact lenses prepared from Si- and Ti-alkoxides by the sol-gel process. J. Non Cryst. Solids 1984, 63, 283–292. [Google Scholar] [CrossRef]
- Brennan, A.B.; Wilkes, G.L. Structure-property behaviour of sol-gel derived hybrid materials: Effect of a polymeric acid catalyst. Polymer 1991, 32, 733–739. [Google Scholar] [CrossRef]
- Girard-Reydet, E.; Lam, T.M.; Pascault, J.P. In situ polymerization of tetraethoxysilane in poly(vinyl acetate). Macromol. Chem. Phys. 1994, 195, 149–158. [Google Scholar] [CrossRef]
- Matějka, L.; Dukh, O.; Kamišová, H.; Hlavatá, D.R.; Špı́rková, M.; Brus, J. Block-copolymer organic-inorganic networks. Structure, morphology and thermomechanical properties. Polymer 2004, 45, 3267–3276. [Google Scholar] [CrossRef]
- Rekondo, A.; Fernández-Berridi, M.J.; Irusta, L. Photooxidation and stabilization of silanised poly(ether-urethane) hybrid systems. Polym. Degrad. Stable 2007, 92, 2173–2180. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F.; Rozes, L.; Alonso, B. Design of Hybrid organic-inorganic nanocomposites synthesized via sol-gel chemistry. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A 2000, 354, 143–158. [Google Scholar] [CrossRef]
- Benvenutti, E.V.; Moro, C.C.; Costa, T.M.H.; Gallas, M.R. Silica based hybrid materials obtained by the sol-gel method. Quím. Nova 2009, 32, 1926–1933. [Google Scholar] [CrossRef]
- Franken, L.; Santos, L.S.; Caramão, E.B.; Costa, T.M.H.; Benvenutti, E.V. p-Anisidinepropylsilica xerogel: Thermal stability and resistance to leaching by solvents. Quím. Nova 2002, 25, 563–566. [Google Scholar] [CrossRef]
- Nassar, E.J.; Ávila, L.R.; Pereira, P.F.S.; Nassor, E.C.O.; Cestari, A.; Ciuffi, K.J.; Calefi, P.S. Phenylsilicate doped with Eu III obtained by sol-gel methodology. Quím. Nova 2007, 30, 1567–1572. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid sol-gel-derived polymers: Applications of multifunctional materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Kawakami, K.; Ebara, M.; Kotsuchibashi, Y.; Ji, Q.; Hill, J.P. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J. Chem. 2014, 38, 5149–5163. [Google Scholar] [CrossRef]
- Brinker, J.C.; Scherer, G.W. Sol-Gel: The Physiscs and Chemystry of Sol-Gel; Academic Press, Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- García, O.; Garrido, L.; Sastre, R.; Costela, A.; García-Moreno, I. Synthetic Strategies for hybrid materials to improve properties for optoelectronic applications. Adv. Funct. Mater. 2008, 18, 2017–2025. [Google Scholar] [CrossRef]
- Franville, A.-C.; Zambon, D.; Mahiou, R.; Troin, Y. Luminescence behavior of sol-gel-derived hybrid materials resulting from covalent grafting of a chromophore unit to different organically modified alkoxysilanes. Chem. Mater. 2000, 12, 428–435. [Google Scholar] [CrossRef]
- Radin, S.; Falaize, S.; Lee, M.H.; Ducheyne, P. In vitro bioactivity and degradation behavior of silica xerogels intended as controlled release materials. Biomaterials 2002, 23, 3113–3122. [Google Scholar] [CrossRef]
- De Menezes, E.W.; Lima, E.C.; Royer, B.; de Souza, F.E.; dos Santos, B.D.; Gregório, J.R.; Costa, T.M.H.; Gushikem, Y.; Benvenutti, E.V. Ionic silica based hybrid material containing the pyridinium group used as an adsorbent for textile dye. J. Colloid Interface Sci. 2012, 378, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.F.; Parreira, R.L.T.; de Faria, E.H.; de Carvalho, H.W.P.; Caramori, G.F.; Coimbra, D.F.; Nassar, E.J.; Ciuffi, K.J. Ureasil-poly(ethylene oxide) hybrid matrix for selective adsorption and separation of dyes from water. Langmuir 2014, 30, 3857–3868. [Google Scholar] [CrossRef] [PubMed]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Lin, C.X.; Qiao, S.Z.; Yu, C.Z.; Ismadji, S.; Lu, G.Q. Periodic mesoporous silica and organosilica with controlled morphologies as carriers for drug release. Microporous Microporous Mater. 2009, 117, 213–219. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Vallet-Regí, M. Time-delayed release of bioencapsulates: A novel controlled delivery concept for bone implant technologies. Chem. Mater. 2008, 20, 4826–4834. [Google Scholar] [CrossRef]
- Web of Science. Available online: https://apps.webofknowledge.com/UA_GeneralSearch_input.do?product=UA&search_mode=GeneralSearch&SID=3EUUQncnjuXFJIG5vRe&preferencesSaved= (accessed on 28 August 2015).
- Gao, S.F.; Chen, H.G.; Wang, Z.H.; Cui, Z.J.; Zhang, H.Y. Preparation, characterisation and controlled drug release from thermosensitive hybrid hydrogels. Plast. Rubber Compos. 2012, 41, 13–17. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials 2007, 28, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Díaz, U.; Arrica, M.; Fernández, E.; Ortega, Í. Organic–inorganic nanospheres with responsive molecular gates for drug storage and release. Angew. Chem. 2009, 48, 6247–6250. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Liu, J.; Lu, G.Q.; Qiao, S.Z. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 11173–11178. [Google Scholar] [CrossRef]
- Wan, X.; Wang, D.; Liu, S. Fluorescent pH-sensing organic/inorganic hybrid mesoporous silica nanoparticles with tunable redox-responsive release capability. Langmuir 2010, 26, 15574–15579. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.F.; Jesus, C.R.N.; Chiavacci, L.A.; Pulcinelli, S.H.; Briois, V.; Santilli, C.V. Ureasil-polyether hybrid blend with tuneable hydrophilic/hydrophobic features based on U-PEO 1900 and U-PPO400 mixtures. J. Sol Gel Sci. Technnol. 2014, 70, 317–328. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Colilla, M.; Gonzalez, B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Phosphorus-containing SBA-15 materials as bisphosphonate carriers for osteoporosis treatment. Microporous Mesoporous Mater. 2010, 135, 51–59. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Rogers, M.J. Bisphosphonates: From the laboratory to the clinic and back again. Bone 1999, 25, 97–106. [Google Scholar] [CrossRef]
- Santilli, C.V.; Chiavacci, L.A.; Lopes, L.; Pulcinelli, S.H.; Oliveira, A.G. Controlled drug release from ureasil-polyether hybrid materials. Chem. Mater. 2009, 21, 463–467. [Google Scholar] [CrossRef]
- Xue, J.M.; Shi, M. PLGA/mesoporous silica hybrid structure for controlled drug release. J. Control Release 2004, 98, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Zurdo Schroeder, I.; Franke, P.; Schaefer, U.F.; Lehr, C.-M. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.K.; Bruno, C.H.; Lopes, L.; Pulcinelli, S.H.; Santilli, C.V.; Chiavacci, L.A. Ureasil–polyether hybrid film-forming materials. Colloids Surf. B. 2013, 101, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Oshiro-Junior, J.A.; Carvalho, F.C.; Soares, C.P.; Chorilli, M.; Chiavacci, L.A. Development of cutaneous bioadhesive ureasil-polyether hybrid films. Int. J. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Junior, J.A.O.; Shiota, L.M.; Chiavacci, L.A. Development of organic-inorganic polymeric film formers for controlled drug release andwound care. Matéria 2014, 19, 24–32. [Google Scholar]
- Paredes, M.; Pulcinelli, S.H.; Peniche, C.; Gonçalves, V.; Santilli, C.V. Chitosan/(ureasil-PEO hybrid) blend for drug delivery. J. Sol Gel Sci. Technnol. 2014, 72, 233–238. [Google Scholar] [CrossRef]
- Palm, C.; Jayamanne, M.; Kjellander, M.; Hällbrink, M. Peptide degradation is a critical determinant for cell-penetrating peptide uptake. Biochem. Biophys. Acta 2007, 1768, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Izquierdo-Barba, I.; Nakase, I.; Futaki, S.; Ruan, J.; Sakamoto, K.; Sakamoto, Y.; Kuroda, K.; Terasaki, O.; Che, S. Mesostructured silica based delivery system for a drug with a peptide as a cell-penetrating vector. Microporous Microporous Mater. 2009, 122, 201–207. [Google Scholar] [CrossRef]
- Muñoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. MCM-41 organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Lin, M.; Wang, H.; Meng, S.; Zhong, W.; Li, Z.; Cai, R.; Chen, Z.; Zhou, X.; Du, Q. Structure and release behavior of PMMA/silica composite drug delivery system. J. Pharm. Sci. 2007, 96, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.F.; Pulcinelli, S.H.; Santilli, C.V.; Blanchandin, S.; Briois, V. Controlled cisplatin delivery from Ureasil-PEO 1900 hybrid matrix. J. Phys. Chem. 2010, 114, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.; Molina, E.F.; Chiavacci, L.A.; Santilli, C.V.; Briois, V.; Pulcinelli, S.H. Drug-matrix interaction of sodium diclofenac incorporated into ureasil-poly(ethylene oxide) hybrid materials. RSC Adv. 2012, 2, 5629–5636. [Google Scholar] [CrossRef]
- Herculano, R.D.; Brunello, C.A.; Melo, J.P., Jr.; Martins, M.; Borges, F.A.; Chiavacci, L.A.; Graeff, C.F.O. Novel solid state nitric oxide sensor using siloxano-poly(oxypropylene) (PPO). Mater. Sci. Appl. 2013, 4, 683–688. [Google Scholar]
- Nava, E.; Lüscher, T.F. Endothelium-derived vasoactive factors in hypertension: Nitric oxide and endothelin. J. Hypertens. 1995, 13, S39–S48. [Google Scholar] [CrossRef]
- Vasta, V.; Meacci, E.; Farnararo, M.; Bruni, P. Identification of a specific transport system for L-Arginine in human platelets. Biochem. Biophys. Res. Commun. 1995, 206, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Herculano, R.D.; Tzu, L.C.; Silva, C.P.; Brunello, C.A.; Queiroz, Á.A.A.D.; Kinoshita, A.; Graeff, C.F.D.O. Nitric oxide release using natural rubber latex as matrix. Mater. Res. 2011, 14, 355–359. [Google Scholar] [CrossRef]
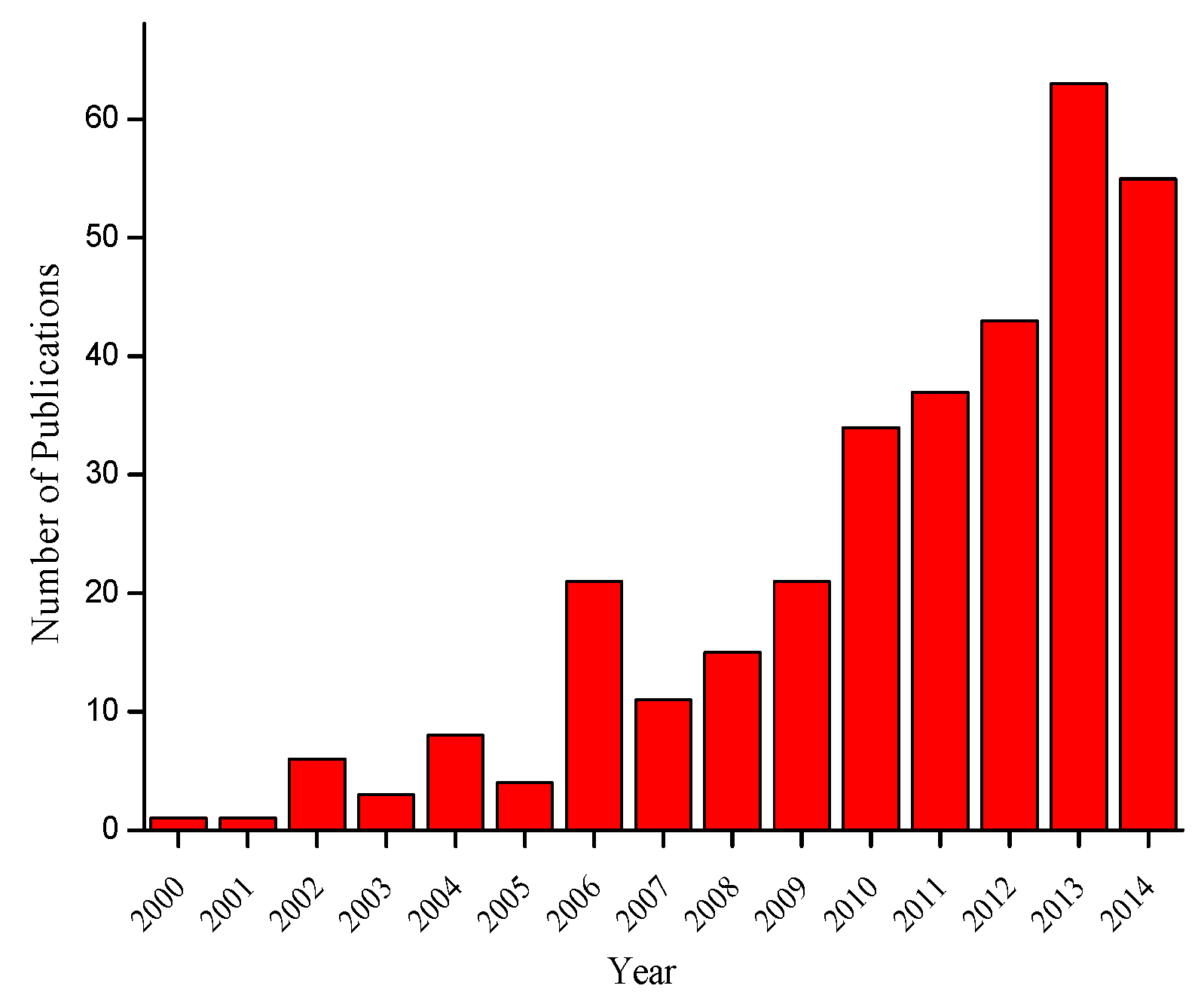

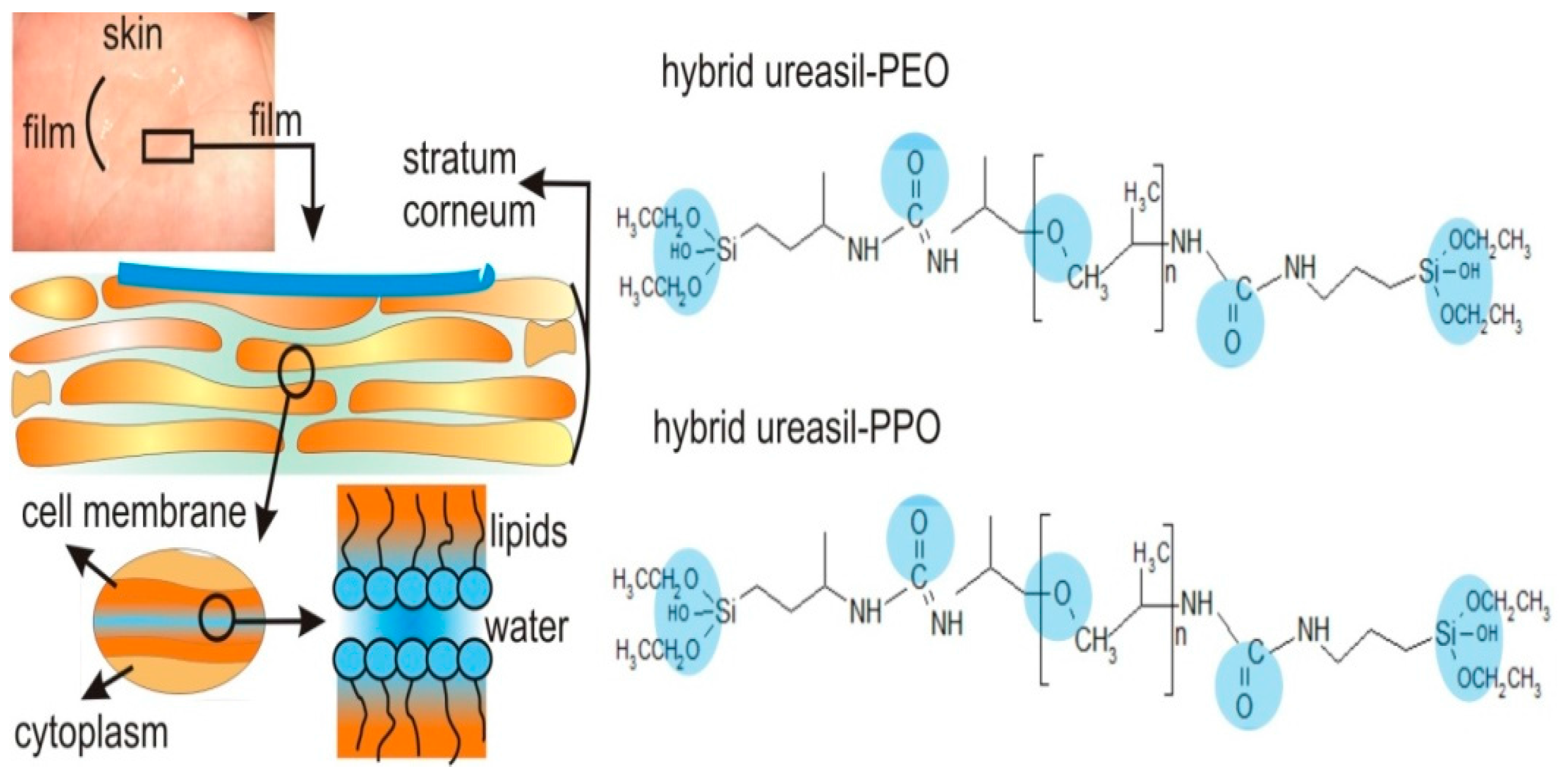
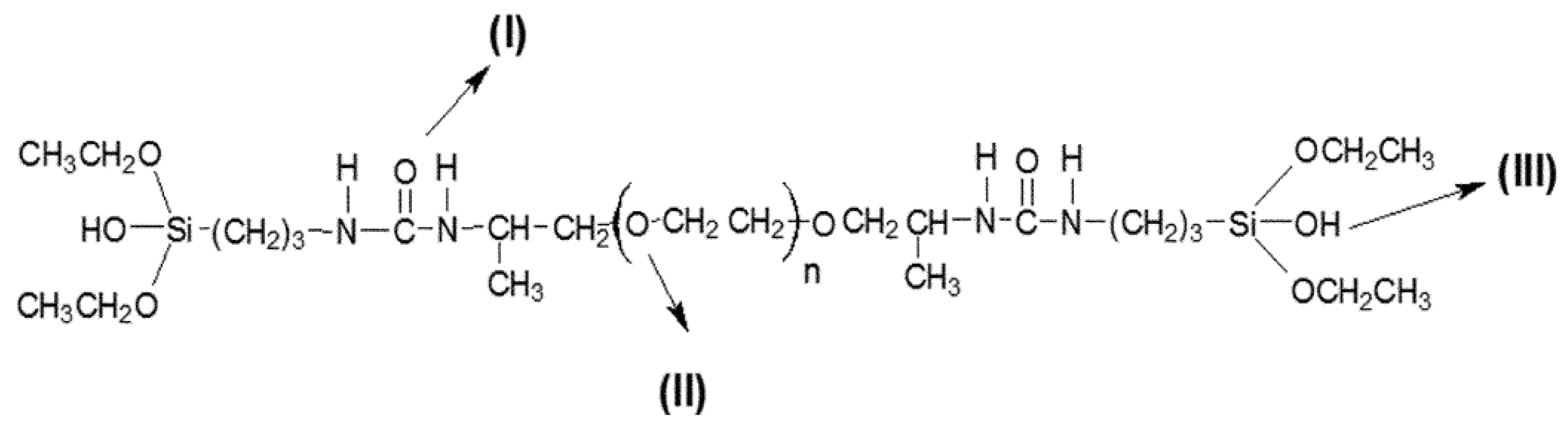
| Organic-Inorganic System Composition | Drug | Time of Released of Drug | Comments | Reference |
|---|---|---|---|---|
| PVA/PNIPAAm | Rhodamine B | 16 h = ± 95% at 38 °C 16 h = ± 70% at 20 °C | Temperature influences in the release behavior | [51] |
| BTEPAA | Doxorubicin | 48 h = ± 10% in pH ≤ 9 and ± 95% in pH ≥ 9. | pH responsive systems | [53] |
| Cs/MSM | Ibuprofen | 4 h = ± 20% in pH 7.4 and ± 90% in pH 5. | pH responsive systems | [54] |
| NAS/NAphMA/OEGMA/MSM | Rhodamine B | 4 h = ± 95% with 20 mM DDT 4 h = ± 5% without DDT | Fluorescence properties | [55] |
| PEO/3-(isocyanatopropyl)-triethoxysilane/Magnetite | Sodium diclofenac | 4 h = ± 95% with the application magnetic and 80% without magnetic field (0.250 T, 220 kHz) | Stimulis with Magnetic field | [56] |
| Pluronic/Tetraethyl orthosilicate/P2O5/H3PO4 | Alendronate | 4 h = 25 µg·m−2 to material without P2O5 and 87 µg·m−2 to material with P2O5 | Implantable systems | [58] |
| PLGA/PS | Gentamicin | 24 h = 77% PLGA pure and 41% PLGA/PS. 25 days = 90% PLGA and 58% PLGA/PS | Implantable Systems | [61] |
| PEO/3-(isocyanatopropyl)-triethoxysilane/Chitosan | Pramoxine | 3 h = ± 100% without chitosan and 30% with 3% of chitosan | Film-forming systems | [66] |
| Pluronic(127)/CPPs/Tetraethyl orthosilicateas | FITC/octaarginine | 5 days = 85% | New devices of DDS | [68] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshiro Junior, J.A.; Paiva Abuçafy, M.; Berbel Manaia, E.; Lallo da Silva, B.; Chiari-Andréo, B.G.; Aparecida Chiavacci, L. Drug Delivery Systems Obtained from Silica Based Organic-Inorganic Hybrids. Polymers 2016, 8, 91. https://doi.org/10.3390/polym8040091
Oshiro Junior JA, Paiva Abuçafy M, Berbel Manaia E, Lallo da Silva B, Chiari-Andréo BG, Aparecida Chiavacci L. Drug Delivery Systems Obtained from Silica Based Organic-Inorganic Hybrids. Polymers. 2016; 8(4):91. https://doi.org/10.3390/polym8040091
Chicago/Turabian StyleOshiro Junior, João Augusto, Marina Paiva Abuçafy, Eloísa Berbel Manaia, Bruna Lallo da Silva, Bruna Galdorfini Chiari-Andréo, and Leila Aparecida Chiavacci. 2016. "Drug Delivery Systems Obtained from Silica Based Organic-Inorganic Hybrids" Polymers 8, no. 4: 91. https://doi.org/10.3390/polym8040091