Structural Properties and Antifungal Activity against Candida albicans Biofilm of Different Composite Layers Based on Ag/Zn Doped Hydroxyapatite-Polydimethylsiloxanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Deposition of PDMS LAyers on Ti Substrates
2.3. Deposition of HAp Layers on a Titanium Substrate Previously Coated with a PDMS Layer
2.4. Deposition of Ag:HAp and Zn:HAp Layers on a Titanium Substrate Previously Coated with a PDMS Layer
2.5. Structural Characterizations
2.6. In Vitro Antifungal Activity
3. Results and Discussions
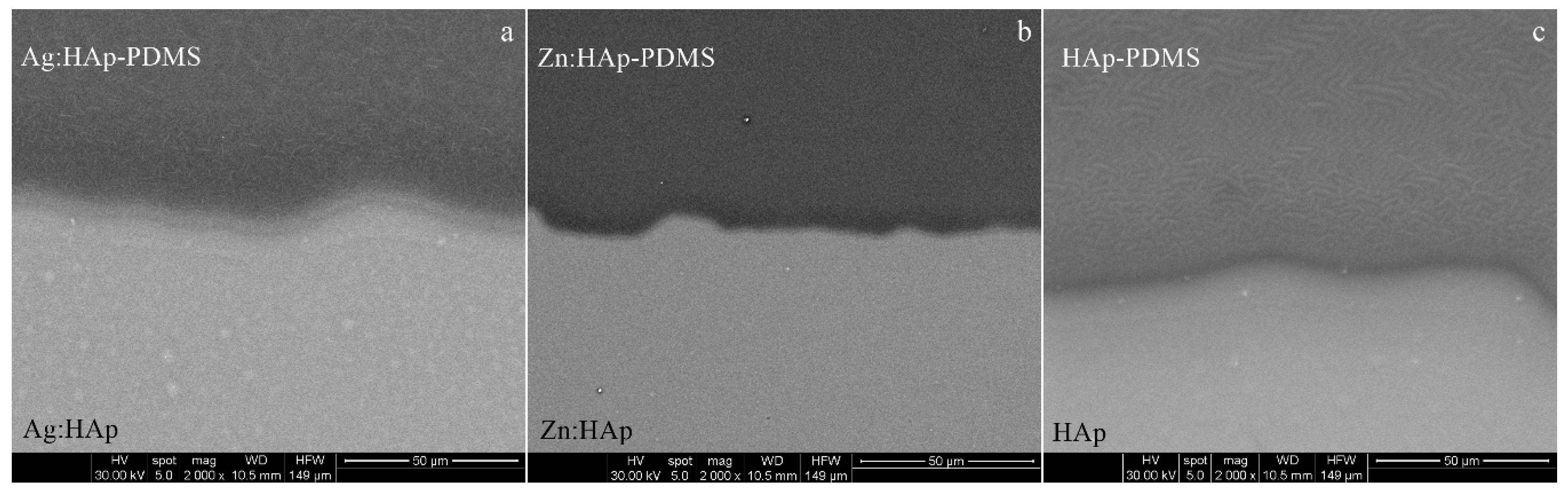
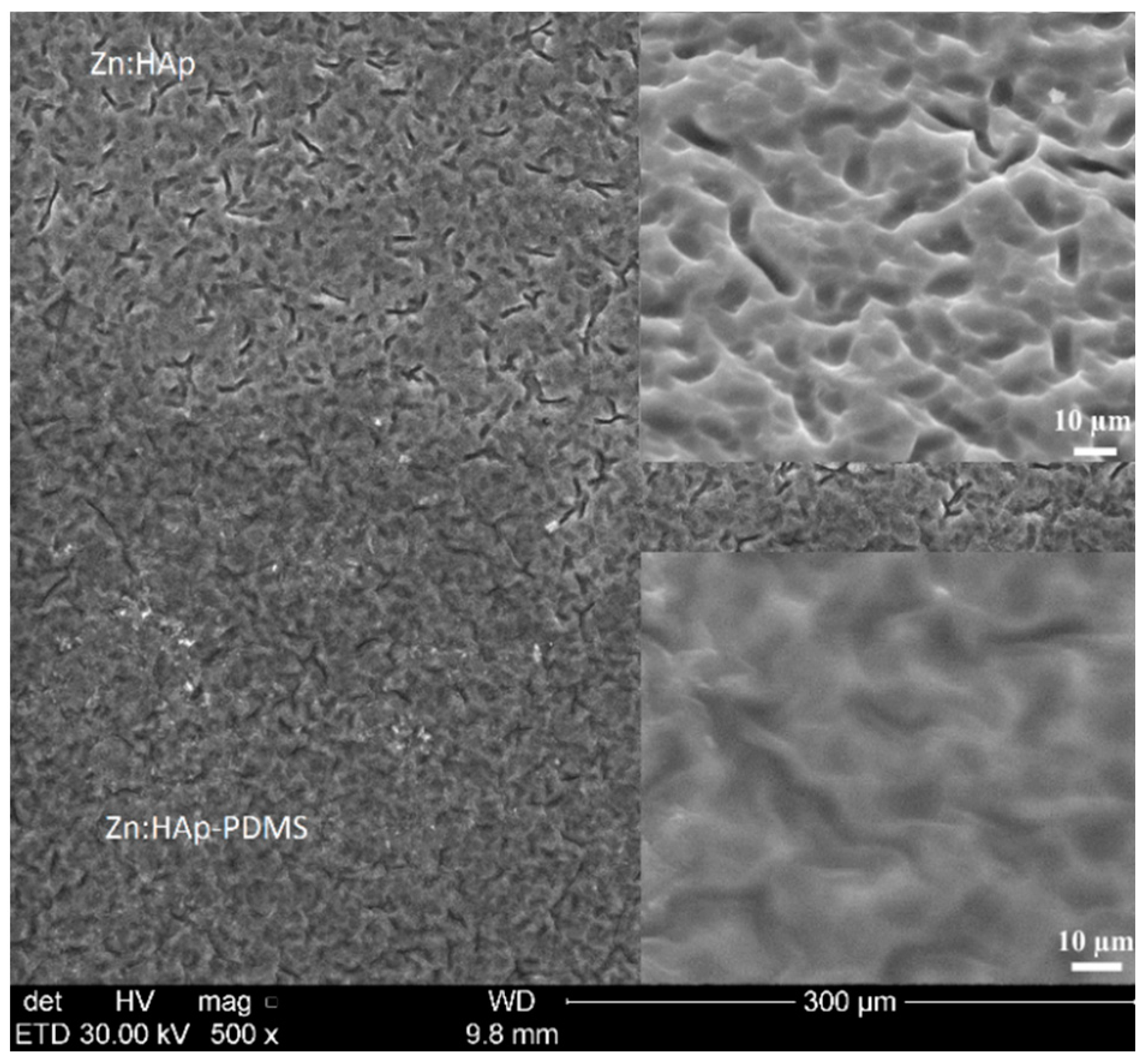
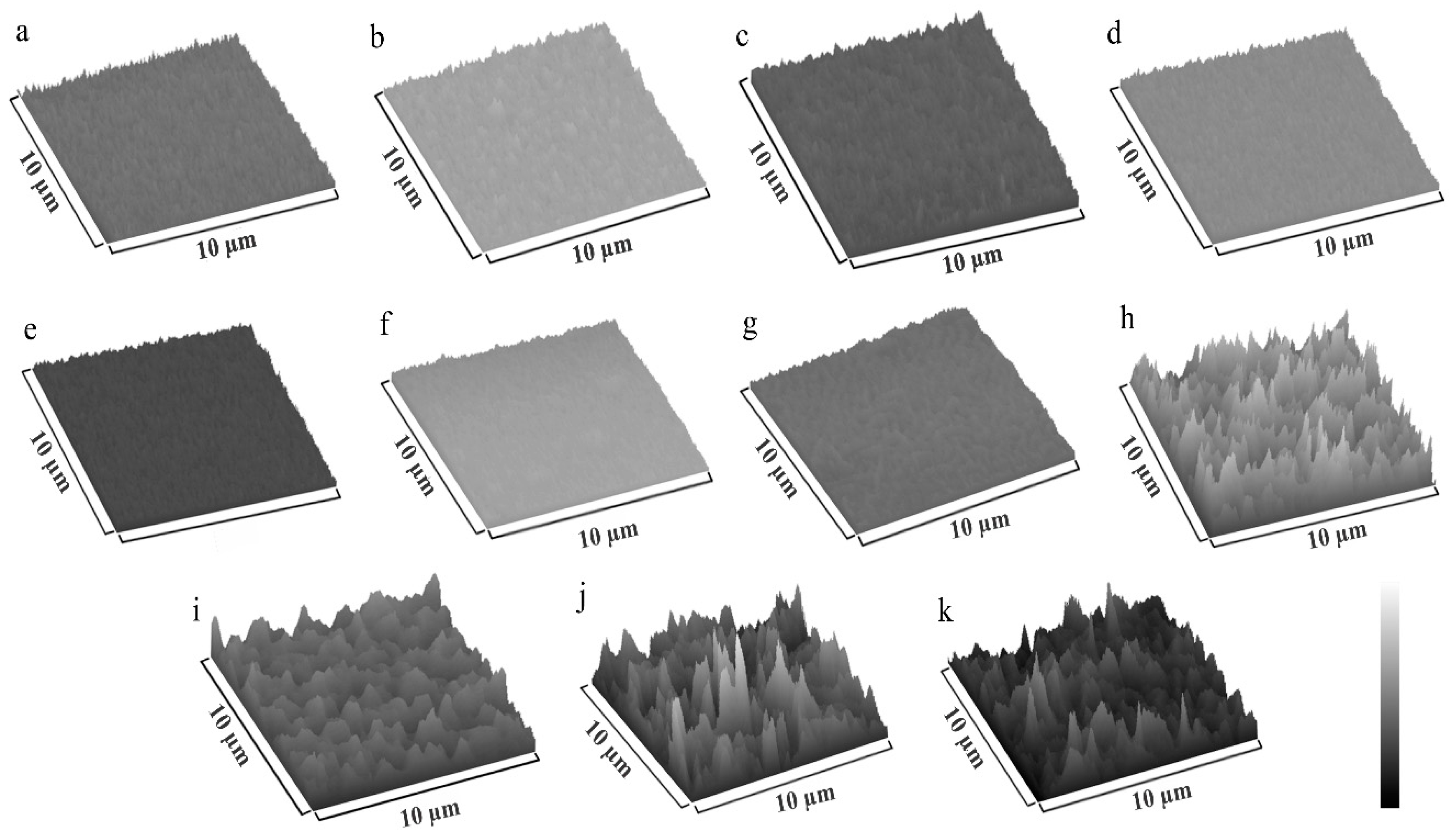
3.1. Scanning Electron Microscopy
3.2. Glow Discharge Optical Emission Spectrometry (GDOES)
3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.4. Biofilm Thickness and Cell Density Analysis Using Confocal Laser Scanning Microscopy (CLSM)
3.5. Biofilm Morphology Analysis Using Scanning Electron Microcopy (SEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PDMS | Poly(dimethylsiloxane) |
| Ti | Titanium |
| HAp | Hydroxyapatite |
| Ag:HAp | Silver doped hydroxyapatite |
| Zn:HAp | Zinc doped hydroxyapatite |
| HAp-PDMS | Hydroxyapatite on a Titanium substrate previously coated with polydimethylsiloxane |
| Ag:HAp-PDMS | Silver doped hydroxyapatite on a Titanium substrate previously coated with polydimethylsiloxane |
| Zn:HAp-PDMS | Zinc doped hydroxyapatite on a Titanium substrate previously coated with polydimethylsiloxane |
| SEM | Scanning Electron Microscopy |
| GDOES | Glow Discharge Optical Emission Spectroscopy |
| GD | Glow Discharge |
| FTIR | Fourier Transform Infrared Spectroscopy |
| YPG | Yeast peptone glucose medium |
| CLSM | Confocal laser scanning microcopy |
| R | Surface roughness |
| IR | Infrared |
References
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; McCourtie, J.; MacFarlane, T.W. Factors affecting the in vitro adherence of Candida albicans to acrylic surfaces. Arch. Oral Biol. 1980, 25, 611–615. [Google Scholar] [CrossRef]
- Bareiro, O.; Santos, L.A. Poly(dimethyl siloxane)/tetraethyl orthosilicate modified hydroxyapatite composites: Surface energy, roughness and mechanical performance. Available online: metallum.com.br (accessed on 2 October 2016).
- Jovanović, J.; Adnadjević, B.; Kićanović, M.; Uskoković, D. The influence of hydroxyapatite modification on the cross-linking of polydimethylsiloxane/HAp composites. In Calcium Phosphate Ceramics—Bioresorbable Polymer Composite Biomaterials; Uskoković, D.P., Ignjatović, N.L., Eds.; Institute of Technical Sciences of the Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2007; pp. 217–226. [Google Scholar]
- Hayakawa, S.; Kanaya, T.; Tsuru, K.; Shirosaki, Y.; Osaka, A.; Fujii, E.; Kawabata, K.; Gasqueres, G.; Bonhomme, C.; Babonneau, F.; et al. Heterogeneous structure and in vitro degradation behavior of wet-chemically derived nanocrystalline silicon-containing hydroxyapatite particles. Acta Biomater. 2013, 9, 4856–4867. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Kumta, P.N. Sol-gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Liu, D.M.; Troczynski, T.; Tseng, W.J. Water-based sol-gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Rams, T.E.; Roberts, T.W.; Feik, D.; Molzan, A.K.; Slots, J. Clinical and microbiological findings on newly inserted hydroxyapatite-coated and pure titanium human dental implants. Clin. Oral Implant. Res. 1991, 2, 121–127. [Google Scholar] [CrossRef]
- Jankauskaitė, V.; Abzalbekuly, B.; Lisauskaitė, A.; Procyčevas, I.; Fataraitė, E.; Vitkauskienė, A.; Janakhmetov, U. Silicone rubber and microcrystalline cellulose composites with antimicrobial PROPERTIES. Mater. Sci. 2014, 20, 42–49. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X. Silver nanoparticle-modified films versus biomedical device-associated infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, G.; Dhanaraju, M.D. Silver nanoparticles: Real antibacterial bullets. In Antimicrobial Agents; Bobbarala, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 407–422. [Google Scholar]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Kheybari, S.; Samadi, N.; Hosseini, S.V.; Fazeli, A.; Fazeli, M.R. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. DARU 2010, 18, 168–172. [Google Scholar] [PubMed]
- Jallot, E.; Nedelec, J.M.; Grimault, A.S.; Chassot, E.; Grandjean-Laquerriere, A.; Laquerriere, P.; Laurent-Maquin, D. STEM and EDXS characterisation of physico-chemical reactions at the periphery of sol–gel derived Zn-substituted hydroxyapatites during interactions with biological fluids. Colloids Surf. B Biointerfaces 2005, 42, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jallot, E.; Irigaray, J.L.; Oudadesse, H.; Brun, V.; Weber, G.; Frayssinet, P. Resorption kinetics of four hydroxyapatite-based ceramics by particle induced X-ray emission and neutron activation analysis. Eur. Phys. J. Appl. Phys. 1999, 6, 205–215. [Google Scholar] [CrossRef]
- Caleb, O.; Molokwu, B.S.; Yang, V.; Li, M.B. Zinc homeostasis and bone mineral density. Ohio Res. Clin. Rev. 2006, 15, 1–15. [Google Scholar]
- Matsunaga, K.; Murata, H.; Mizoguchi, T.; Nakahira, A. Mechanism of incorporation of Zinc into hydroxyapatite. Acta Biomater. 2010, 6, 2289–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, H.; Ito, A.; Miyakawa, S.; Layrolle, P.; Ojima, K.; Ichinose, N.; Tateishi, T. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J. Biomed. Mater. Res. 2000, 50, 184–190. [Google Scholar] [CrossRef]
- Bareiro, O.; Santos, L.A. Tetraethylorthosilicate (TEOS) applied in the surface modification ofhydroxyapatite to develop polydimethylsiloxane/hydroxyapatitecomposites. Colloids Surf. B 2014, 115, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.W.; Shah, J.; Misra, R.D.K. Superior in vitro biological response and mechanical properties of an implantable nanostructured biomaterial: Nanohydroxyapatite-silicone rubber composite. Acta Biomater. 2009, 5, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bhowmick, A.K. Tailor-made fibrous hydroxyapatite/polydimethylsiloxane composites: Insight into the kinetics of polymerization in the presence of filler and structure-property relationship. J. Phys. Chem. C 2012, 116, 26551–26560. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Andriot, M.; Chao, S.H.; Colas, A.R.; Cray, S.E.; de Buyl, F.; de Groot, J.V.; Dupont, A.; Easton, T.; Garaud, J.L.; Gerlach, E.; et al. Silicones in industrial application. Dow Corning Corp 2009, 84, 1–106. [Google Scholar]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X. Mechanical properties evolution of polydimethylsiloxane during crosslinking process. MRS Proc. 2006, 975. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Q.; Cheng, L.; Lei, Y.; Zhang, A. Synthesis and antimicrobial activities of polysiloxane-containing quaternary ammonium salts on bacteria and phytopathogenic fungi. React. Funct. Polym. 2014, 85, 36–44. [Google Scholar] [CrossRef]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.L.; McDonald, A.; Gourley, P.L.; Sasaki, D.Y. Poly(dimethylsiloxane) thin films as biocompatible coatings for microfluidic devices: Cell culture and flow studies with glial cells. J. Biomed. Mater. Res. A 2005, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ceseracciu, L.; Heredia-Guerrero, J.A.; Dante, S.; Athanassiou, A.; Bayer, I.S. Robust and biodegradable elastomers based on corn starch and polydimethylsiloxane (PDMS). ACS Appl. Mater. Interfaces 2015, 7, 3742–3753. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.; Adamczyk, L. Properties of hydroxyapatite layers used for implant coatings. Opt. Appl. 2013, 43, 143–151. [Google Scholar]
- Kim, D.H.; Kong, Y.M.; Lee, S.H.; Lee, I.S.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Composition and crystallization of hydroxyapatite coating layer formed by electron beam deposition. J. Am. Ceram. Soc. 2003, 86, 186–188. [Google Scholar] [CrossRef]
- Mediaswanti, K.; Wen, C.; Ivanova, E.P.; Berndt, C.C.; Wang, J. Sputtered hydroxyapatite nanocoatings on novel titanium alloys for biomedical applications. In Titanium Alloys—Advances in Properties Control; Sieniawski, J., Ziaja, W., Eds.; InTech: Rijeka, Croatia, 2013; pp. 21–44. [Google Scholar]
- Popa, C.L.; Groza, A.; Chapon, P.; Ciobanu, C.S.; Ghita, R.V.; Trusca, R.; Ganciu, M.; Predoi, D. Physicochemical analysis of the polydimethylsiloxane interlayer influence on a hydroxyapatite doped with silver coating. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Groza, A.; Iconaru, S.L.; Popa, C.L.; Chapon, P.; Chifiriuc, M.C.; Hristu, R.; Stanciu, G.A.; Negrila, C.C.; Ghita, R.V.; et al. Antimicrobial activity evaluation on silver doped hydroxyapatite/polydimethylsiloxane composite layer. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.-P.; Rocha, L.A. Wear and corrosion interactions on titanium in oral environment: Literature review. J. Bio- Tribo- Corros. 2015, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dhir, S. Biofilm and dental implant: The microbial link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Surmeian, A.; Diplasu, C.; Luculescu, C.; Chapon, P.; Tempez, A.; Ganciu, M. Physico-chemical processes occurring during polymerization of liquid polydimethylsiloxane films on metal substrates under atmospheric pressure air corona discharges. Surf. Coat. Technol. 2012, 2012, 145–151. [Google Scholar] [CrossRef]
- Groza, A.; Surmeian, A. Characterization of the oxides present in a polydimethylsiloxane layer obtained by polymerisation of its liquid precursor in corona discharge. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Costescu, A.; Pasuk, I.; Ungureanu, F.; Dinischiotu, A.; Costache, M.; Huneau, F.; Galaup, S.; Le Coustumer, P.; Predoi, D. Physico-chemical properties of nano-sized hexagonal hydroxyapatite powder synthesized by sol-gel. Dig. J. Nanomater. Biostruct. 2010, 5, 989–1000. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Bartha, C.M.; Albu, M.; Guegan, R.; Motelica-Heino, M.; Chifiriuc, M.C.; Bleotu, C.; Badea, M.L.; Antohe, S. Synthesis, characterization and cytotoxicity evaluation on Zinc doped hydroxyapatite in collagen matrix. Dig. J. Nanomater. Biostruct. 2015, 10, 681–691. [Google Scholar]
- Iconaru, S.L.; Chapon, P.; Le Coustumer, P.; Predoi, D. Antimicrobial activity of thin solid films of silver doped hydroxyapatite prepared by sol-gel method. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared Characteristic Group Frequencies, 2nd ed.; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]
- Aminian, A.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Bakhshi, F.; Gorjipour, F.; Farzadi, A.; Moztarzadeh, F.; Schmucker, M. Synthesis of silicon-substituted hydroxyapatite by a hydrothermal method with two different phosphorous sources. Ceram. Int. 2011, 37, 1219–1229. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Rebelo, A.H.S.; Lemos, A.F.; Rocha, J.H.G.; Ventura, J.M.G.; Ferreira, J.M.F. Suitability evaluation of sol-gel derived Si-substituted hydroxyapatite for dental and maxillofacial applications through in vitro osteoblasts response. Dent. Mater. 2008, 24, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Hijon, N.; Cabanas, M.V.; Pena, J.; Vallet-Regi, M. Dip coated silicon-substituted hydroxyapatite films. Acta Biomater. 2006, 5, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.J.; Parthasarathy, G.; Sarmah, N.C. Fourier transform infrared spectroscopic estimation of crystallinity in SiO2 based rocks. Bull. Mater. Sci. 2008, 31, 775–779. [Google Scholar] [CrossRef]
- Hilonga, A.; Kim, J.K.; Sarawade, P.B.; Kim, H.T. Influence of annealing conditions on the properties of reinforced silver embedded silica matrix from the cheap silica source. Appl. Surf. Sci. 2010, 256, 2849–2855. [Google Scholar] [CrossRef]
- Jovanovic, J.; Adnadjevic, B.; Kicanovic, M.; Uskokovic, D. The influence of hydroxyapatite modification on the crosslinking of polydimethylsiloxane/HAp composites. Colloids Surf. B Biointerfaces 2014, 39, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-pottuz, L.; Babonneau, F.; Esnoufc, C.; Chevalier, J.; Bernache-assollant, D. Accurate characterization of pure silicon-substituted hydroxyapatite powders synthesized by a new precipitation route. Acta Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, H.; Serra, J.; Gonzalez, P.; Leon, B. Structural study of sol-gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- López-Alvarez, M.; Solla, E.L.; González, P.; Serra, J.; León, B.; Marques, A.P.; Reis, R.L. Silicon-hydroxyapatite bioactive coatings (Si-HA) from diatomaceous earth and silica. Study of adhesion and proliferation of osteoblast-like cells. J. Mater. Sci. Mater. Med. 2009, 20, 1131–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, D.M.; Mostafa, A.A.; Korowash, S.I. Chemical characterization of some substituted hydroxyapatites. Chem. Cent. J. 2011, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.G.; Phoenix, V.R.; Yee, N.; Tobin, M.J. Molecular characterization of cyanobacterial silicification using synchrotron infrared micro-spectroscopy. Geochim. Cosmochim. Acta 2004, 68, 729–741. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields, C.W., IV; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 28, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Liu, T.; Lai, D.; Feng, X.; Zhu, H.; Chen, J. Synthesis and characterization of a novel mesoporous bioactive glass/hydroxyapatite nanocomposite. Mater. Lett. 2013, 92, 444–447. [Google Scholar] [CrossRef]
- Armelao, L.; Bertoncello, R.; de Dominicis, M. Silver nanocluster formation in silica coatings by the sol-gel route. Adv. Mater. 1997, 9, 736–741. [Google Scholar] [CrossRef]
- Shevchenko, G.P.; Vashchanka, S.V.; Bokshits, Y.V.; Rakhmanov, S.K. On the nature of the processes occurring in silver doped SiO2 films under heat treatment. J. Sol Gel Sci. Technol. 2008, 45, 143–149. [Google Scholar] [CrossRef]
- Salvi, G.E.; Furst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res. 2008, 19, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Penk, A.; Pittrow, L. Role of fluconazole in the long-term suppressive therapy of fungal infections in patients with artificial implants. Mycoses 1999, 42, 91–96. [Google Scholar] [PubMed]
- Ramage, G.; Martinez, J.P.; Lopez-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Graybill, J.R.; Burgess, D.; Hardin, T. Key issues concerning fungistatic versus fungicidal drugs. Eur. J. Clin. Microbiol. 1997, 16, 42–50. [Google Scholar] [CrossRef]
- Miranda, M.; Fernandez, A.; Lopez-Esteban, S.; Malpartida, F.; Moya, J.S.; Torrecillas, R. Ceramic/metal biocidal nanocomposites for bone-related applications. J. Mater. Sci. 2012, 23, 1655–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.A.; Kalmodia, S.; Kesarwani, P.; Basu, B.; Balani, K. Bactericidal effect of silver-reinforced carbon nanotube and hydroxyapatite composites. J. Biomater. Appl. 2012, 27, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. BioMed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, F.; Boeglib, L.; Agostinho, A.; Morales, E.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Zamperini, C.A.; André, R.S.; Longo, V.M.; Mima, E.G.; Vergani, C.E.; Machado, A.L.; Varela, J.A.; Longo, E. Antifungal applications of ag-decorated hydroxyapatite nanoparticles. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; SuiMaeb, L.; Kit, A.Y. ZnO deposited/encapsulated halloysite-poly(lactic acid) (PLA) nanocomposites for high performance packaging films with improved mechanical and antimicrobial properties. Appl. Clay Sci. 2015, 111, 10–20. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. Nanoantibiotics: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Le, A.T.; Le, T.T.; Tran, H.H.; Dang, D.A.; Tran, Q.H.; Vu, D.L. Powerful colloidal silver nanoparticles for the prevention of gastrointestinal bacterial infections. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 1–10. [Google Scholar] [CrossRef]


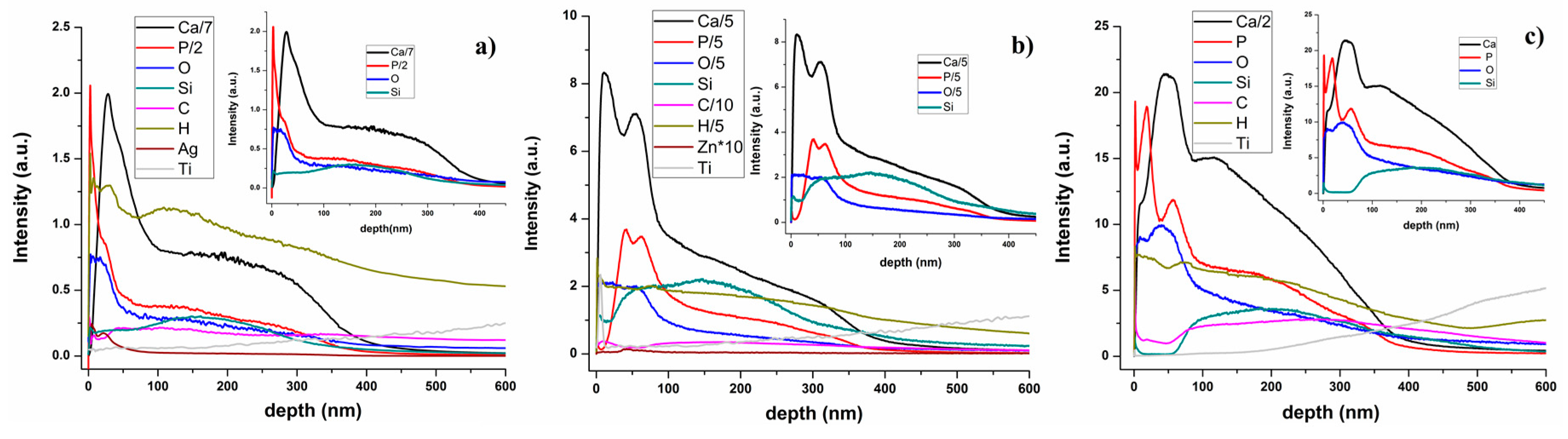

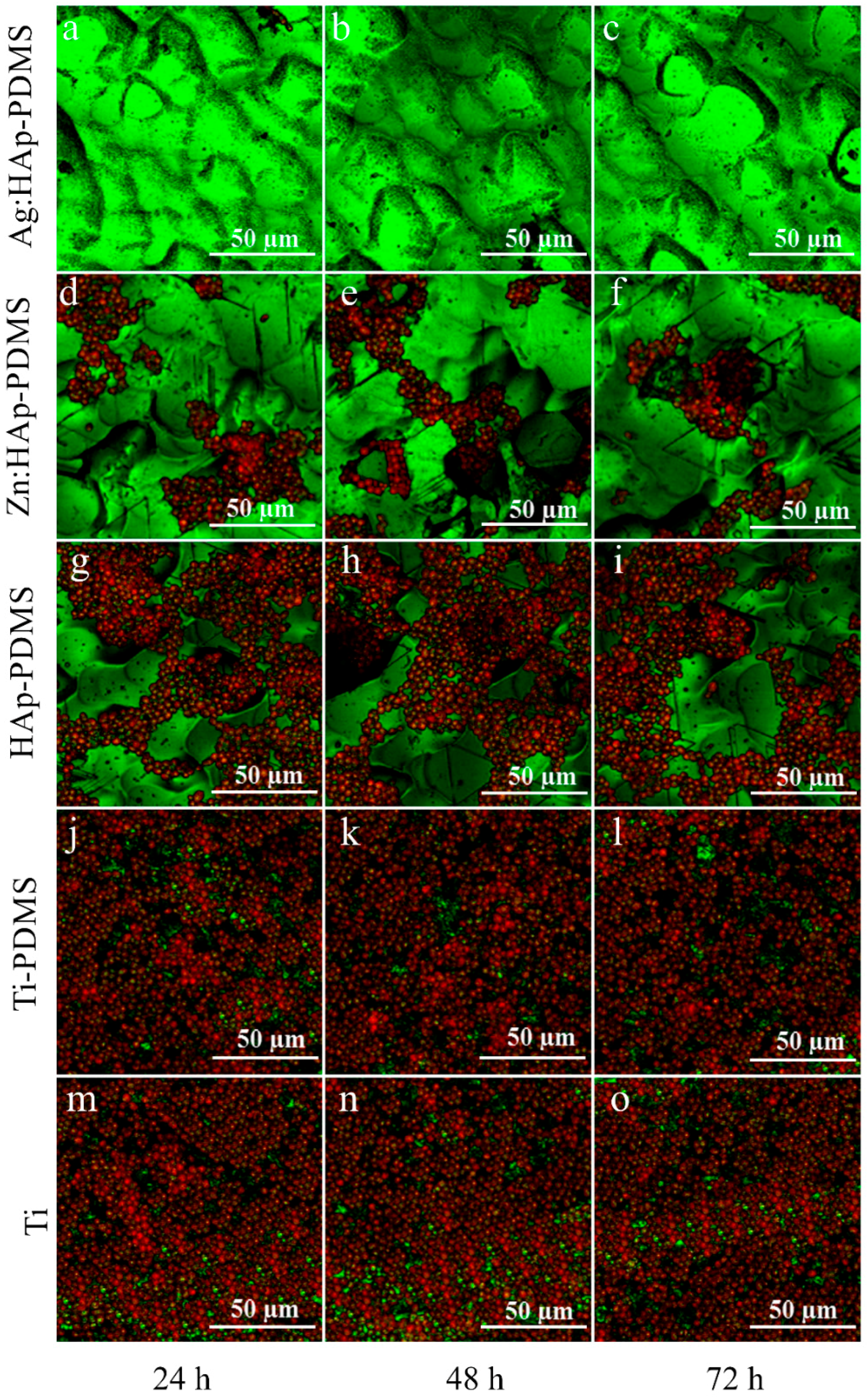

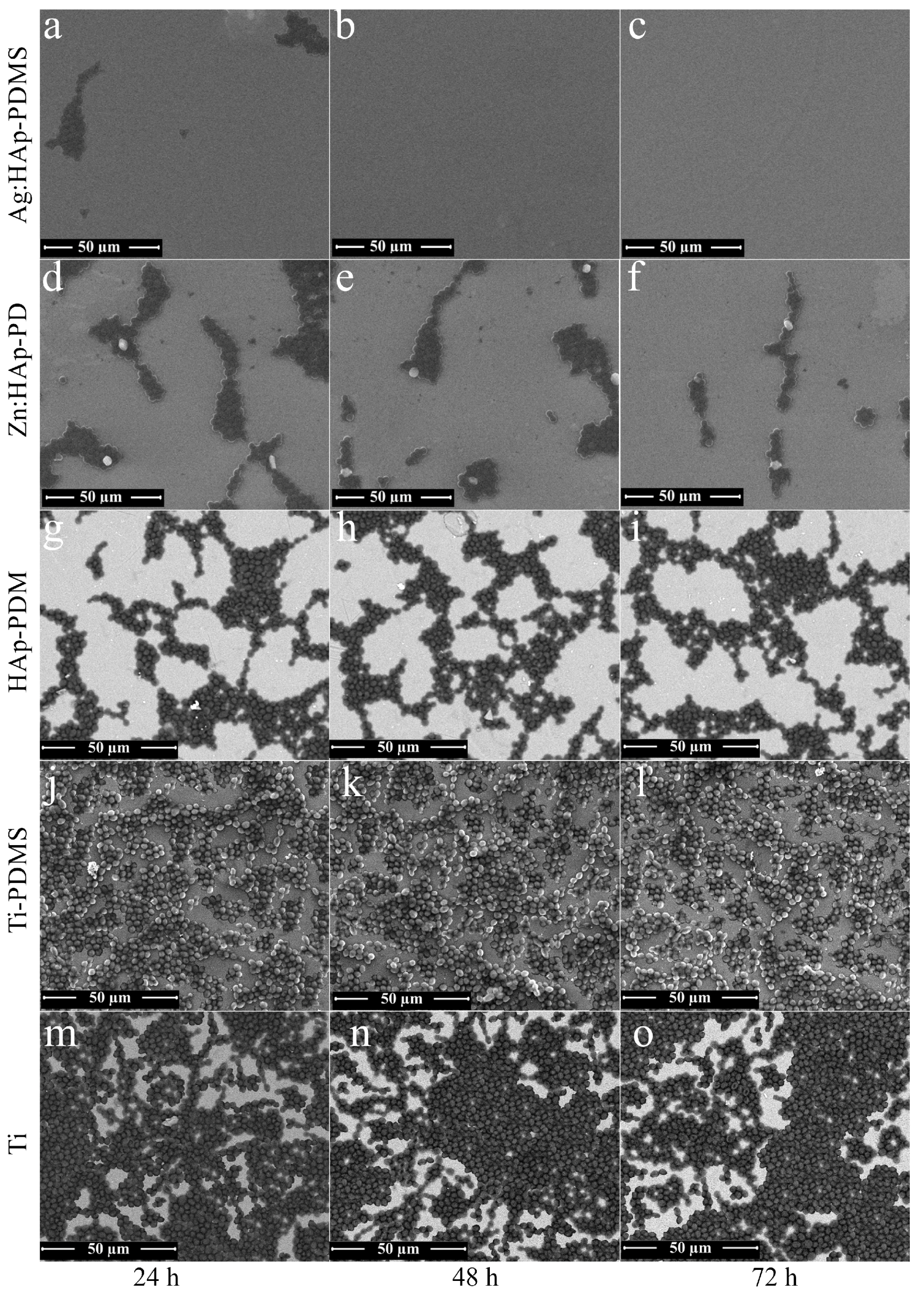
| IR Bands Assigment | HAp-PDMS Wavenumber (cm−1) | Ag:HAp-PDMS Wavenumber (cm−1) | Zn:HAp-PDMS Wavenumber (cm−1) |
|---|---|---|---|
| SiO44− | 498 | 489 | 511 |
| ν4 PO43− | 600 | 570 | 567 |
| C–H in Si–CH3 | 701 | 737 | – |
| Si(CH3)2 | – | – | 619 |
| Si–O in SiO2 | 794 | 775 | 774 |
| Si–C/Si–O | 862 | 857 | 852 |
| Si–O–Si in siloxane | 1,014 | 984 | 989 |
| ν3 PO43− | – | – | 1,053 |
| Si–O–Si siloxane | 1,086 | 1,070 | – |
| C–H bond in Si=CH3 group | 1,260 | 1,255 | 1,252 |
| 1,413 | 1,413 | 1,397 | |
| H2O, OH, Si–OH | 1,650 | 1,655 | 1,600 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groza, A.; Ciobanu, C.S.; Popa, C.L.; Iconaru, S.L.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural Properties and Antifungal Activity against Candida albicans Biofilm of Different Composite Layers Based on Ag/Zn Doped Hydroxyapatite-Polydimethylsiloxanes. Polymers 2016, 8, 131. https://doi.org/10.3390/polym8040131
Groza A, Ciobanu CS, Popa CL, Iconaru SL, Chapon P, Luculescu C, Ganciu M, Predoi D. Structural Properties and Antifungal Activity against Candida albicans Biofilm of Different Composite Layers Based on Ag/Zn Doped Hydroxyapatite-Polydimethylsiloxanes. Polymers. 2016; 8(4):131. https://doi.org/10.3390/polym8040131
Chicago/Turabian StyleGroza, Andreea, Carmen Steluta Ciobanu, Cristina Liana Popa, Simona Liliana Iconaru, Patrick Chapon, Catalin Luculescu, Mihai Ganciu, and Daniela Predoi. 2016. "Structural Properties and Antifungal Activity against Candida albicans Biofilm of Different Composite Layers Based on Ag/Zn Doped Hydroxyapatite-Polydimethylsiloxanes" Polymers 8, no. 4: 131. https://doi.org/10.3390/polym8040131







