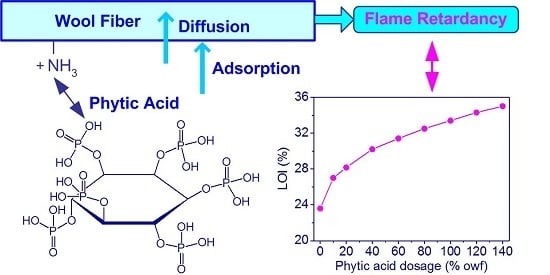
Adsorption and Flame Retardant Properties of Bio-Based Phytic Acid on Wool Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments of the Adsorption of PA
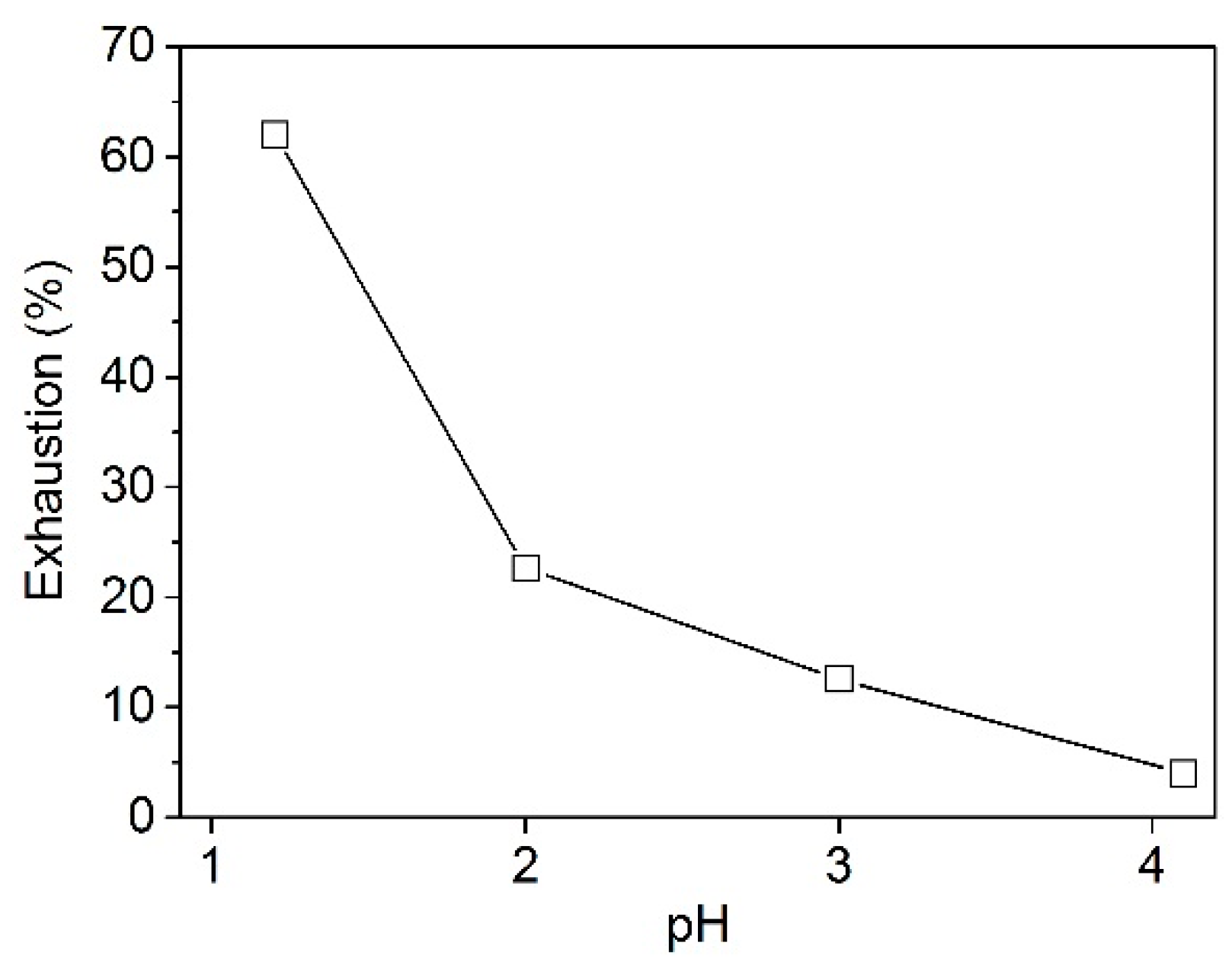
2.2.1. Effect of pH on the Adsorption of PA
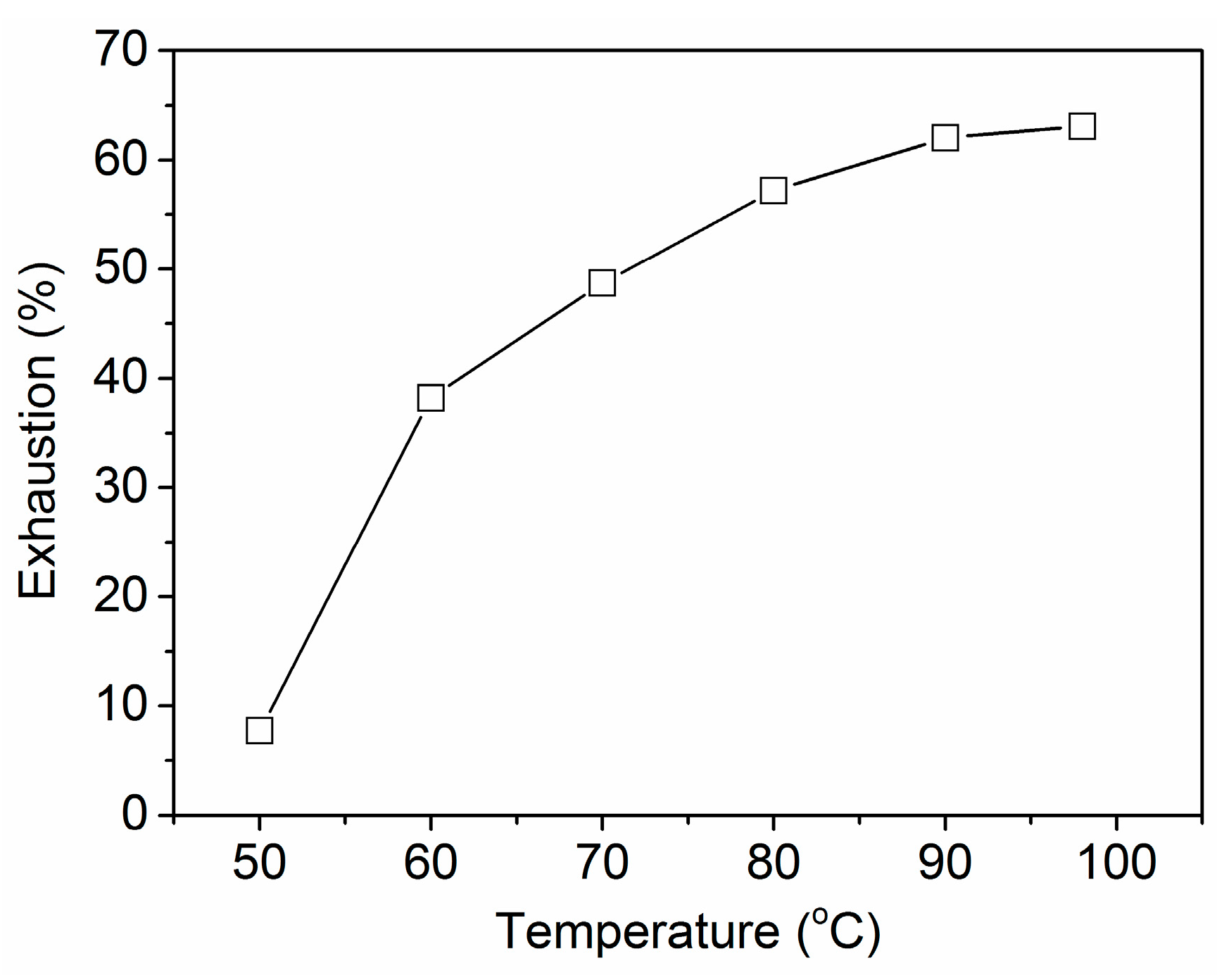
2.2.2. Effect of Temperature on the Adsorption of PA
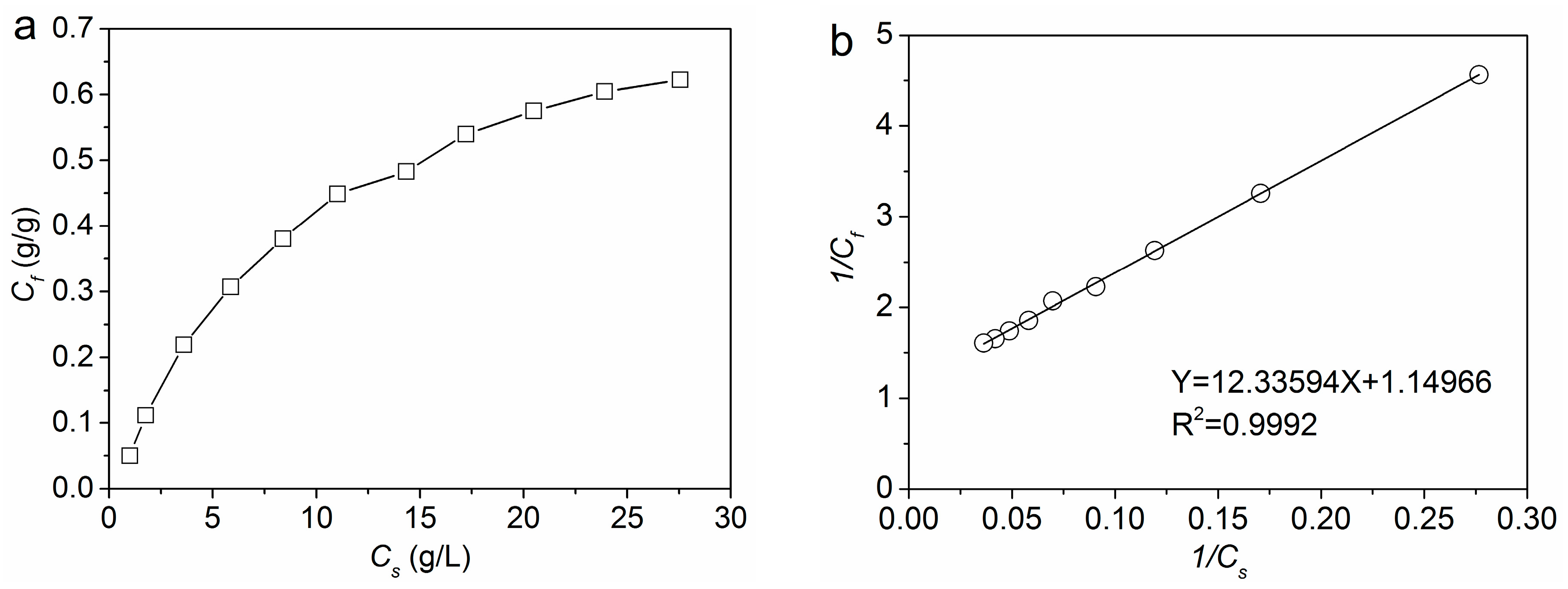
2.2.3. Equilibrium Adsorption Isotherm of PA
2.2.4. Building-up Property of PA
2.3. FR Treatment of Wool Fabric
2.4. Measurements
2.4.1. Adsorptions of PA
2.4.2. Weight Gain
2.4.3. LOI Test
2.4.4. Vertical Burning Test
2.4.5. PCFC Test
2.4.6. TG Analysis
2.4.7. FT-IR Spectra
2.4.8. SEM Observation
2.4.9. ICP-OES
2.4.10. Whiteness Index
2.4.11. Mechanical Performance
2.4.12. Durability to Washing
3. Results and Discussion
3.1. Adsorption Properties of PA
3.1.1. Effect of pH on the Uptake of PA
3.1.2. Effect of Temperature on the Uptake of PA
3.1.3. Equilibrium Adsorption Isotherm of PA
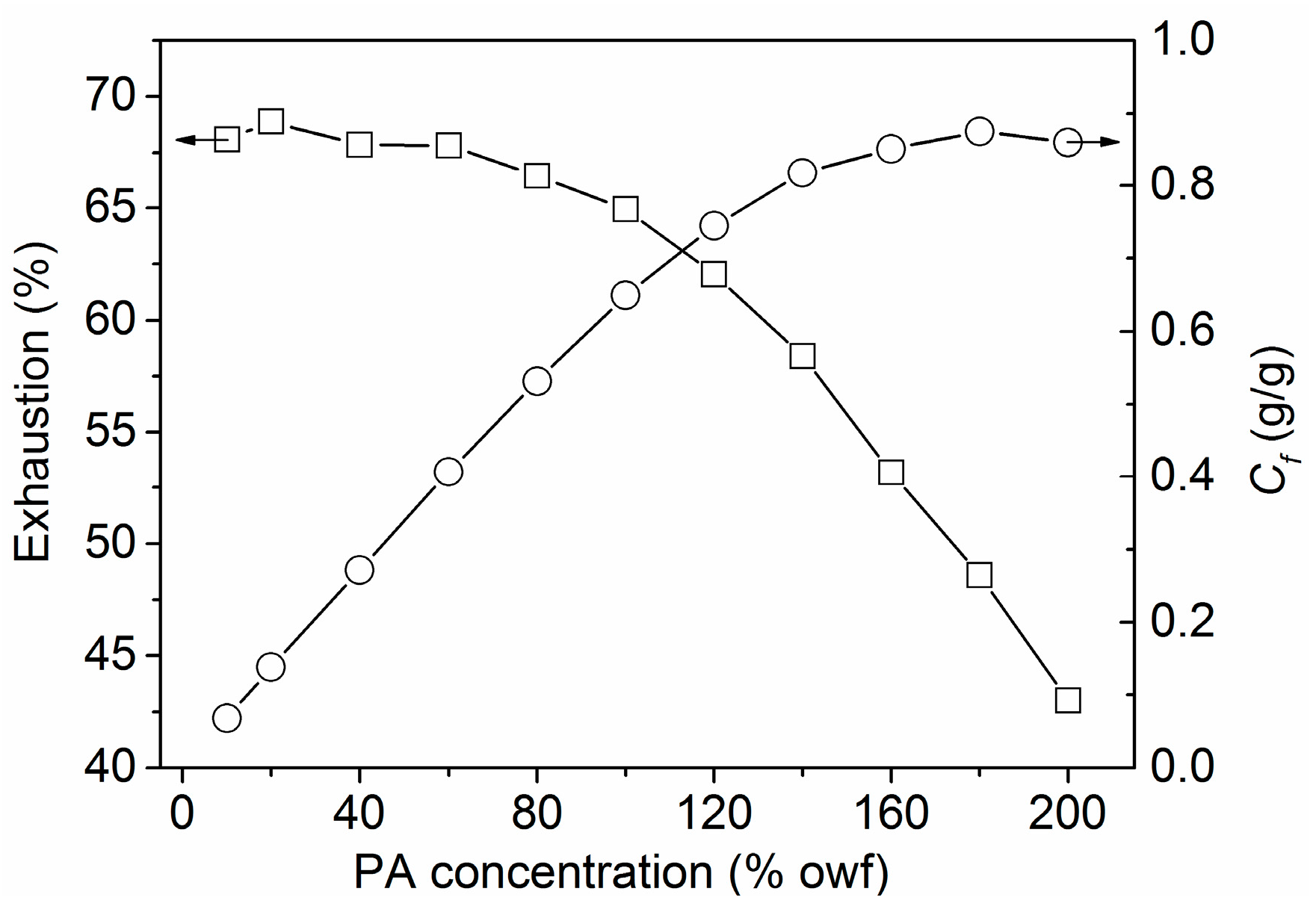
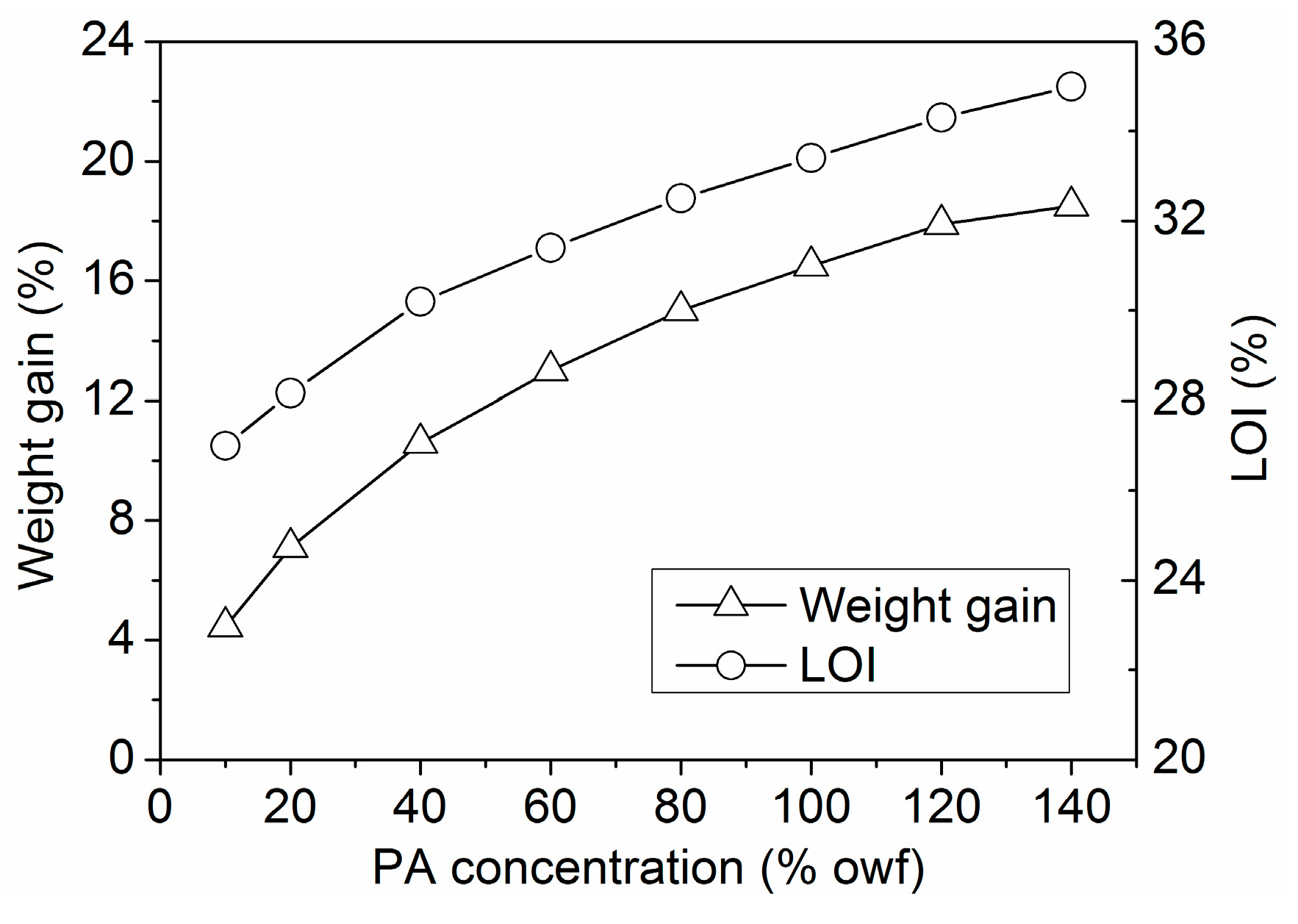
3.1.4. Building-up Property of PA
3.2. Flame Retardancy
3.2.1. Flammability Test
3.2.2. PCFC Analyses
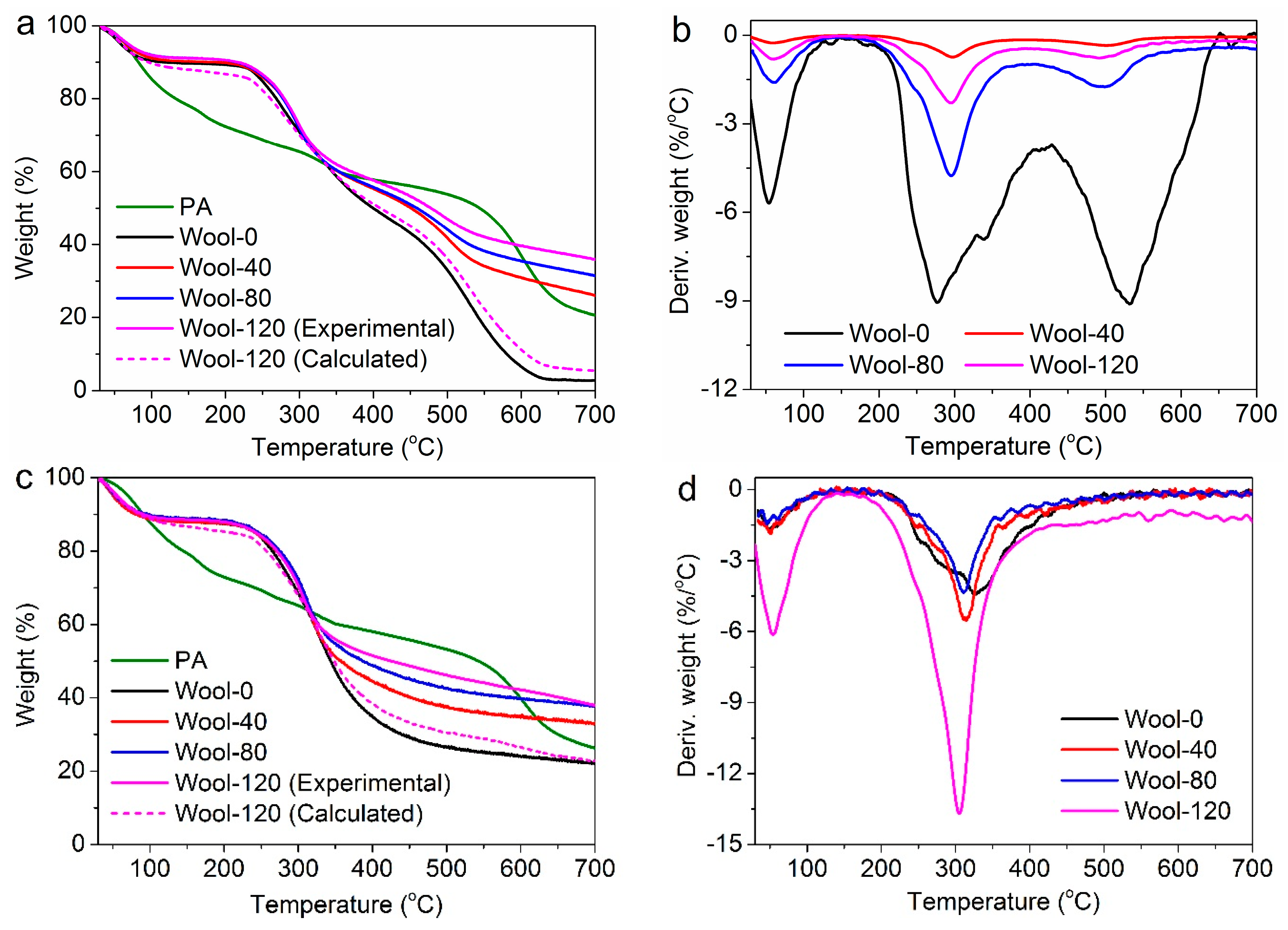
3.2.3. TG Analyses
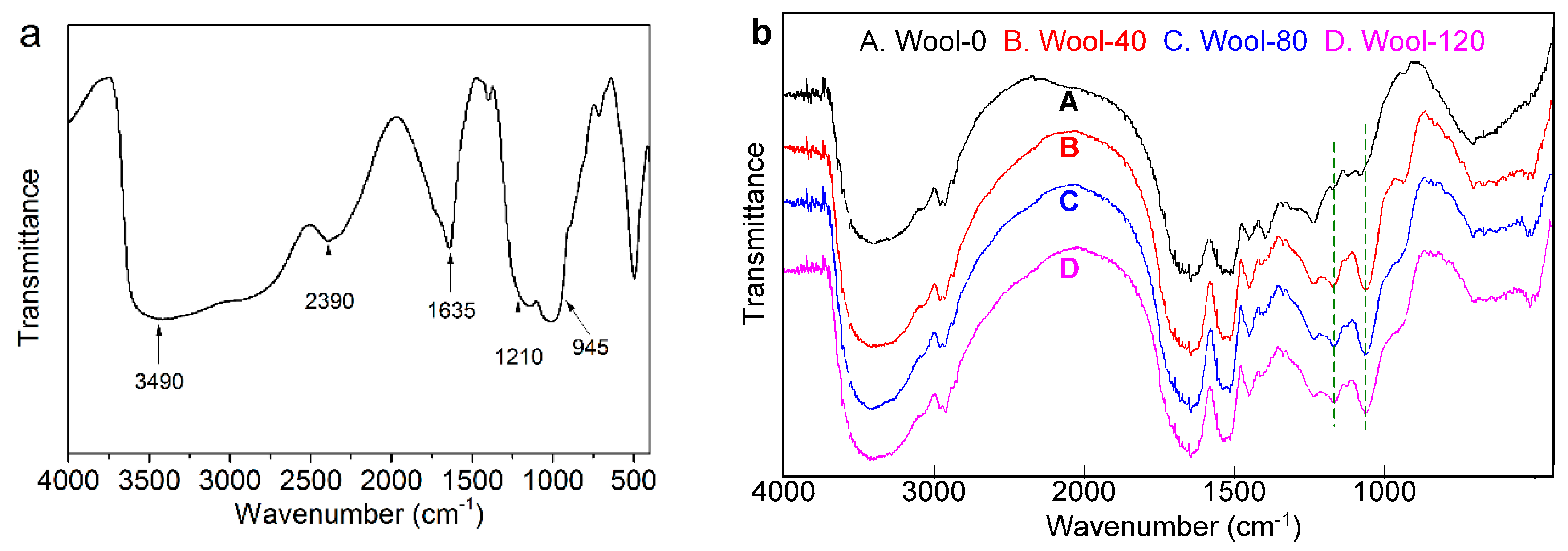
3.2.4. FT-IR Analyses
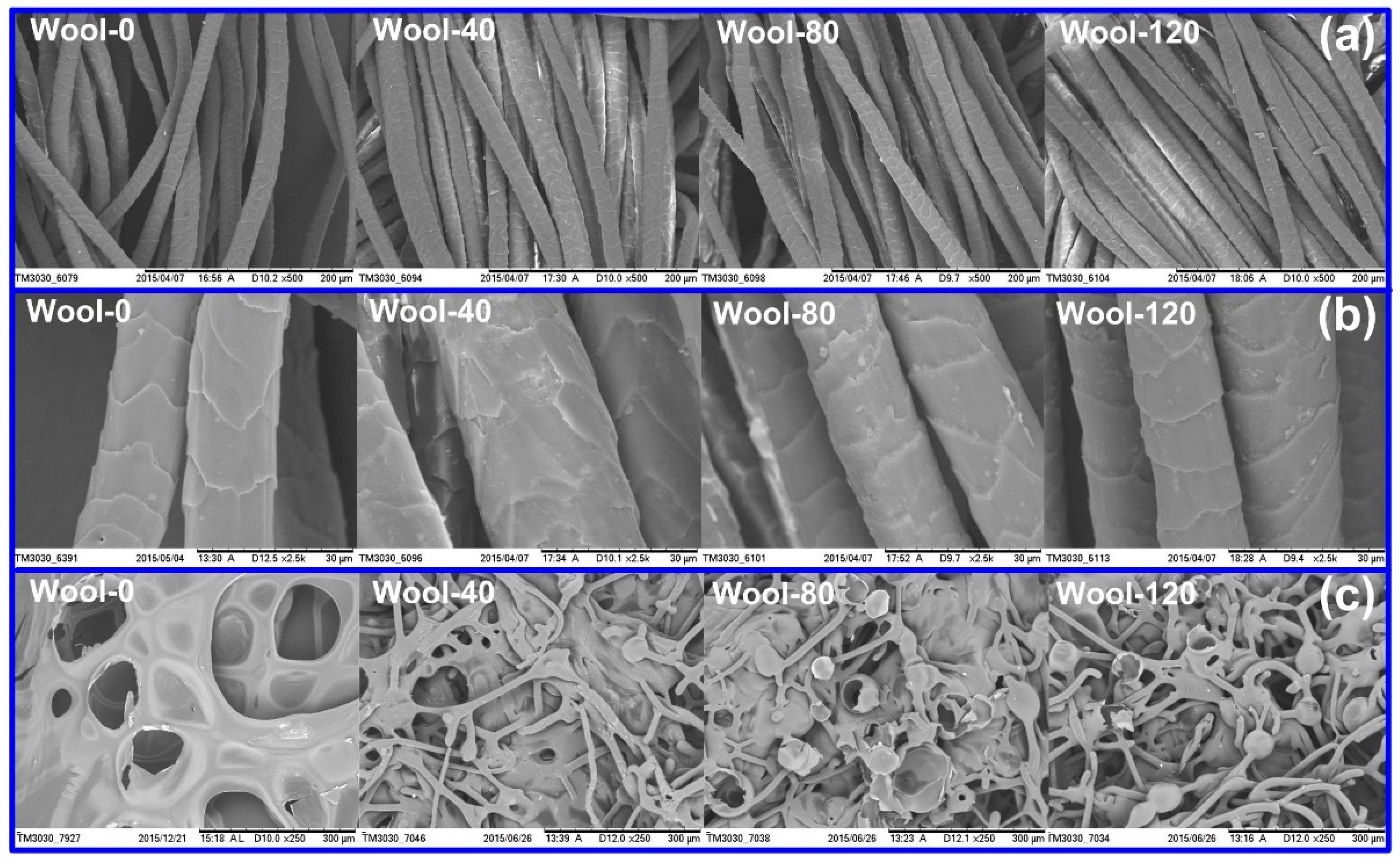
3.2.5. SEM Analyses
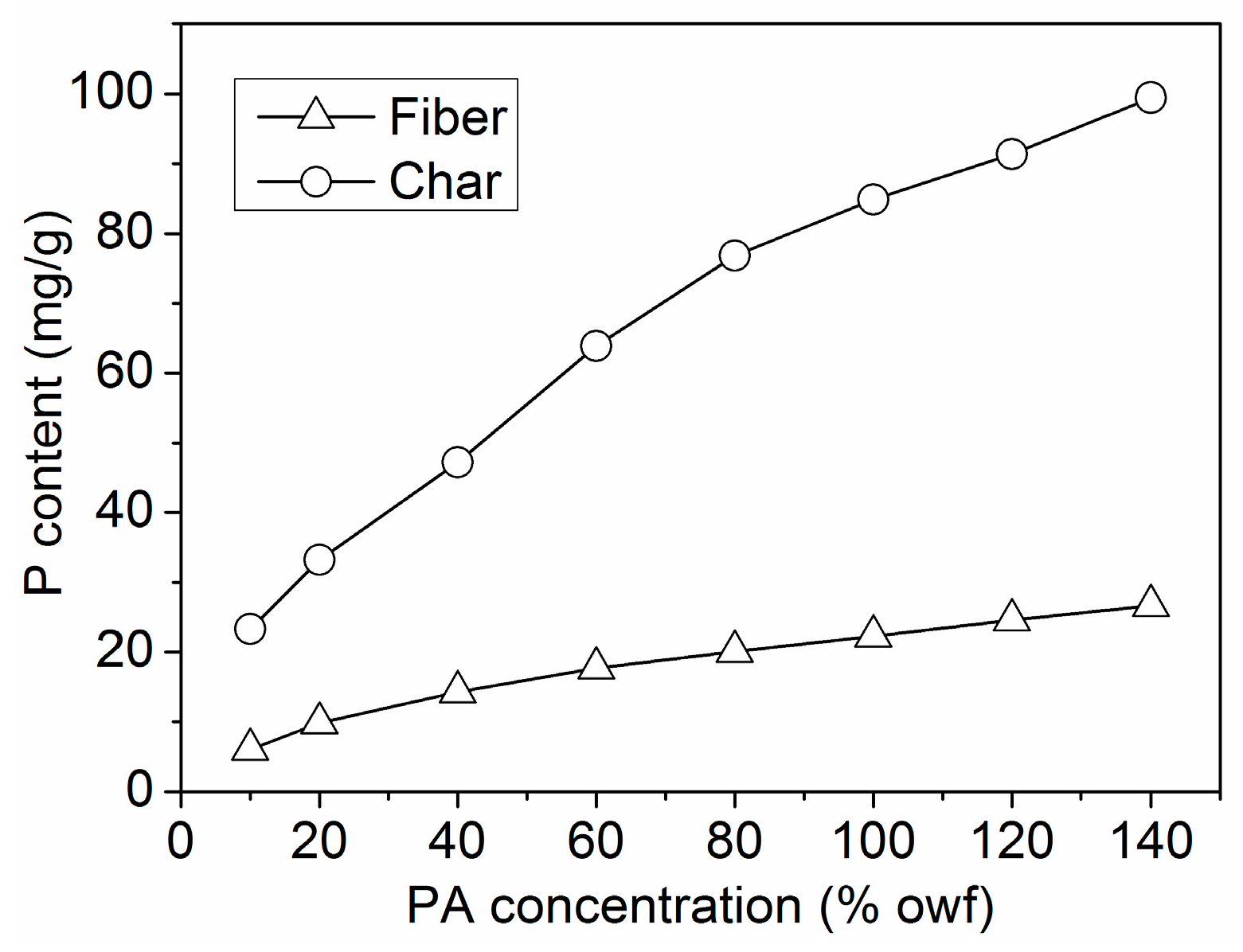
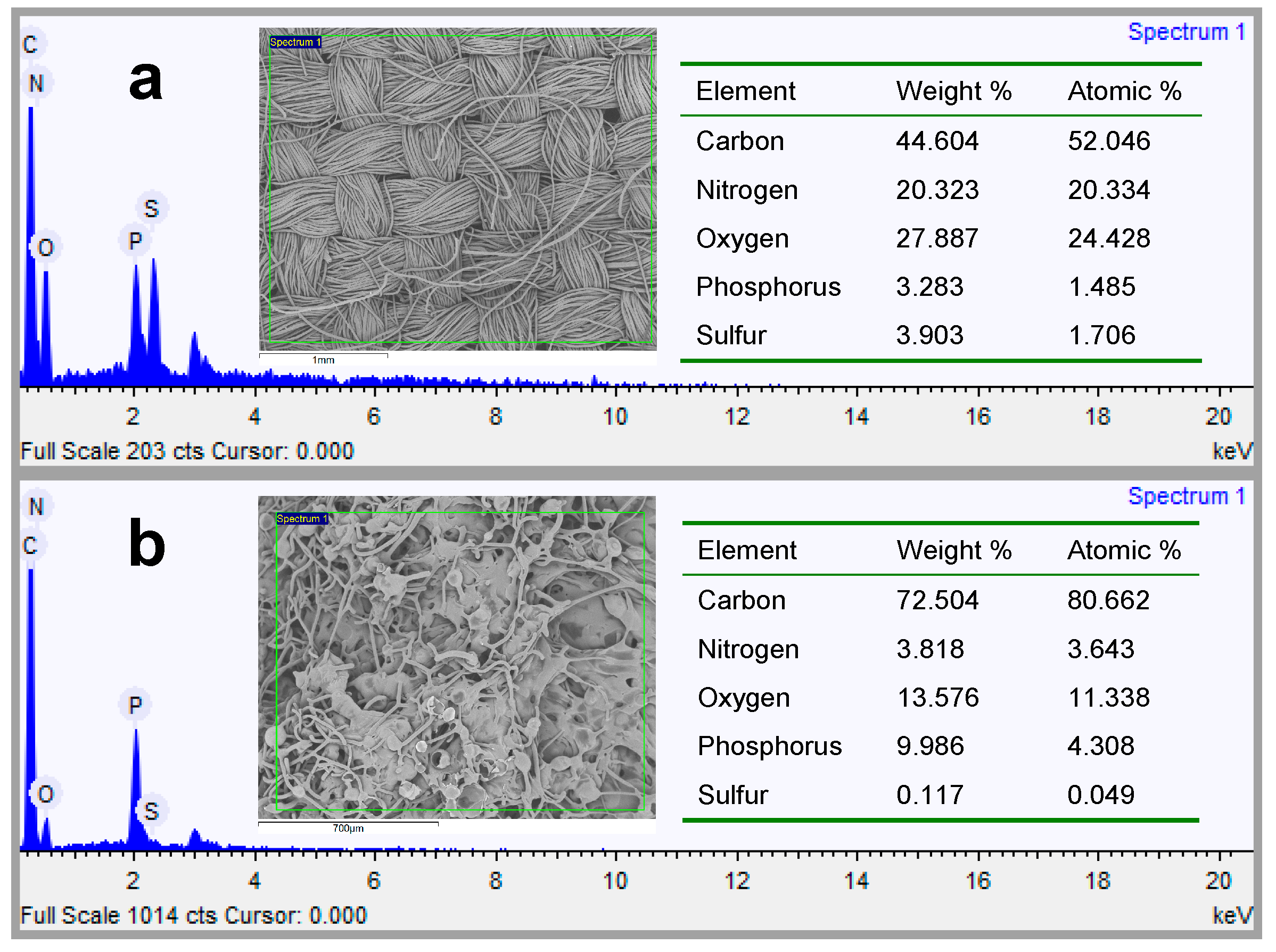
3.2.6. P Content Determination
3.3. Physical Properties
3.3.1. Whiteness Index
3.3.2. Mechanical Performance
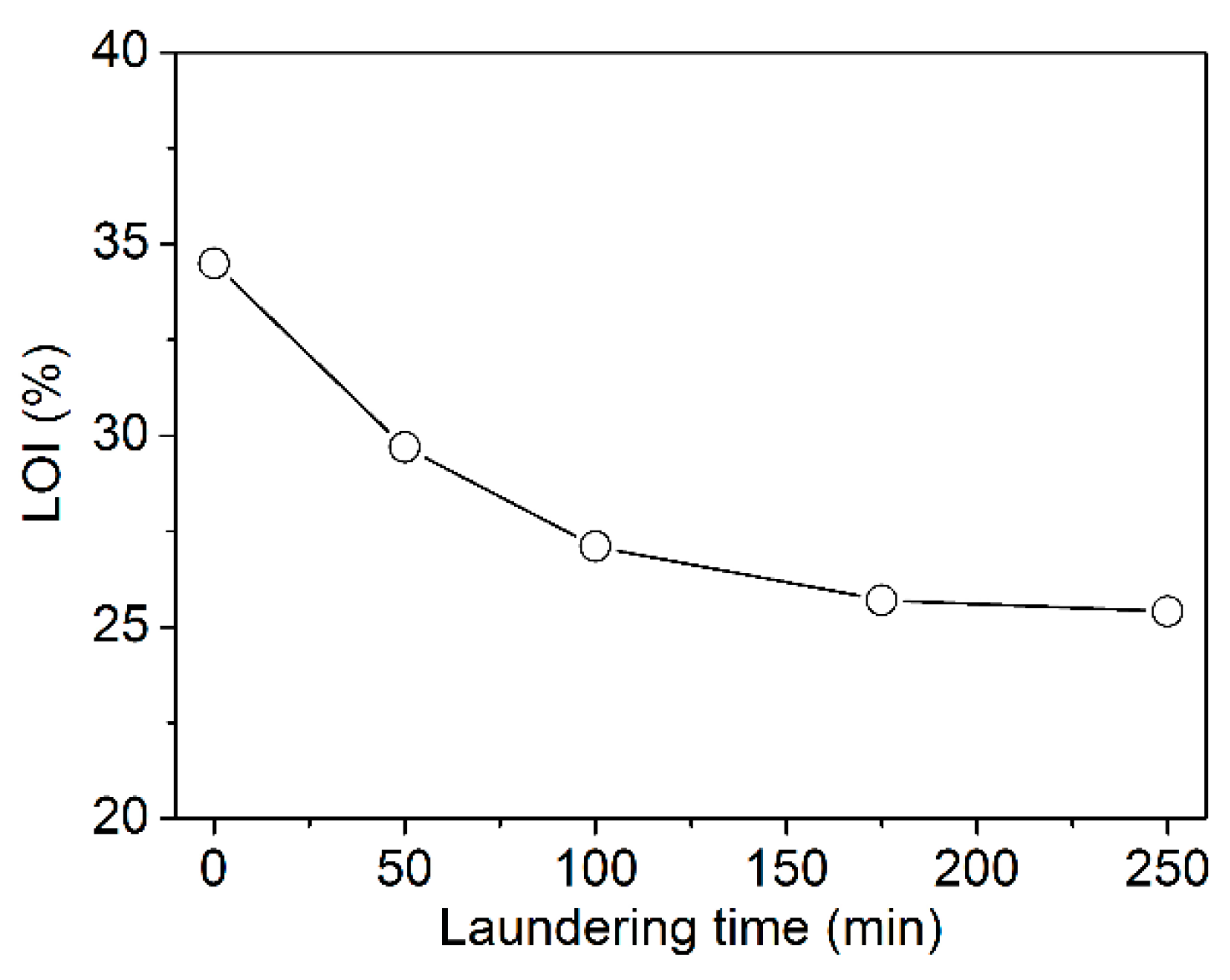
3.4. Durability of FR Effect
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DTG | Derivative Theremogravimetric |
| EDS | Energy Dispersive X-ray Spectroscopy |
| FR | Flame Retardant |
| FT-IR | Fourier Transform Infrared |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| LOI | Limiting Oxygen Index |
| PCFC | Pyrolysis Combustion Flow Calorimetry |
| PA | Phytic Acid |
| SEM | Scanning Electron Microscopy |
| TG | Thermogravimetry |
References
- Cardamone, J.M. Flame resistant wool and wool blends. In Handbook of Fire Resistant Textiles, 1st ed.; Selcen Kilinc, F., Ed.; Woodhead Publishing Limited: Cambridge, 2013; pp. 245–271. [Google Scholar]
- Forouharshad, M.; Montazer, M.; Moghaddam, M.B.; Saligheh, O.; Roudbari, B.Y. Optimization of zirconium acetate on the flame retardant properties of wool. J. Appl. Polym. Sci. 2012, 125, 1261–1266. [Google Scholar] [CrossRef]
- Flambard, X.; Bourbigot, S.; Kozlowski, R.; Muzyczek, M.; Mieleniak, B.; Ferreira, M.; Vermeulen, B.; Poutch, F. Progress in safety, flame retardant textiles and flexible fire barriers for seats in transportation. Polym. Degrad. Stabil. 2005, 88, 98–105. [Google Scholar] [CrossRef]
- Benisek, L. Use of titanium complexes to improve natural flame retardancy of wool. J. Soc. Dyers Color. 1971, 87, 277–278. [Google Scholar]
- Benisek, L. Improvement of the natural flame retardants of wool part I: Metal-Complex applications. J. Text. Inst. 1974, 65, 102–108. [Google Scholar] [CrossRef]
- Martini, P.; Spearpoint, M.J.; Ingham, P.E. Low-cost wool-based fire blocking inter-liners for upholstered furniture. Fire. Saf. J. 2010, 45, 238–248. [Google Scholar] [CrossRef]
- Moghadam, M.B. A comparative optimization study of flame retardancy of wool fabric with and without robust parameter design approach. World Appl. Sci. J. 2011, 13, 2252–2257. [Google Scholar]
- Forouharshad, M.; Montazer, M.; Moghadam, M.B.; Saligheh, O. Flame retardancy of wool fabric with zirconium oxychloride optimized by central composite design. J. Fire Sci. 2010, 28, 561–572. [Google Scholar] [CrossRef]
- Forouharshad, M.; Montazer, M.; Moghadam, M.B.; Saligheh, O. Preparation of flame retardant wool using zirconium acetate optimized by CCD. Thermochim. Acta 2011, 520, 134–138. [Google Scholar] [CrossRef]
- Tian, C.M.; Li, Z.; Guo, H.Z.; Xu, J.Z. Study on the thermal degradation of flame retardant wools. J. Fire Sci. 2003, 21, 155–162. [Google Scholar] [CrossRef]
- Horrocks, A.R. Flame-retardant finishing of textile. Rev. Prog. Color. 1986, 16, 62–101. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Davies, P.J. Char formation in flame-retarded wool fibres. Part 1. Effect of intumescent on thermogravimetric behaviour. Fire. Mater. 2000, 24, 151–157. [Google Scholar] [CrossRef]
- Davies, P.J.; Horrocks, A.R.; Miraftab, M. Scanning electron microscopic studies of wool/intumescent char formation. Polym. Int. 2000, 49, 1125–1132. [Google Scholar] [CrossRef]
- Tian, C.M.; Zhang, H.Y.; Xu, J.Z.; Gua, H.Z.; Shi, J.R. Studies on the flame retardation and thermal degradation of wool. J. Text. Inst. 1998, 89, 591–594. [Google Scholar] [CrossRef]
- Gashti, M.P.; Almasian, A.; Gashti, M.P. Preparation of electromagnetic reflective wool using nano-ZrO2/citric acid as inorganic/organic hybrid coating. Sens. Actuat. A 2012, 187, 1–9. [Google Scholar]
- Malucelli, G.; Bosco, F.; Alongi, J.; Carosio, F.; Di Blasio, A.; Mollea, C.; Cuttica, F.; Casale, A. Biomacromolecules as novel green flame retardant systems for textiles: An overview. RSC Adv. 2014, 4, 46024–46039. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Di Blasio, A.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Bosco, F.; Carletto, R.A.; Alongi, J.; Marmo, L.; Di Blasio, A.; Malucelli, G. Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohyd. Polym. 2013, 94, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Cuttica, F.; Carosio, F.; Bosco, F.; Malucelli, G. Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohyd. Polym. 2013, 96, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Di Blasio, A.; Cuttica, F.; Alongi, J.; Malucelli, G. Flame retardancy of polyester and polyester-cotton blends treated with caseins. Ind. Eng. Chem. Res. 2014, 53, 3917–3923. [Google Scholar] [CrossRef]
- Duskovar, D.; Marounek, M.; Brezina, P. Determination of phytic acid in feeds and faeces of pigs and poultry by capillary isotachophoresis. J. Sci. Food Agric. 2000, 81, 36–41. [Google Scholar]
- Moccelini, S.K.; Fernandes, S.C.; Vieira, I.C. Bean sprout peroxidase biosensor based on l-cysteine self-assembled monolayer for the determination of dopamine. Sensor. Actuat. B Chem. 2008, 133, 364–369. [Google Scholar] [CrossRef]
- Jo, S.; Jeong, H.; Bae, S.R.; Jeon, S. Modified platinum electrode with phytic acid and single-walled carbon nanotube: Application to the selective determination of dopamine in the presence of ascorbic and uric acids. Microchem. J. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Ye, C.H.; Zheng, Y.F.; Wang, S.Q.; Xi, T.F.; Li, Y.D. In vitro corrosion and biocompatibility study of phytic acid modified WE43 magnesium alloy. Appl. Surf. Sci. 2012, 258, 3420–3427. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ding, C.; Qian, X.; An, X. Further improvement of flame retardancy of polyaniline-deposited paper composite through using phytic acid as dopant or co-dopant. Carbohyd. Polym. 2015, 115, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Romero, M.Q.; Zhang, X.-Q.; Wang, R.; Wang, D.-Y. Intumescent multilayer hybrid coating for flame retardant cotton fabrics based on layer-by-layer assembly and sol-gel process. RSC Adv. 2015, 5, 10647–10655. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Dumazert, L.; Lopez-cuesta, J.M.; Brohez, S.; Delvosalle, C.; Dubois, P. Metallic phytates as efficient bio-based phosphorous flame retardant additives for poly (lactic acid). Polym. Degrad. Stabil. 2015, 119, 217–227. [Google Scholar] [CrossRef]
- Yang, L.Z.; Liu, H.Y.; Hu, N.F. Assembly of electroactive layer-by-layer films of myoglobin and small-molecular phytic acid. Electrochem. Commun. 2007, 9, 1057–1061. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Liu, B.; Chen, M.; Mao, J.; He, H.; Zhao, Y.; Huang, N.; Wan, G. Corrosion-Controlling and Osteo-Compatible Mg Ion-Integrated Phytic Acid (Mg-PA) Coating on Magnesium Substrate for Biodegradable Implants Application. ACS Appl. Mater. Interfaces 2014, 6, 19531–19543. [Google Scholar] [CrossRef] [PubMed]
- Schartel, B.; Pawlowski, K.H.; Lyon, R.E. Pyrolysis combustion flow calorimeter: A tool to assess flame retarded PC/ABS materials? Thermochim. Acta 2007, 462, 1–14. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I. Screening flame retardants for plastics using microscale combustion calorimetry. Polym. Eng. Sci. 2007, 47, 1501–1510. [Google Scholar] [CrossRef]
- Razmjooei, F.; Singh, K.P.; Bae, E.J.; Yu, J.-S. A new class of electroactive Fe- and P- functionalized graphene for oxygen reduction. J. Mater. Chem. A 2015, 3, 11031–11039. [Google Scholar] [CrossRef]
- Jiang, G.; Qiao, J.; Hong, F. Application of phosphoric acid and phytic acid-doped bacterial cellulose as novel proton-conducting membranes to PEMFC. Int. J. Hydrogen. Energ. 2012, 37, 9182–9192. [Google Scholar] [CrossRef]
- Guo, X.; Miao, Y.; Ye, P.; Wen, Y.; Yang, H. Multi-walled carbon nanotubes in aqueous phytic acid for enhancing biosensor. Mater. Res. Express 2014, 1, 025403. [Google Scholar] [CrossRef]
- Carran, R.S.; Ghosh, A.; Dyer, J.M. The effects of zeolite molecular sieve based surface treatments on the properties of wool fabrics. Appl. Surf. Sci. 2013, 287, 467–472. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Flammability and combustion properties of ammonium polyphosphate-/poly(acrylic acid)-based layer by layer architectures deposited on cotton, polyester and their blends. Polym. Degrad. Stabil. 2013, 98, 1626–1637. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly (lactic acid). Polym. Eng. Sci. 2012, 52, 2620–2626. [Google Scholar] [CrossRef]
- Daneluti, A.L.M.; Matos, J.D.R. Study of thermal behavior of phytic acid. Braz. J. Pharm. Sci. 2013, 49, 275–283. [Google Scholar] [CrossRef]





| Samples | HRC (J/(g K)) | pHRR (W/g) | THR (kJ/g) | Tmax (°C) |
|---|---|---|---|---|
| Wool-0 | 130 ± 4 | 131.7 ± 3.5 | 14.0 ± 0.5 | 331.7 ± 3.4 |
| Wool-40 | 85 ± 1 | 89.8 ± 1.9 | 8.2 ± 0.1 | 300.3 ± 0.5 |
| Wool-80 | 78 ± 2 | 80.7 ± 0.4 | 7.4 ± 0.2 | 296.6 ± 0.5 |
| Wool-120 | 74 ± 2 | 76.7 ± 1.6 | 6.7 ± 0.2 | 296.1 ± 2.7 |
| Samples | T20% (°C) | T50% (°C) | Char Residues at 700 °C (%) | ||
|---|---|---|---|---|---|
| Experimental | Calculated | ||||
| Air | PA | 132.9 | 543.8 | 20.6 | – |
| Wool-0 | 270.0 | 399.9 | 2.8 | 2.8 | |
| Wool-40 | 278.3 | 453.9 | 26.1 | 4.6 | |
| Wool-80 | 277.8 | 459.6 | 31.5 | 5.1 | |
| Wool-120 | 279.8 | 478.1 | 36.0 | 5.5 | |
| Nitrogen | PA | 145.4 | 543.2 | 26.3 | – |
| Wool-0 | 264.9 | 343.7 | 22.3 | 22.3 | |
| Wool-40 | 270.6 | 357.6 | 33.2 | 23.7 | |
| Wool-80 | 274.8 | 386.9 | 37.6 | 24.2 | |
| Wool-120 | 271.5 | 425.9 | 38.0 | 24.5 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.-W.; Guan, J.-P.; Chen, G.; Yang, X.-H.; Tang, R.-C. Adsorption and Flame Retardant Properties of Bio-Based Phytic Acid on Wool Fabric. Polymers 2016, 8, 122. https://doi.org/10.3390/polym8040122
Cheng X-W, Guan J-P, Chen G, Yang X-H, Tang R-C. Adsorption and Flame Retardant Properties of Bio-Based Phytic Acid on Wool Fabric. Polymers. 2016; 8(4):122. https://doi.org/10.3390/polym8040122
Chicago/Turabian StyleCheng, Xian-Wei, Jin-Ping Guan, Guoqiang Chen, Xu-Hong Yang, and Ren-Cheng Tang. 2016. "Adsorption and Flame Retardant Properties of Bio-Based Phytic Acid on Wool Fabric" Polymers 8, no. 4: 122. https://doi.org/10.3390/polym8040122