Structural and Spectroscopic Characterization of A Nanosized Sulfated TiO2 Filler and of Nanocomposite Nafion Membranes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
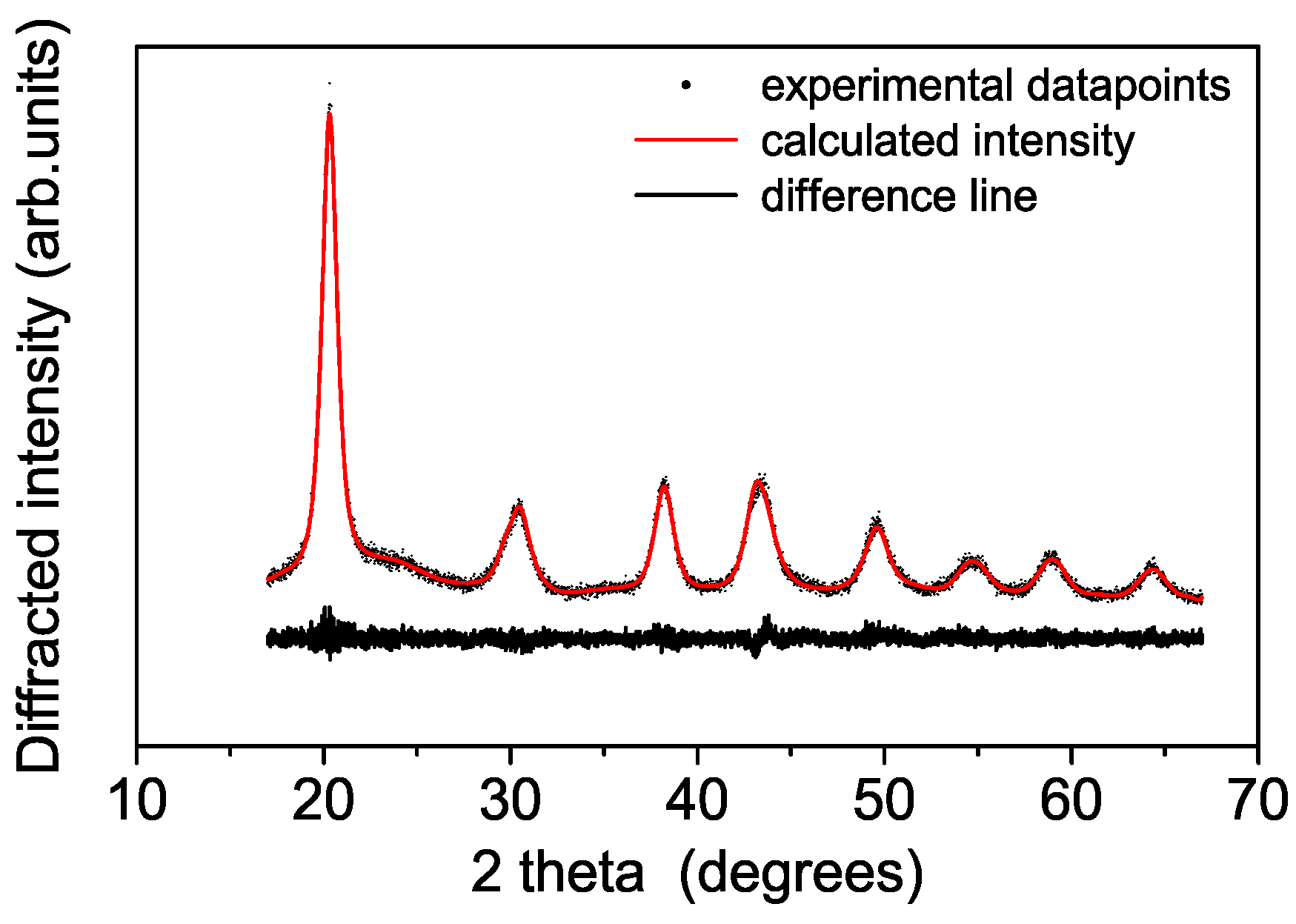
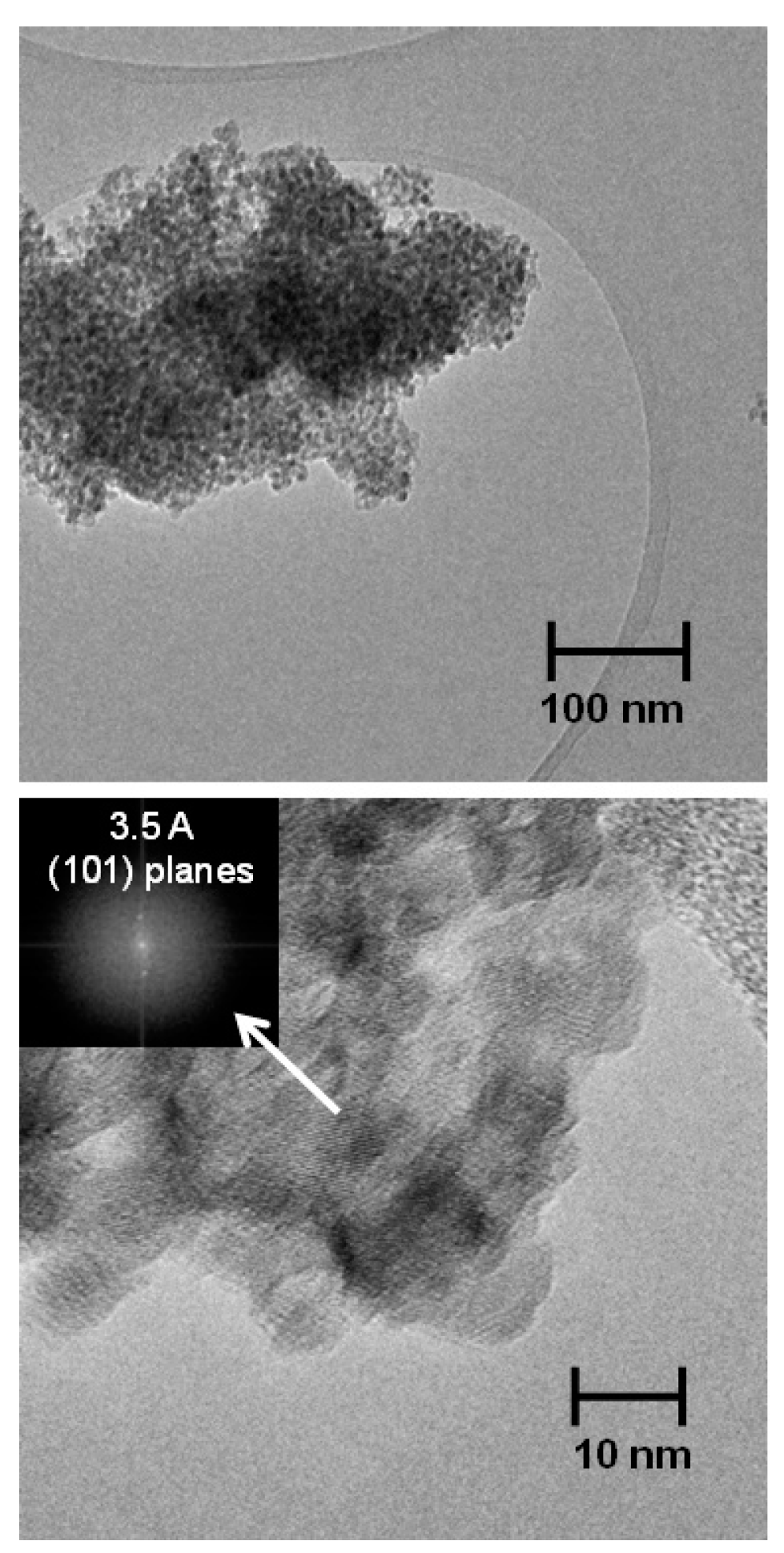
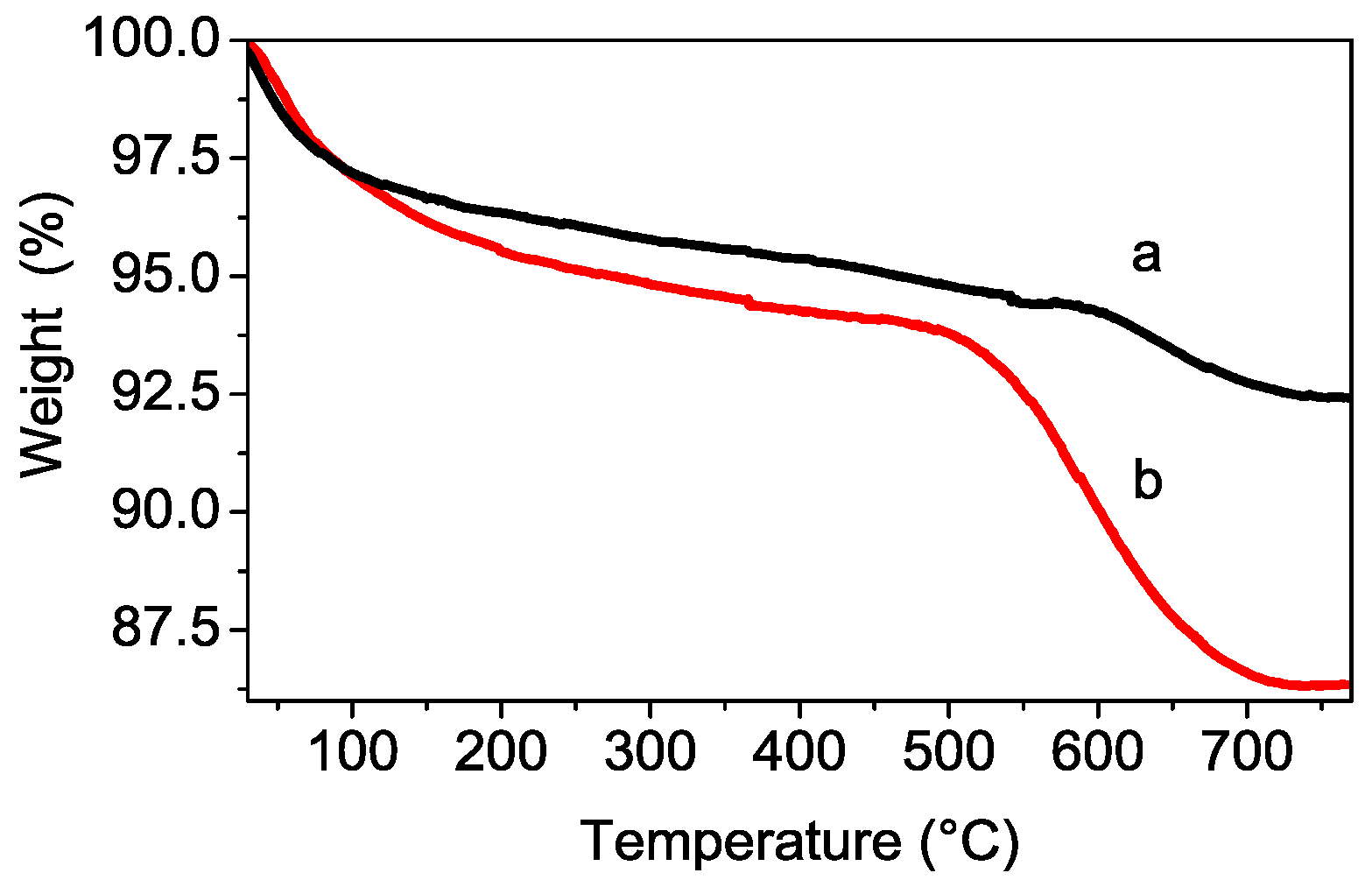
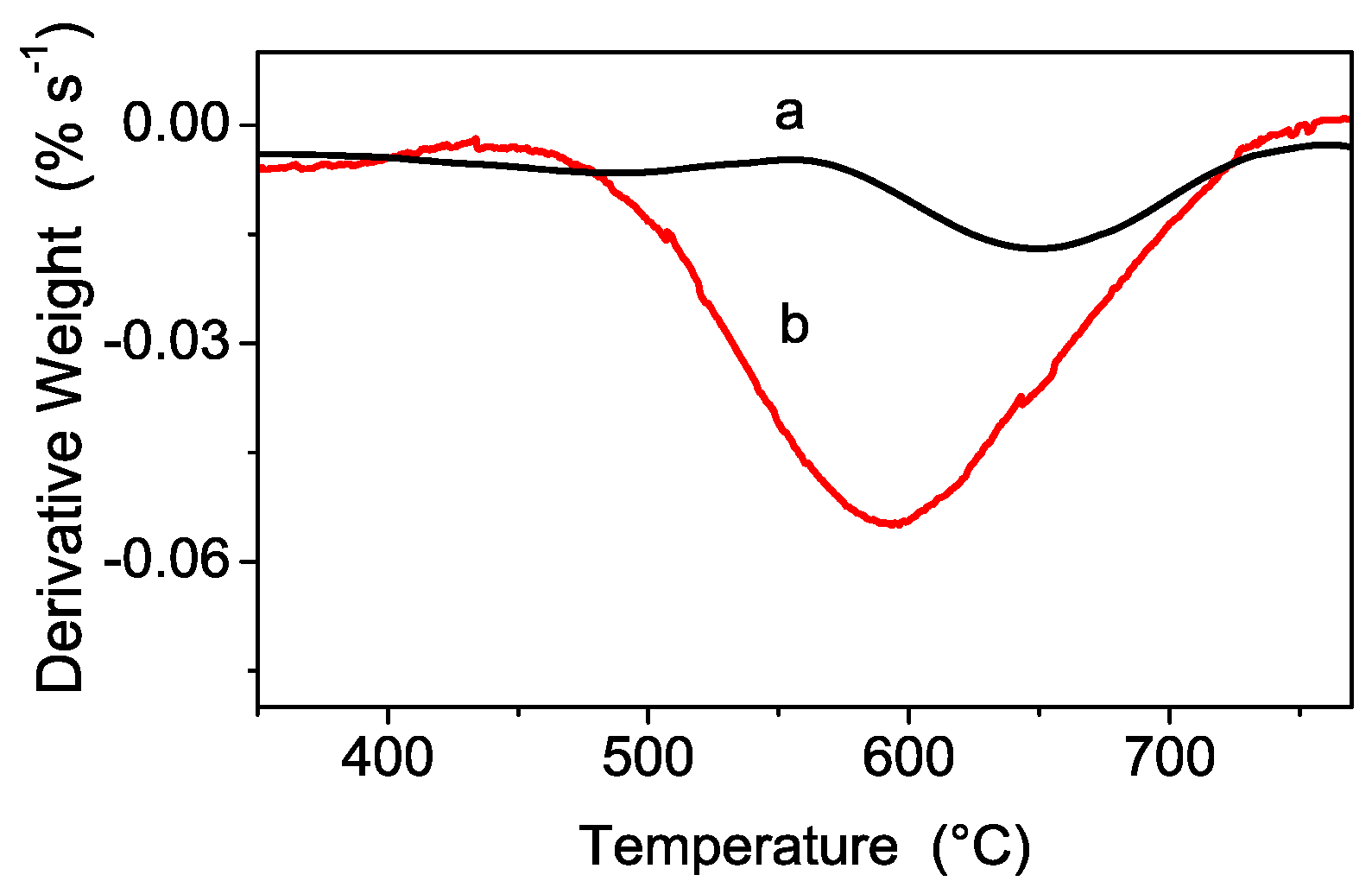
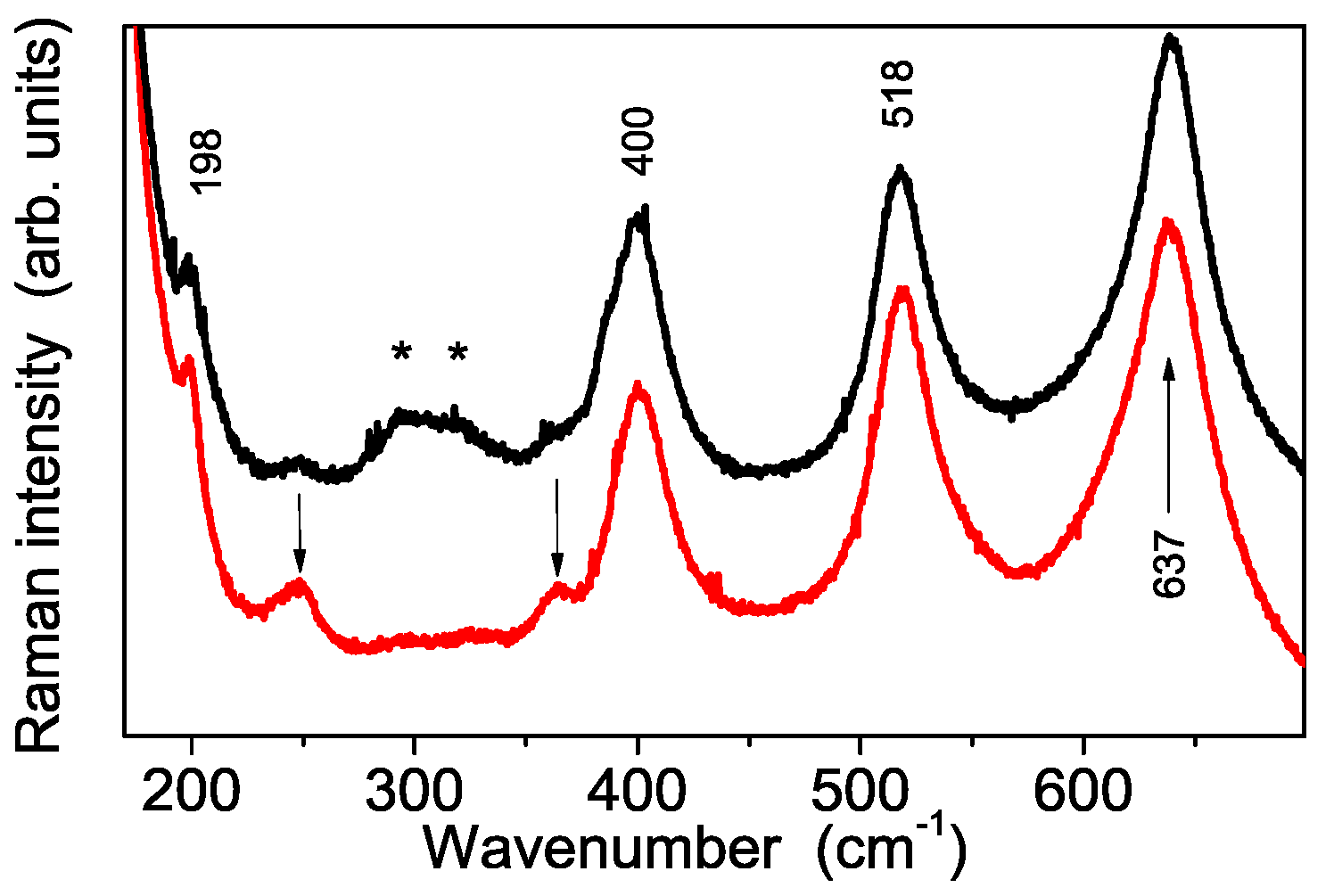
3.1. Structural and Thermal Characterization of the Sulfated Powders
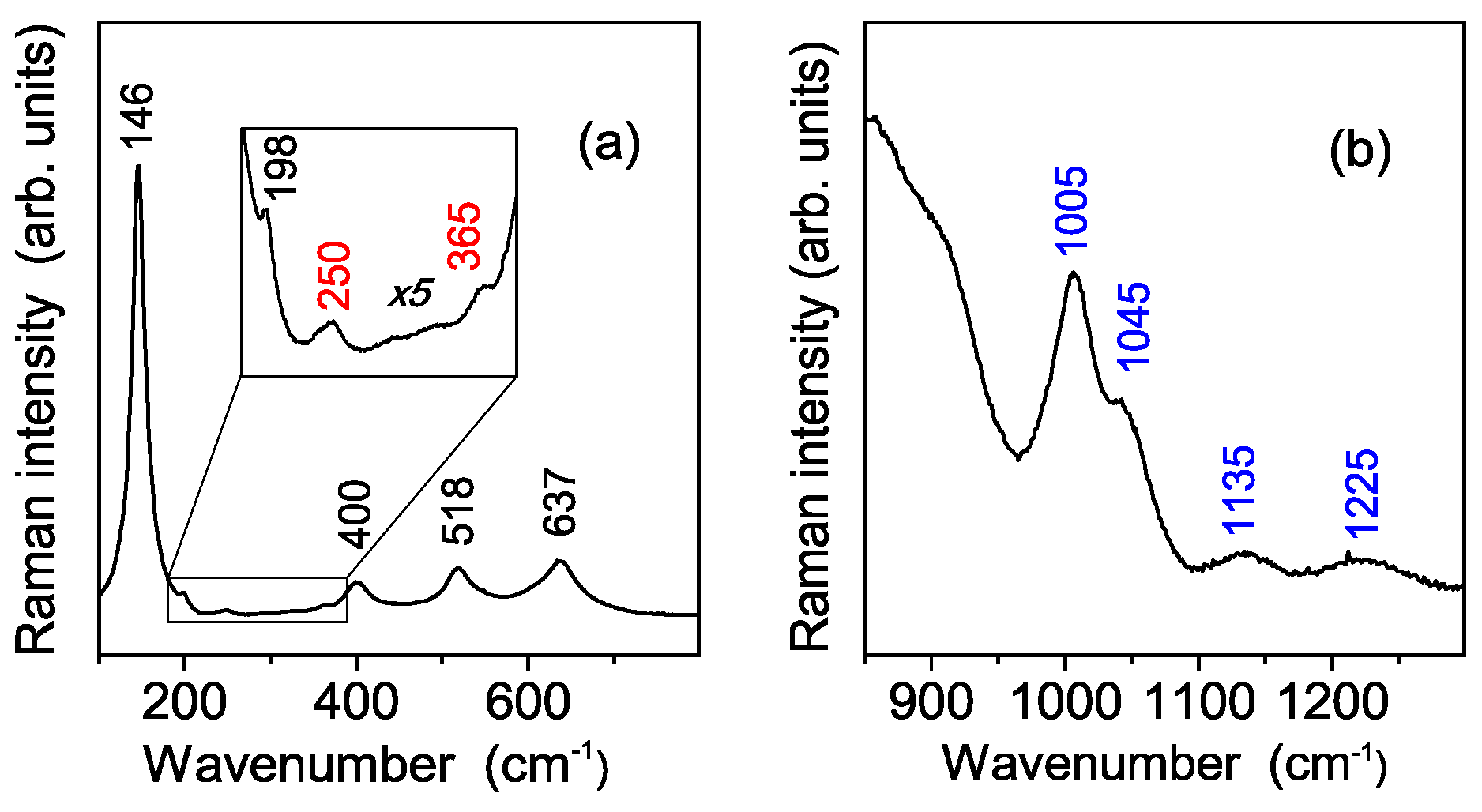
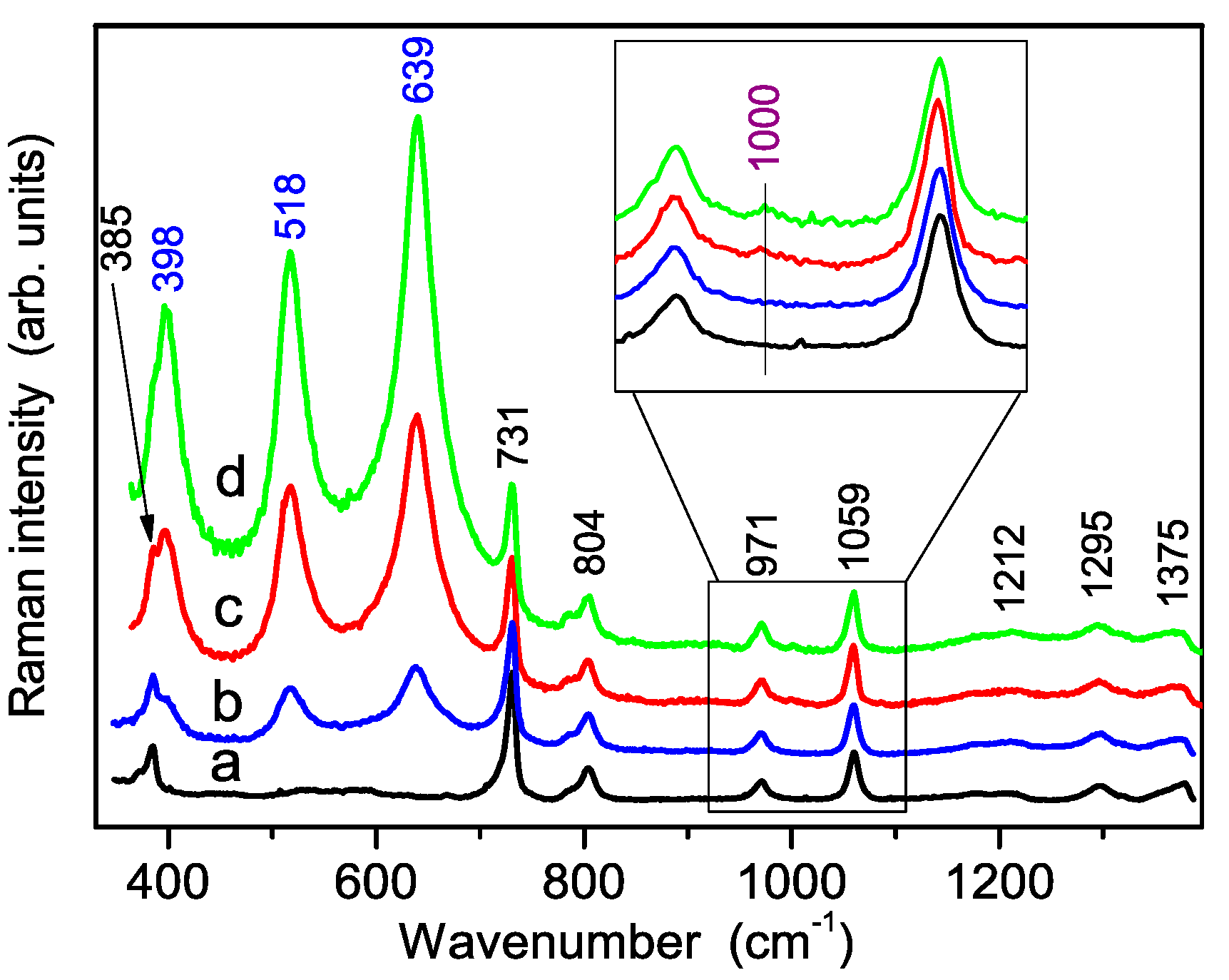
3.2. Vibrational Characterization of the Synthesized Powders
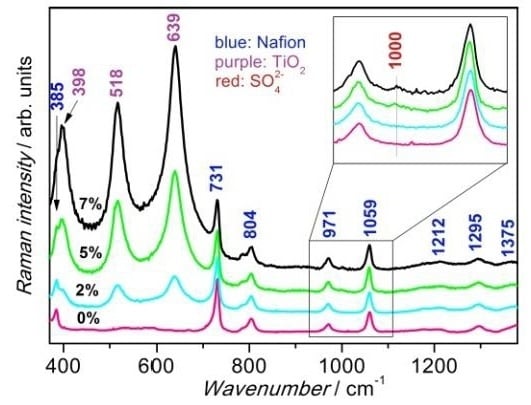
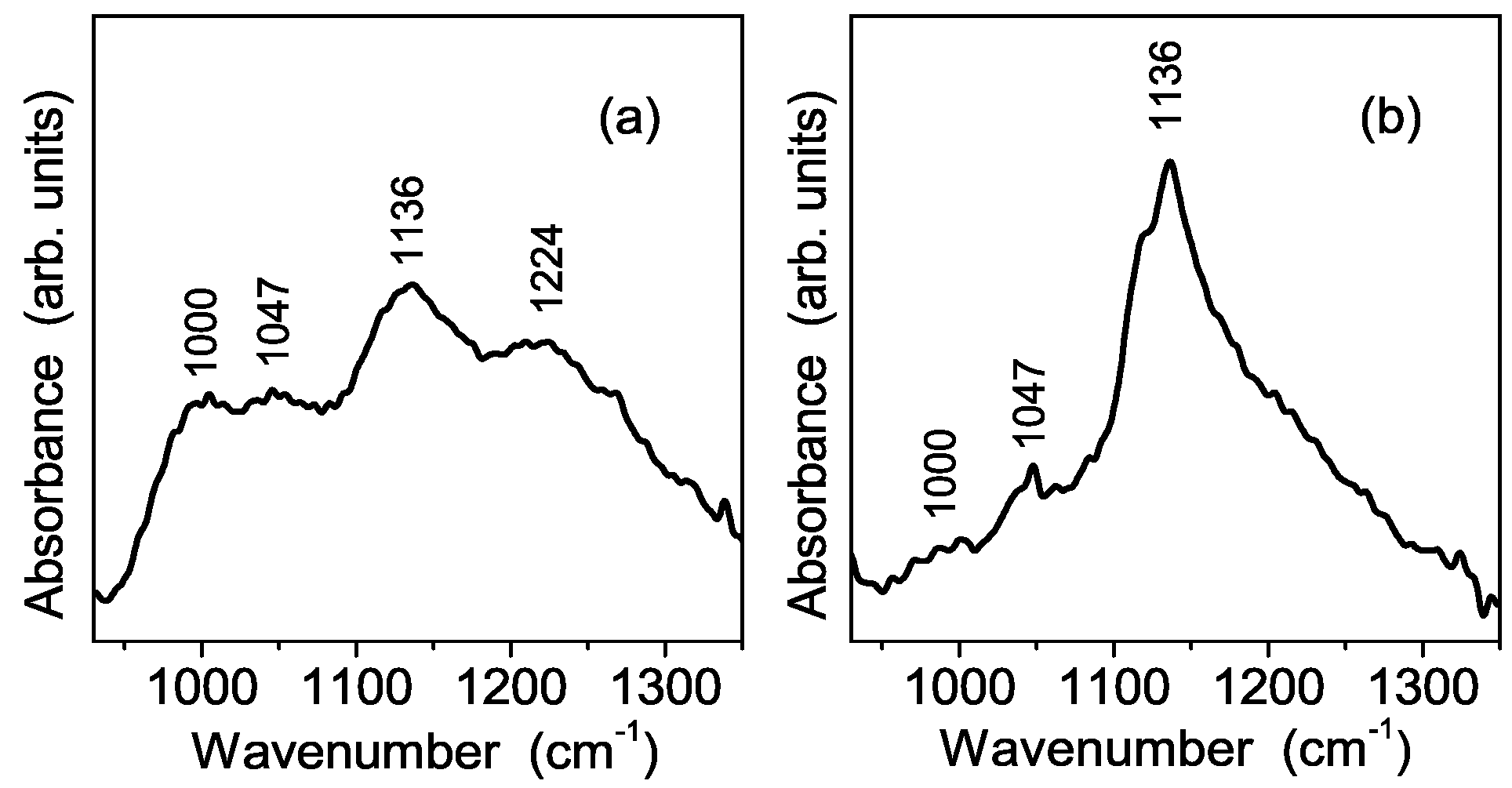
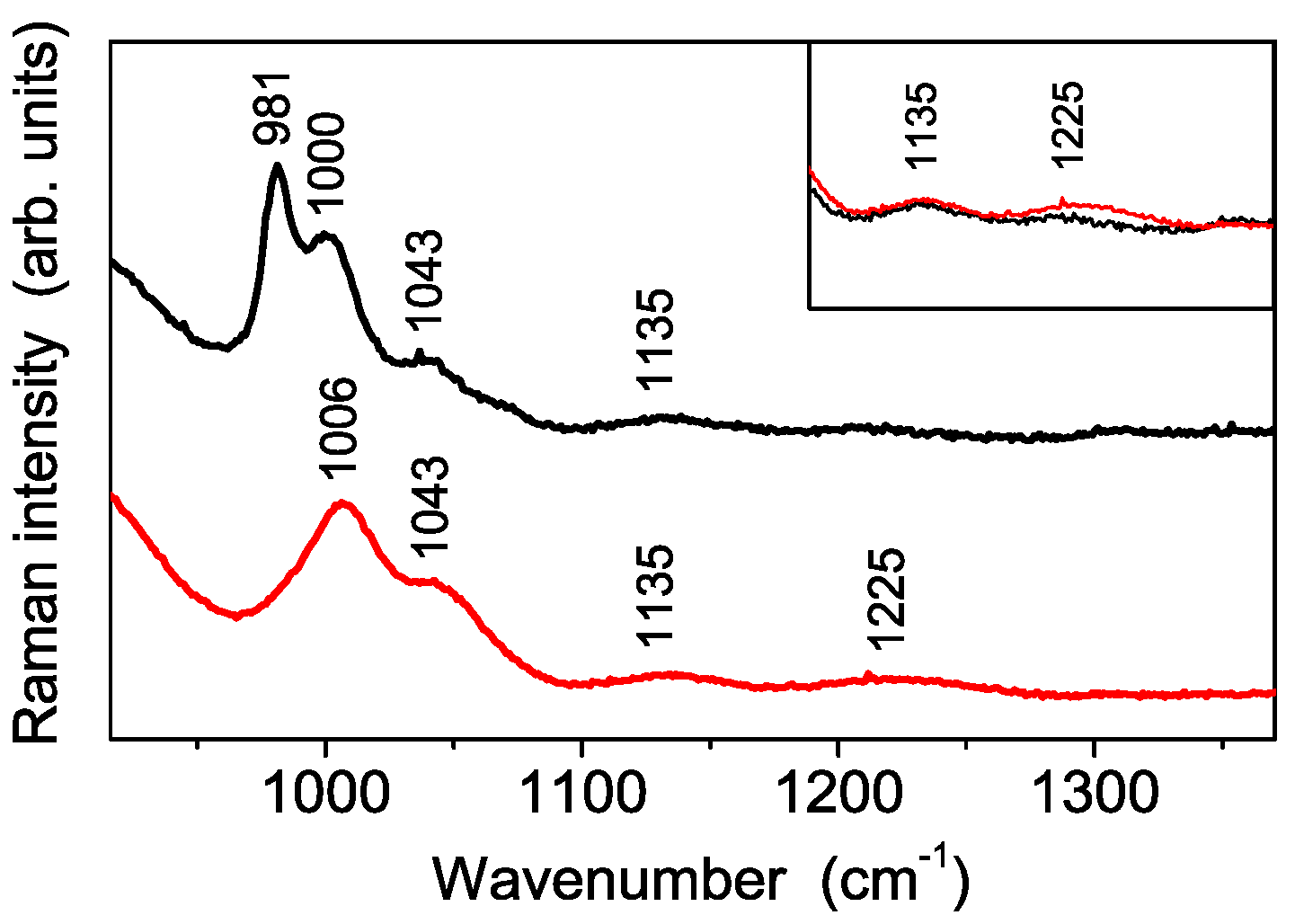
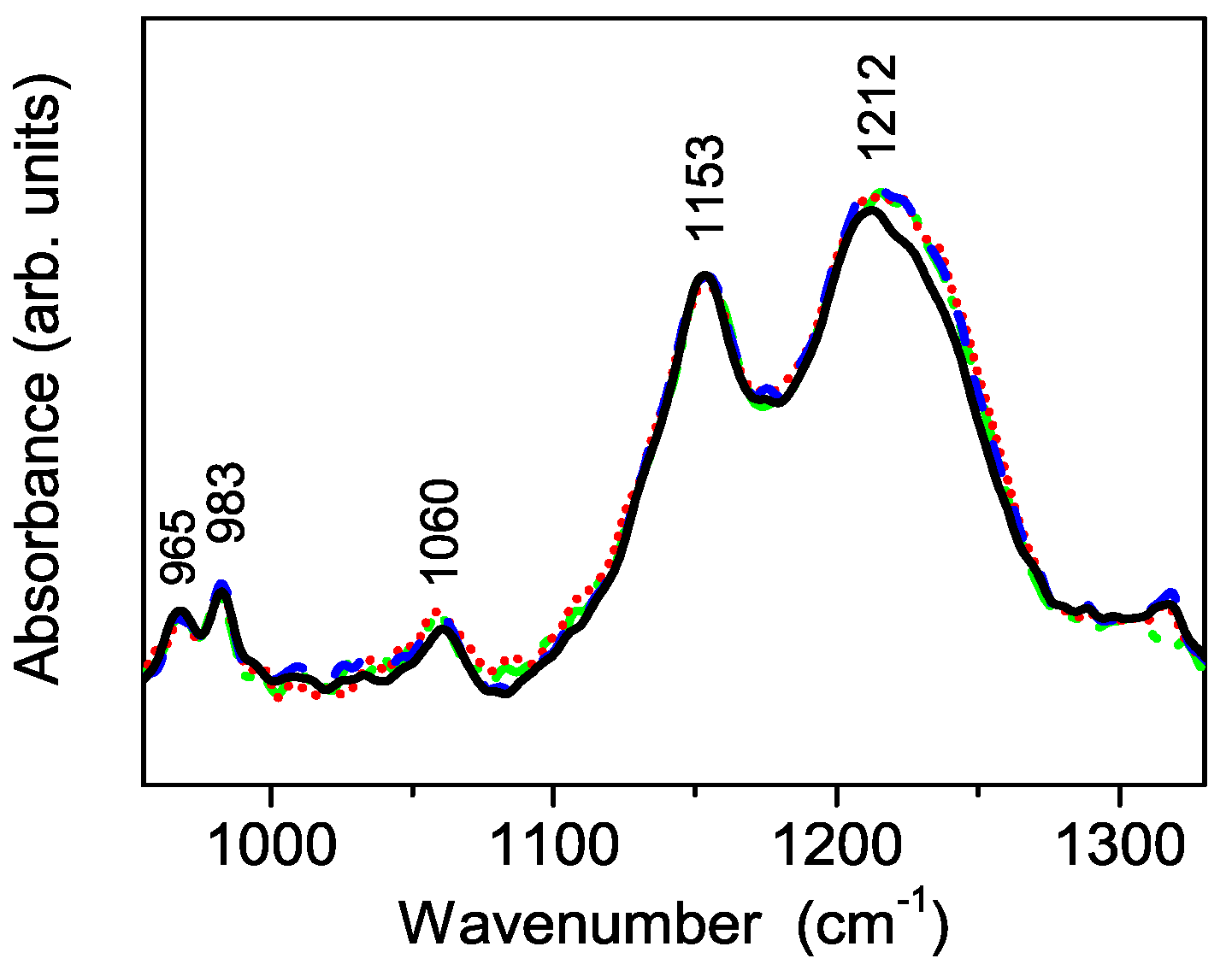
3.3. Vibrational Characterization of Composite Membranes
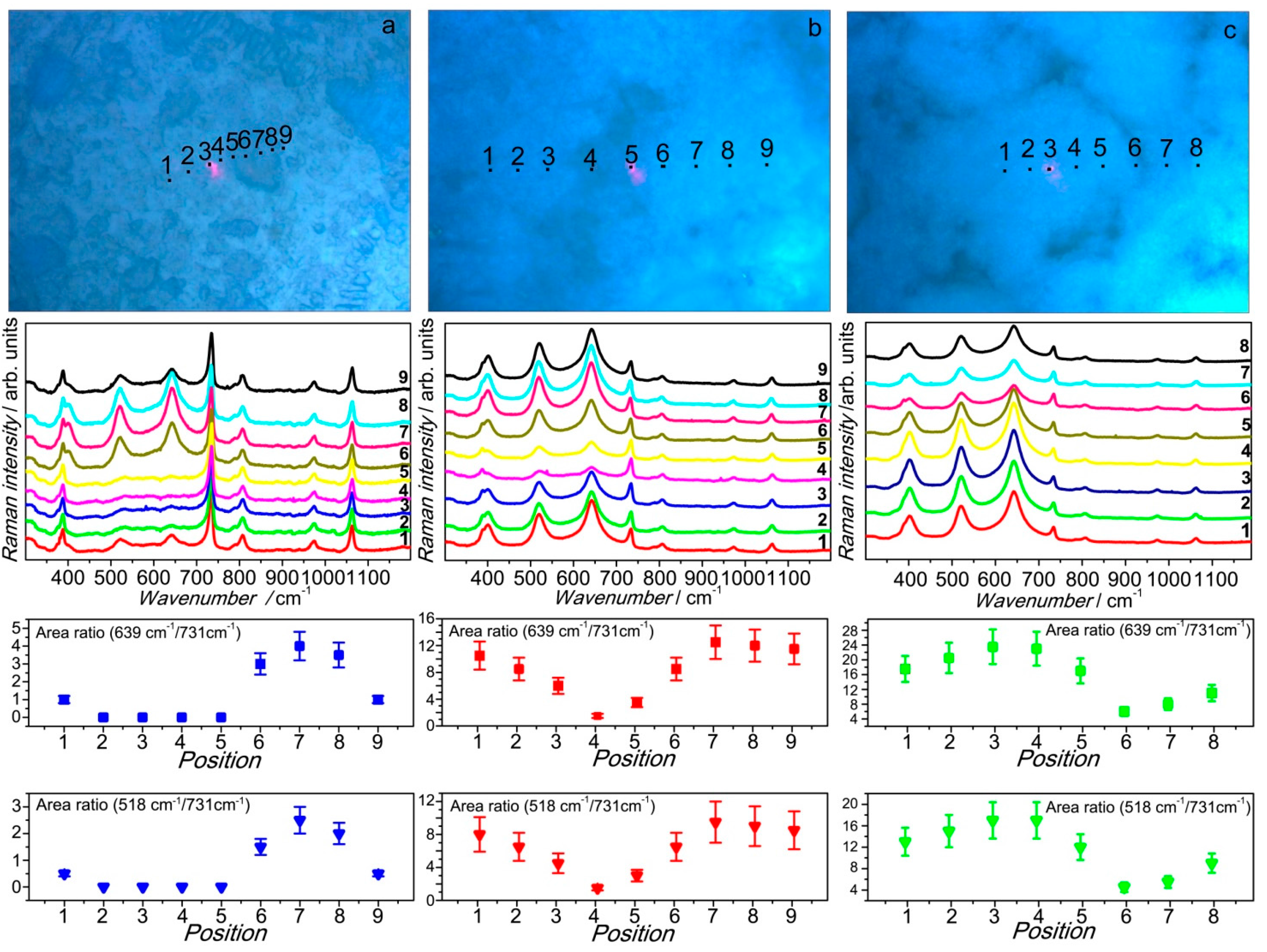
3.4. Membrane Morphology Related Raman Mapping
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giffin, G.A.; Piga, M.; Lavina, S.; Navarra, M.A.; D’Epifanio, A.; Scrosati, B.; di Noto, V. Characterization of sulfated-zirconia/Nafion composite membranes for proton exchange membrane fuel cells. J. Power Sources 2012, 198, 66–75. [Google Scholar] [CrossRef]
- Tominaka, S.; Akiyama, N.; Croce, F.; Momma, T.; Scrosati, B.; Osaka, T. Sulfated zirconia nanoparticles as a proton conductor for fuel cell electrodes. J. Power Sources 2008, 185, 656–663. [Google Scholar] [CrossRef]
- Navarra, M.A.; Croce, F.; Scrosati, B. New, high temperature superacid zirconia-doped Nafion™ composite membranes. J. Mater. Chem. 2007, 17, 3210–3215. [Google Scholar] [CrossRef]
- Scipioni, R.; Gazzoli, D.; Teocoli, F.; Palumbo, O.; Paolone, A.; Ibris, N.; Brutti, S.; Navarra, M.A. Preparation and characterization of nanocomposite polymer membranes containing functionalized SnO2 additives. Membranes 2014, 4, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Brutti, S.; Scipioni, R.; Navarra, M.A.; Panero, S.; Allodi, V.; Giarola, M.; Mariotto, G. SnO2-Nafion® nanocomposite polymer electrolytes for fuel cell applications. Int. J. Nanotechnol. 2014, 11, 882–896. [Google Scholar] [CrossRef]
- Sgambetterra, M.; Panero, S.; Hassoun, J.; Navarra, M.A. Hybrid membranes based on sulfated titania nanoparticles as low cost proton conductors. Ionics 2013, 19, 1203–1206. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Munuera, G.; Ferino, I.; Cutrufello, M.G.; Navío, J.A. Structural and surface approach to the enhanced photocatalytic activity of sulfated TiO2 photocatalyst. Appl. Catal. B 2006, 63, 45–59. [Google Scholar] [CrossRef]
- Sakai, T.; Kajitani, S.; Hamagami, S.J.; Oda, H.; Matsuka, M.; Matsumoto, H.; Ishihara, T. Proton conduction properties of hydrous sulfated nano-titania synthesized by hydrolysis of titanyl sulfate. Solid State Ionics 2010, 181, 1746–1749. [Google Scholar] [CrossRef]
- Aslan, A.; Bozkurt, A. An investigation of proton conductivity of nanocomposite membranes based on sulfated nano-titania and polymer. Solid State Ionics 2013, 239, 21–27. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; Ranieri, G.A.; Sgambetterra, M.; Panero, S.; Navarra, M.A. An NMR study on the molecular dynamic and exchange effects in composite Nafion/sulfated titania membranes for PEMFCs. Int. J. Hydrogen. Energ. 2015, 40, 14651–14660. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Velmurugan, R.; Swaminathan, M. TiO2-SO42− as a novel solid acid catalyst for highly efficient, solvent free and easy synthesis of chalcones under microwave irradiation. Catal. Commun. 2011, 12, 375–379. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR: Los Alamos, NM, USA, 1994. [Google Scholar]
- Banfield, J.F.; Veblen, R. Conversion of perovskite to anatase and TiO2(B): A TEM study and the use of fundamental building blocks for understanding relationships among the TiO2 minerals. Am. Miner. 1992, 77, 545–557. [Google Scholar]
- Young, R.A. The Rietveld Method; Oxford University Press: London, UK, 1993. [Google Scholar]
- Cromer, D.T.; Herrington, K. The structures of anatase and rutile. J. Am. Chem. Soc. 1955, 77, 4708–4709. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6726. [Google Scholar] [CrossRef]
- Liu, Z.; Andreev, Y.; G. Armstrong, A.R.; Brutti, S.; Ren, Y.; Bruce, P.G. Nanostructured TiO2(B): The effect of size and shape on anode properties for Li-ion batteries. Progr. Nat. Sci. 2013, 23, 235–244. [Google Scholar] [CrossRef]
- Beuvier, T.; Richard-Plouet, M.; Brohan, L. Accurate methods for quantifying the relative ratio of anatase and TiO2(B) nanoparticles. J. Phys. Chem. C 2009, 113, 13703–13706. [Google Scholar] [CrossRef]
- Gentili, V.; Brutti, S.; Hardwick, L.J.; Armstrong, A.R.; Panero, S.; Bruce, P.G. Lithium insertion into anatase nanotubes. Chem. Mater. 2012, 24, 4468–4476. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics. Int. 2004, 11, 36–42. [Google Scholar]
- Rasband, W.S. Image J.; National Institutes of Health: Bethesda, MD, USA, 1997. [Google Scholar]
- Bersani, D.; Lottici, P.P.; Ding, X.-Z. Phonon confinement effects in the Raman scattering by TiO2 nanocrystals. Appl. Phys. Letts 1998, 82, 73–75. [Google Scholar] [CrossRef]
- Giarola, M.; Sanson, A.; Monti, F.; Mariotto, G.; Bettinelli, M.; Speghini, A.; Salviulo, G. Vibrational dynamics of anatase TiO2: Polarized Raman spectroscopy and ab initio calculations. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef]
- Ben Yahia, M.; Lemoigno, F.; Beuvier, T.; Filhol, J.; Richard-Plouet, M.; Brohan, L.; Doublet, M.-L. Updated references for the structural, electronic, and vibrational properties of TiO2(B) bulk using first-principles density functional theory calculations. J. Chem. Phys. 2009, 130, 204501. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Petrykin, V.V.; Kakihana, M. One-step synthesis of TiO2(B) nanoparticles from a water-soluble titanium complex. Chem. Mater. 2007, 19, 5373–5376. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and raman spectra of inorganic and coordination compounds. In Handbook of Vibrational Spectroscopy; J. Wiley&Sons: New York, NY, USA, 1986. [Google Scholar]
- Arata, K.; Hino, M. Preparation of superacids by metal-oxides and their cathalitic action. Mater. Chem. Phys. 1990, 26, 213–237. [Google Scholar] [CrossRef]
- Bolis, V.; Magnavacca, G.; Cerrato, G.; Morterra, C. Surface heterogeneity on hydrophilic and hydrophobic silicas: Water and alcohols as probes for H-bonding and dispersion forces. Langmuir 1997, 13, 888–894. [Google Scholar] [CrossRef]
- Myhre, C.E.L.; Christensen, D.H.; Nicolaisen, F.M.; Nielsen, C.J. Spectroscopic study of aqueous H2SO4 at different temperatures and compositions: Variations in dissociation and optical properties. J. Phys. Chem. A 2003, 107, 1979–1991. [Google Scholar] [CrossRef]
- Gruger, A.; Régis, A.; Schmatko, T.; Colomban, P. Nanostructure of Nafion (R) membranes at different states of hydration—An IR and Raman study. Vibr. Spectrosc. 2001, 26, 215–225. [Google Scholar] [CrossRef]
- Kunimatsu, K.; Bae, B.; Miyatake, K.; Uchida, H.; Watanabe, M. ATR-FTIR study of water in Nafion membrane combined with proton conductivity measurements during hydration/dehydration cycle. J. Phys. Chem. B 2011, 115, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
| Sample | Filler | Filler content (wt %) | Sample acronym |
|---|---|---|---|
| Plain Nafion | None | 0 | N |
| Composite Nafion | Superacid S-TiO2 | 2 | 2-TiO2-S |
| Composite Nafion | Superacid S-TiO2 | 5 | 5-TiO2-S |
| Composite Nafion | Superacid S-TiO2 | 7 | 7-TiO2-S |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allodi, V.; Brutti, S.; Giarola, M.; Sgambetterra, M.; Navarra, M.A.; Panero, S.; Mariotto, G. Structural and Spectroscopic Characterization of A Nanosized Sulfated TiO2 Filler and of Nanocomposite Nafion Membranes. Polymers 2016, 8, 68. https://doi.org/10.3390/polym8030068
Allodi V, Brutti S, Giarola M, Sgambetterra M, Navarra MA, Panero S, Mariotto G. Structural and Spectroscopic Characterization of A Nanosized Sulfated TiO2 Filler and of Nanocomposite Nafion Membranes. Polymers. 2016; 8(3):68. https://doi.org/10.3390/polym8030068
Chicago/Turabian StyleAllodi, Valentina, Sergio Brutti, Marco Giarola, Mirko Sgambetterra, Maria Assunta Navarra, Stefania Panero, and Gino Mariotto. 2016. "Structural and Spectroscopic Characterization of A Nanosized Sulfated TiO2 Filler and of Nanocomposite Nafion Membranes" Polymers 8, no. 3: 68. https://doi.org/10.3390/polym8030068