Fabrication of Porous Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Monoliths via Thermally Induced Phase Separation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of P3HB3HHx Monoliths
2.3. Measurements
3. Results and Discussion
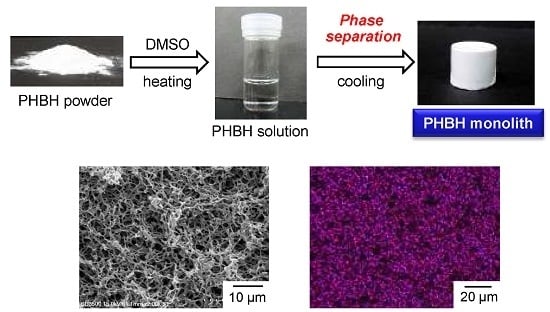
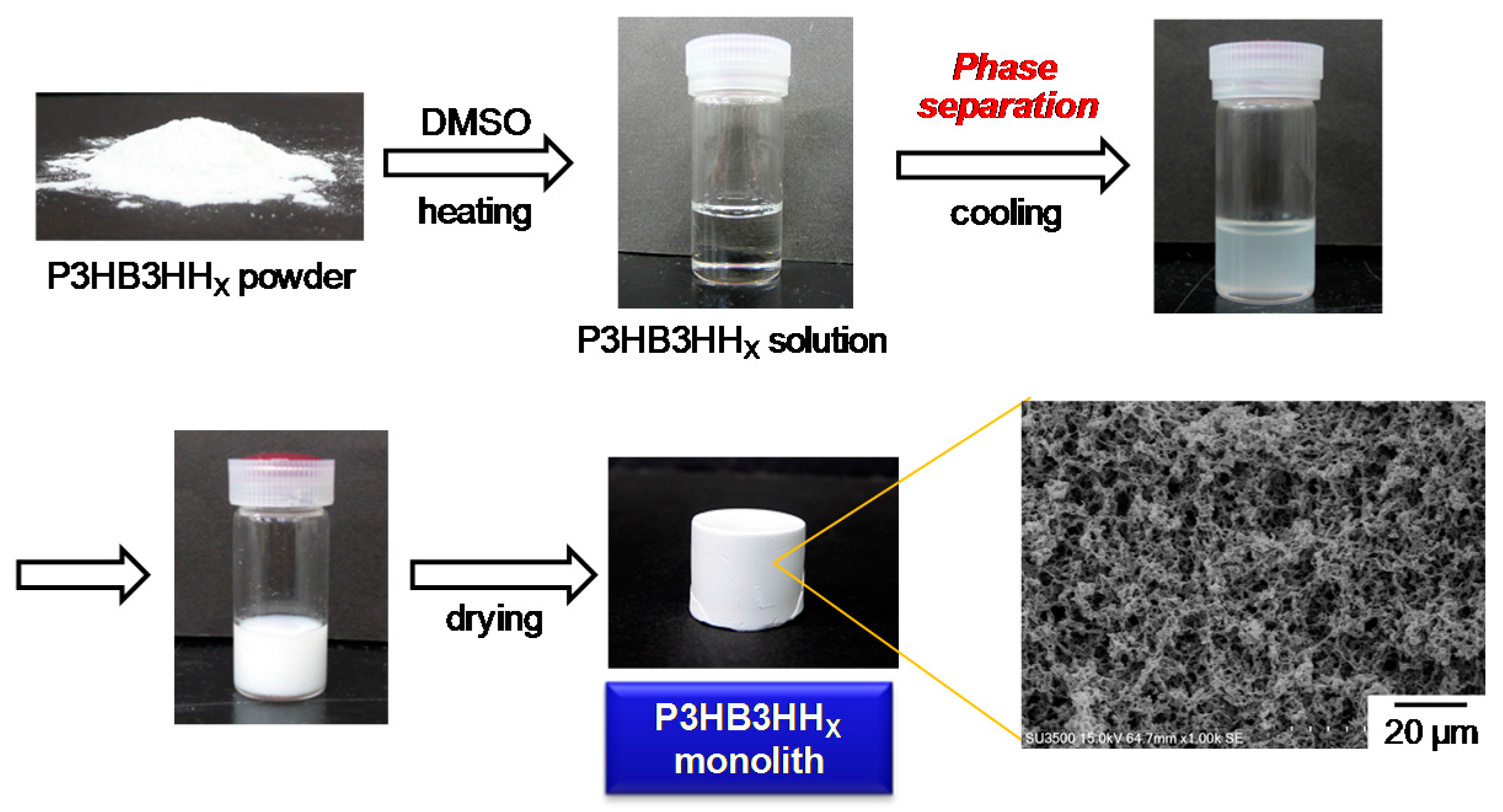
3.1. Fabrication of P3HB3HHx Monoliths
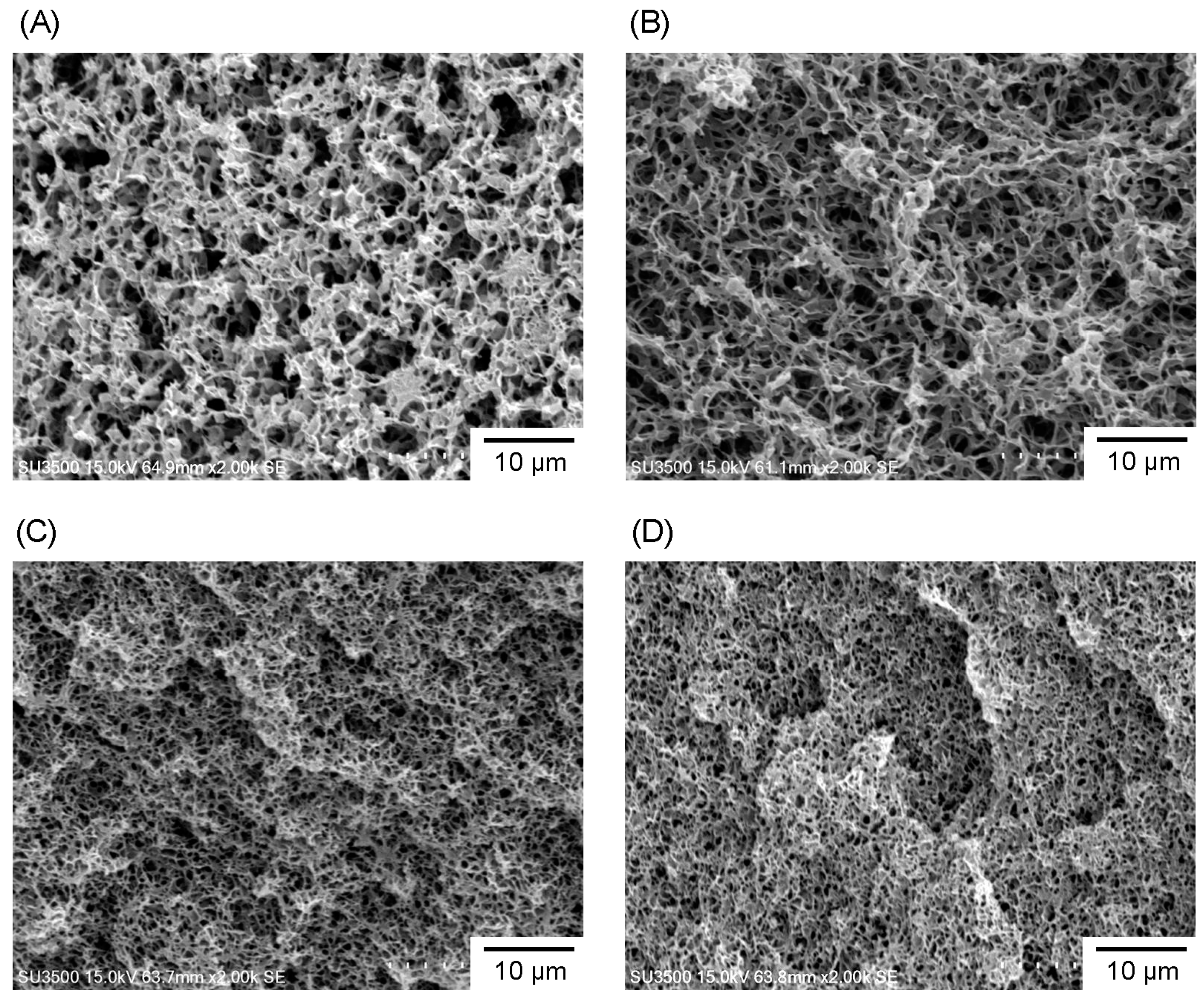
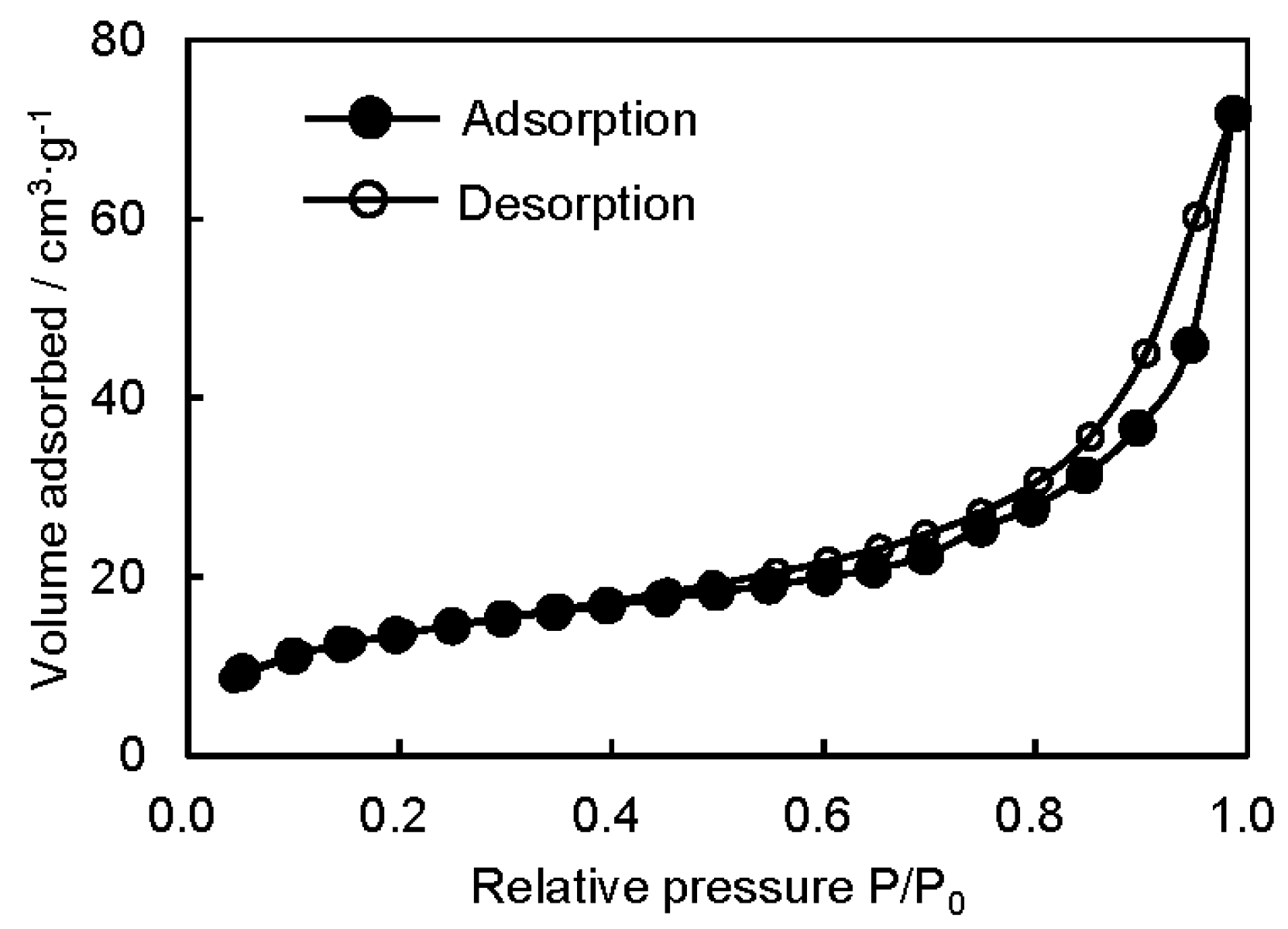
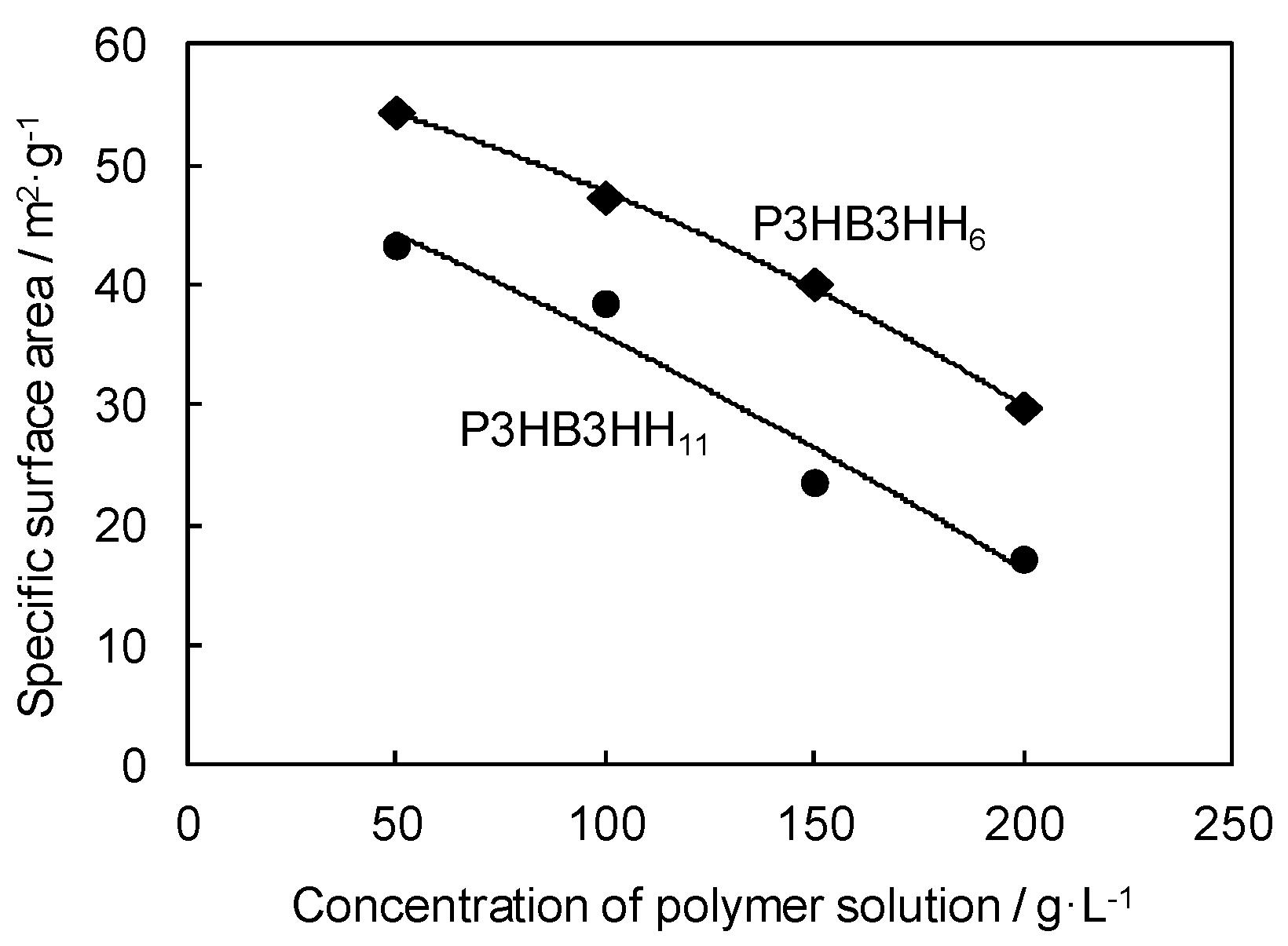
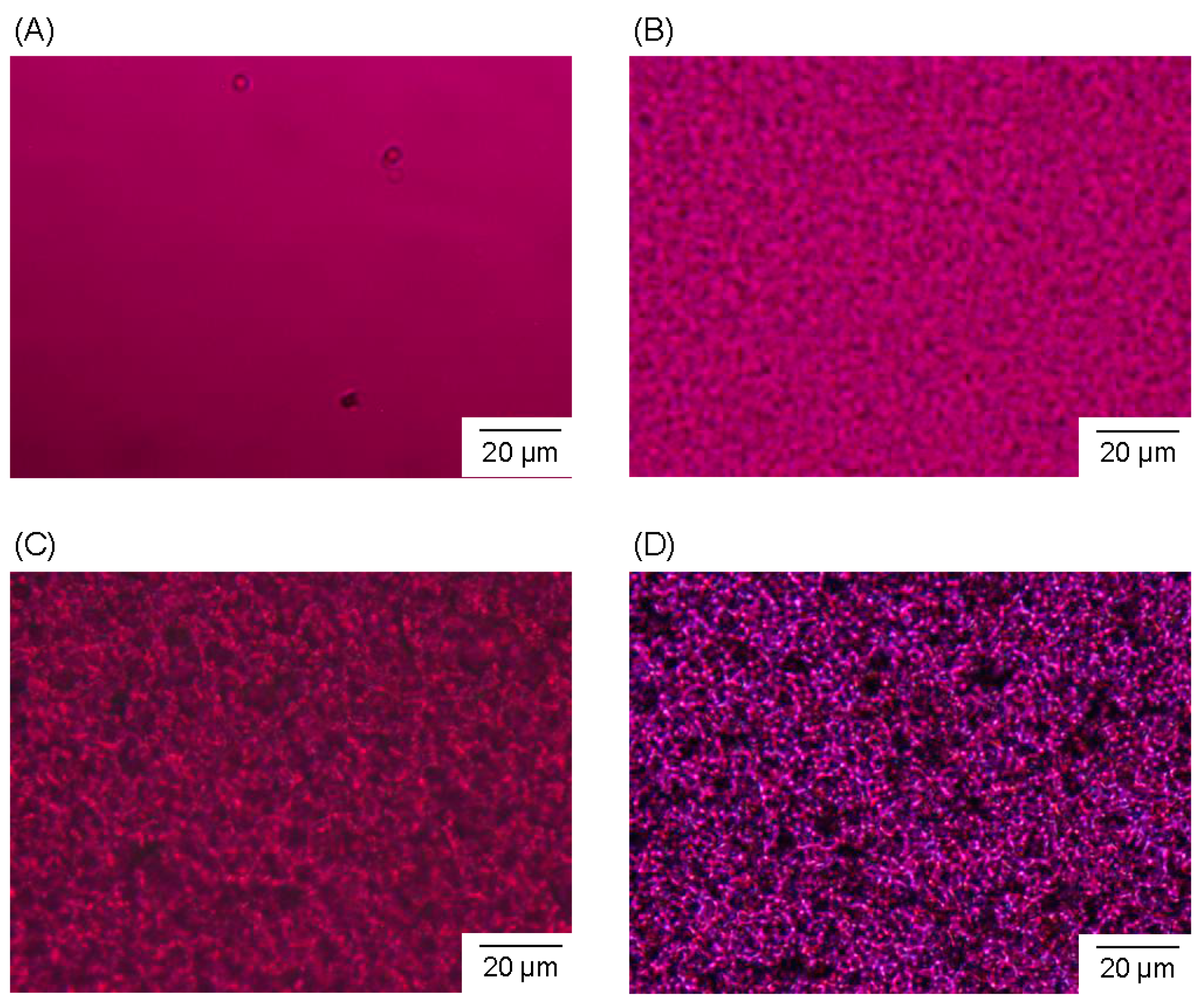
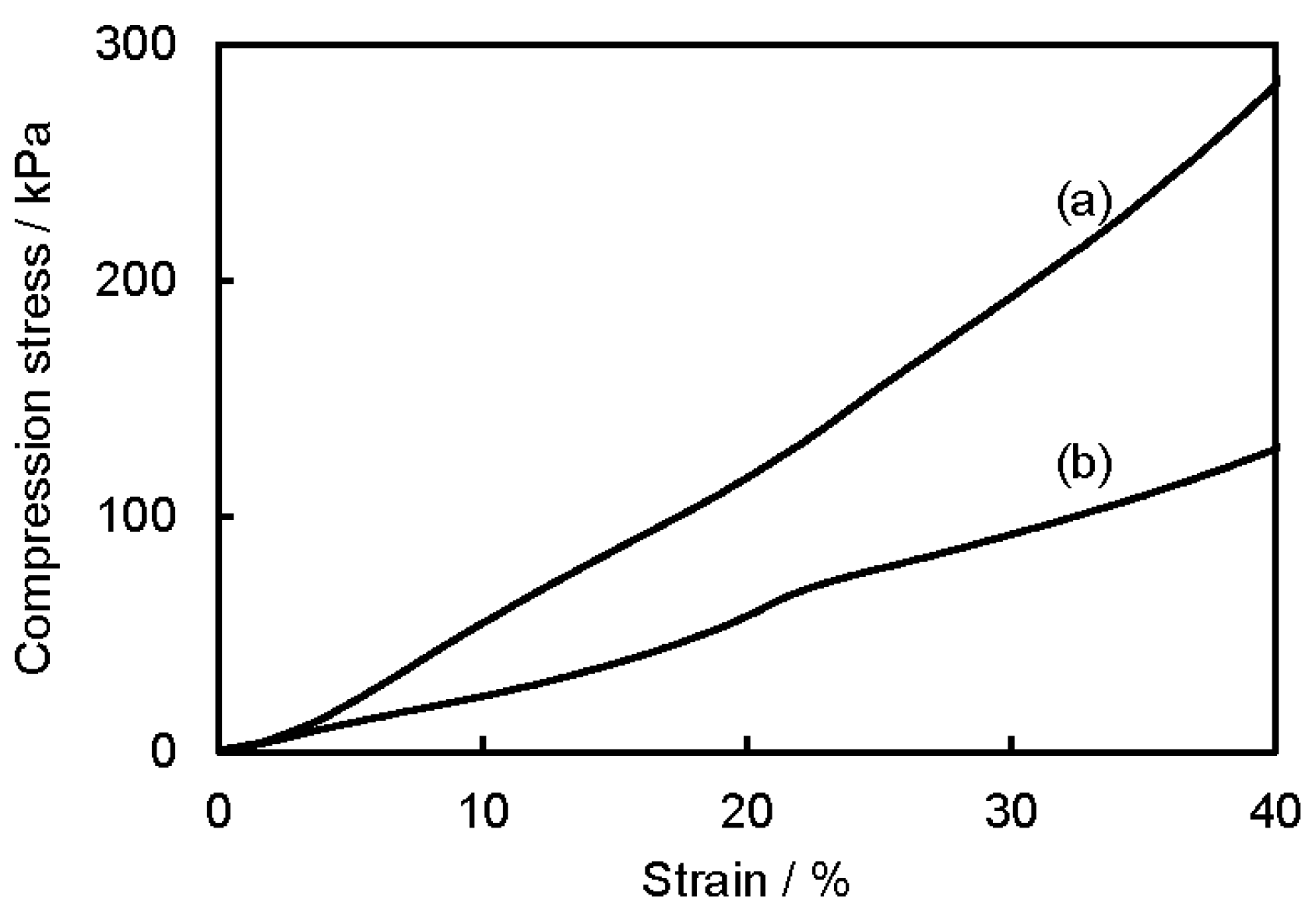
3.2. Microstructure and Properties of P3HB3HHx Monoliths
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hüsing, N.; Schubert, U. Aerogels-airy materials: Chemistry, structure and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Hasegawa, G.; Maeno, A.; Kaji, H.; Nakanishi, K. A superamphiphobic macroporous silicone monolith with marshmallow-like flexibility. Angew. Chem. Int. Ed. 2013, 52, 10788–10791. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Mayr, M.; Mayr, B.; Buchmeiser, M.R. Monolithic materials: New high-performance supports for permanently immobilized metathesis catalysts. Angew. Chem. Int. Ed. 2001, 40, 3839–3842. [Google Scholar] [CrossRef]
- Hentze, H.P.; Antonietti, M. Porous polymers and resins for biotechnological and biomedical applications. Rev. Mol. Biotechnol. 2002, 90, 27–53. [Google Scholar] [CrossRef]
- Shih, Y.H.; Singco, B.; Liu, W.L.; Hsu, C.H.; Huang, H.Y. A rapid synthetic method for organic polymer-based monoliths in a room temperature ionic liquid medium via microwave-assisted vinylization and polymerization. Green Chem. 2011, 13, 296–299. [Google Scholar] [CrossRef]
- Li, T.Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly(l-lactic acid) foams. Polymer 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Lu, M.H.; Zhang, Y. Microbead patterning of on porous films with ordered arrays of pores. Adv. Mater. 2006, 18, 3094–3098. [Google Scholar] [CrossRef]
- Harris, L.D.; Kim, B.S.; Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998, 42, 396–402. [Google Scholar] [CrossRef]
- Taki, K.; Nitta, K.; Kihara, S.; Ohshima, M. CO2 foaming of poly(ethylene glycol)/polystyrene blends: Relationship of the blend morphology, CO2 mass transfer, and cellular structure. J. Appl. Polym. Sci. 2005, 97, 1899–1906. [Google Scholar] [CrossRef]
- Whang, K.; Thomas, C.H.; Healy, K.E.; Nuber, G. A novel method to fabricate bioabsorbable scaffolds. Polymer 1995, 36, 837–842. [Google Scholar] [CrossRef]
- Lee, M.; James, C.Y.; Wu, B.M. Scaffold fabrication by indirect three-dimensional printing. Biomaterials 2005, 26, 4281–4289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hussain, I.; Brust, M.; Butler, M.F.; Rannard, S.P.; Cooper, A.I. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 2005, 4, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Lu, Z.; Li, J.; Zhu, Y.; Zhang, S.; Chen, J. Preparation of poly(l-lactic acid) with aligned structures by unidirectional freezing. Polym. Advan. Technol. 2015, 26, 606–612. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T.T.; Kwei, T.K. Thermally induced phase separation behavior of compatible polymer mixtures. Macromolecules 1975, 8, 227–234. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, T.G. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 1999, 20, 1783–1790. [Google Scholar] [CrossRef]
- Hua, F.J.; Park, T.G.; Lee, D.S. A facile preparation of highly interconnected macroporous poly(d,l-lactic acid-co-glycolic acid) (PLGA) scaffolds by liquid-liquid phase separation of a PLGA-dioxane-water ternary system. Polymer 2003, 44, 1911–1920. [Google Scholar] [CrossRef]
- Tanaka, T.; Lloyd, D.R. Formation of poly(l-lactic acid) microfiltration membranes via thermally induced phase separation. J. Membrane Sci. 2004, 238, 65–73. [Google Scholar] [CrossRef]
- Yoneda, S.; Han, W.; Hasegawa, U.; Uyama, H. Facile fabrication of poly(methyl methacrylate) monolith via thermally induced phase separation by utilizing unique cosolvency. Polymer 2014, 55, 3212–3216. [Google Scholar] [CrossRef]
- Aubert, J.H.; Clough, R.L. Low density, microcellular polystyrene foams. Polymer 1985, 26, 2047–2054. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally induced phase separation. II. Liquid-liquid phase separation. J. Membrane Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res. 1999, 47, 8–17. [Google Scholar] [CrossRef]
- Okada, K.; Nandi, M.; Maruyama, J.; Oka, T.; Tsujimoto, T.; Kondoh, K.; Uyama, H. Fabrication of mesoporous polymer monolith: A template-free approach. Chem. Commun. 2011, 47, 7422–7424. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Liu, X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J. Mater. Chem. B 2013, 1, 4764–4772. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, I.; Myatt, M. Phase separation in ternary polymer solutions induced by solvent loss. Macromolecules 2002, 35, 5153–5160. [Google Scholar] [CrossRef]
- Kim, J.K.; Taki, K.; Ohshima, M. Preparation of a unique microporous structure via two step phase separation in the course of drying a ternary polymer solution. Langmuir 2007, 23, 12397–12405. [Google Scholar] [CrossRef] [PubMed]
- Luccio, M.D.; Nobrega, R.; Borges, C.P. Microporous anisotropic phase inversion membranes from bisphenol-A polycarbonate: Study of a ternary system. Polymer 2000, 41, 4309–4315. [Google Scholar] [CrossRef]
- Ying, L.; Kang, E.T.; Neoh, K.G. Synthesis and characterization of poly(N-isopropylacrylamide)-graft-poly(vinylidene fluoride) copolymers and temperature-sensitive membranes. Langmuir 2002, 18, 6416–6423. [Google Scholar] [CrossRef]
- Xin, Y.; Fujimoto, T.; Uyama, H. Facile fabrication of polycarbonate monolith by non-solvent induced phase separation method. Polymer 2012, 53, 2847–2853. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Isothermal ternary phase diagram of the polylactic acid-dichloromethane-hexane system. Polymer 2014, 55, 3100–3106. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Salerno, A.; Muhr, A.; Reiterer, A.; Braunegg, G. Polyhydroxyalkanoates: Biodegradable polymers and plastics from renewable resources. Mater. Technol. 2013, 47, 5–12. [Google Scholar]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen bonds in polymer blends. Prog. Polym. Sci. 2004, 29, 1021–1051. [Google Scholar] [CrossRef]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaaaini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Xie, Y.; Kohls, D.; Noda, I.; Schaefer, D.W.; Akpalu, Y.A. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanocomposites with optimal mechanical properties. Polymer 2009, 50, 4656–4670. [Google Scholar] [CrossRef]
- Hosoda, N.; Tsujimoto, T.; Uyama, H. Green composite of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with porous cellulose. ACS Sustain. Chem. Eng. 2013, 2, 248–253. [Google Scholar] [CrossRef]
- Kuga, S. The porous structure of cellulose gel regenerated from calcium thiocyanate solution. J. Colloid and Interf. Sci. 1980, 77, 413–417. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Taki, K.; Nagamine, S.; Ohshima, M. Preparation of porous poly(l-lactic acid) honeycomb monolith structure by phase separation and unidirectional freezing. Langmuir 2009, 25, 5304–5312. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Y.; Zeng, X.; Quan, D.; Liao, S.; Zeng, Y.; Lu, J.; Ramakrishna, S. Fabrication and characterization of poly(l-lactic acid) 3D nanofibrous scaffolds with controlled architecture by liquid-liquid phase separation from ternary polymer-solvent system. Polymer 2009, 50, 4128–4138. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Production of porous polylactic acid monoliths via nonsolvent induced phase separation. Polymer 2014, 55, 6743–6753. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Liu, Y.; Liu, R.; Wei, F.; Xiao, R.; Liu, H. 3D Porous poly(l-lactic acid) foams composed of nanofibers, nanofibrous microsheaves and microspheres and their application in oil-water separation. J. Mater. Chem. A 2015, 3, 14054–14062. [Google Scholar] [CrossRef]
- Yin, G.; Zhao, D.; Zhang, L.; Feng, J.; Ren, Y.; Chen, G.; Zhou, Z.; Li, Q. Gradual precipitation to fabricate PLLA materials with high porosity and tunable strength formed by high crystalline nanosheets. Macromol. Mater. Eng. 2015. [Google Scholar] [CrossRef]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujimoto, T.; Hosoda, N.; Uyama, H. Fabrication of Porous Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Monoliths via Thermally Induced Phase Separation. Polymers 2016, 8, 66. https://doi.org/10.3390/polym8030066
Tsujimoto T, Hosoda N, Uyama H. Fabrication of Porous Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Monoliths via Thermally Induced Phase Separation. Polymers. 2016; 8(3):66. https://doi.org/10.3390/polym8030066
Chicago/Turabian StyleTsujimoto, Takashi, Nao Hosoda, and Hiroshi Uyama. 2016. "Fabrication of Porous Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Monoliths via Thermally Induced Phase Separation" Polymers 8, no. 3: 66. https://doi.org/10.3390/polym8030066