Self-Assembly of Discrete Metallocycles versus Coordination Polymers Based on Cu(I) and Ag(I) Ions and Flexible Ligands: Structural Diversification and Luminescent Properties
Abstract
:1. Introduction
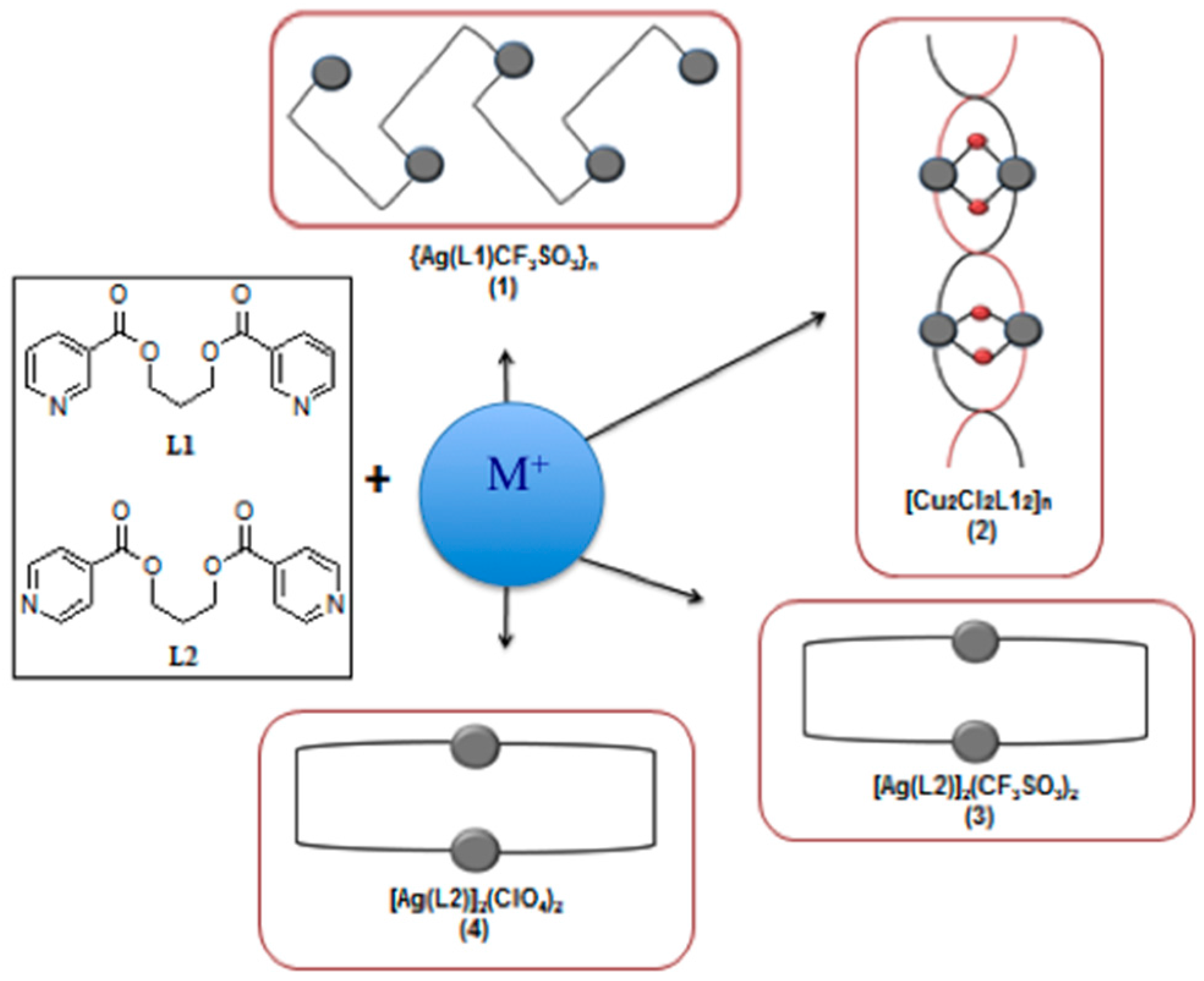
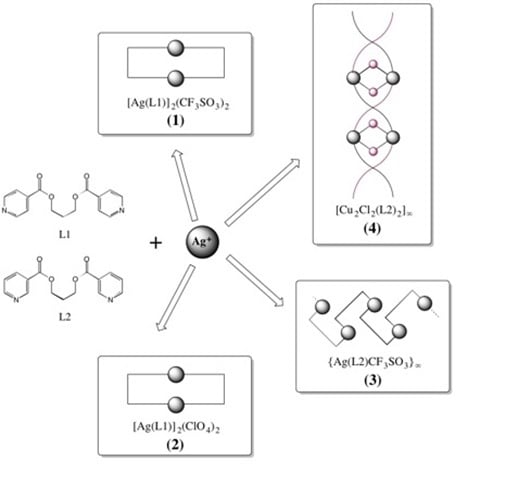
2. Experimental Section
2.1. Coordination Polymer Synthesis and Characterization
2.2. X-ray Crystallographic Measurements
3. Results and Discussion
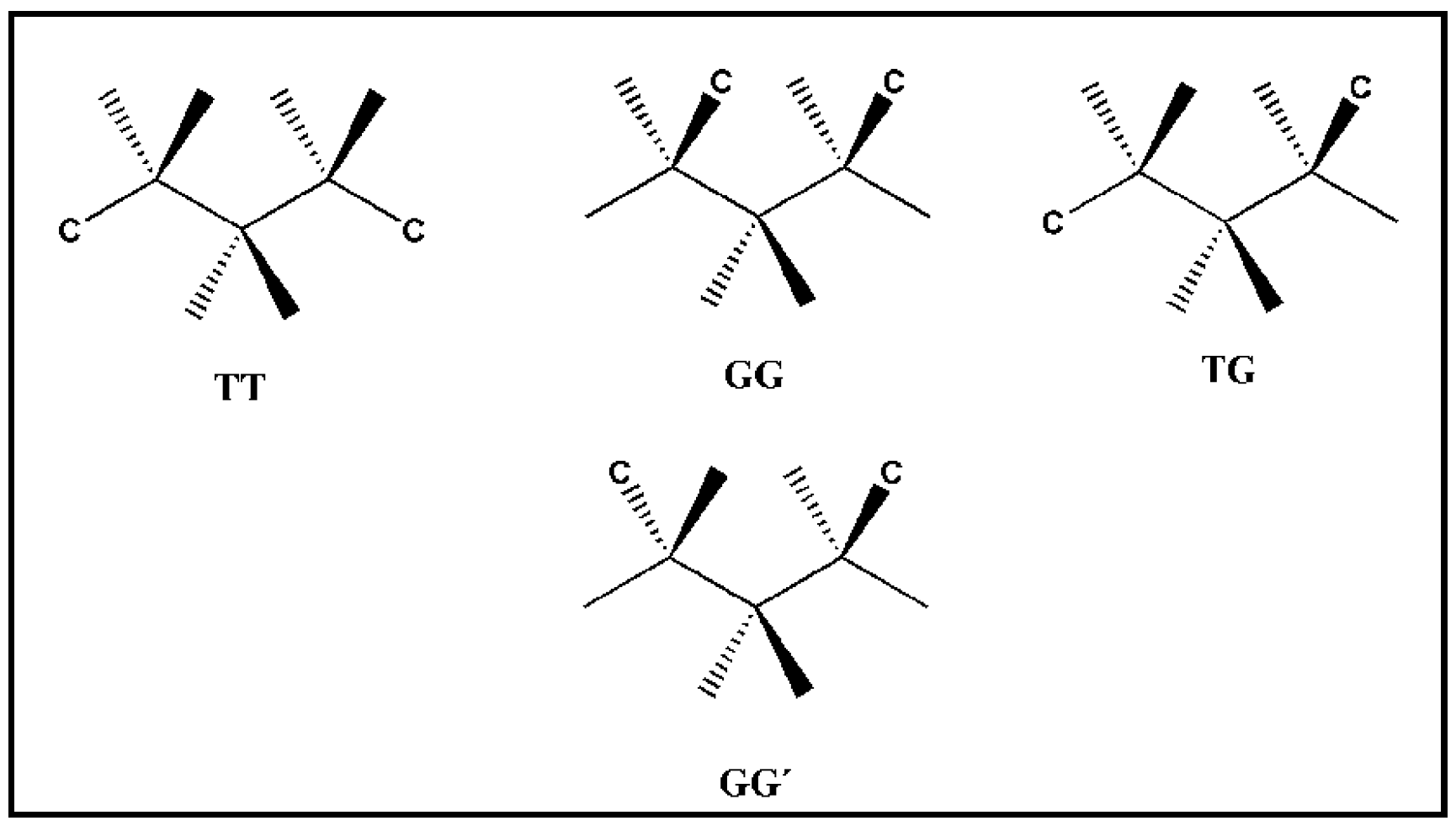
3.1. Conformational Flexibility of the Organic Linkers
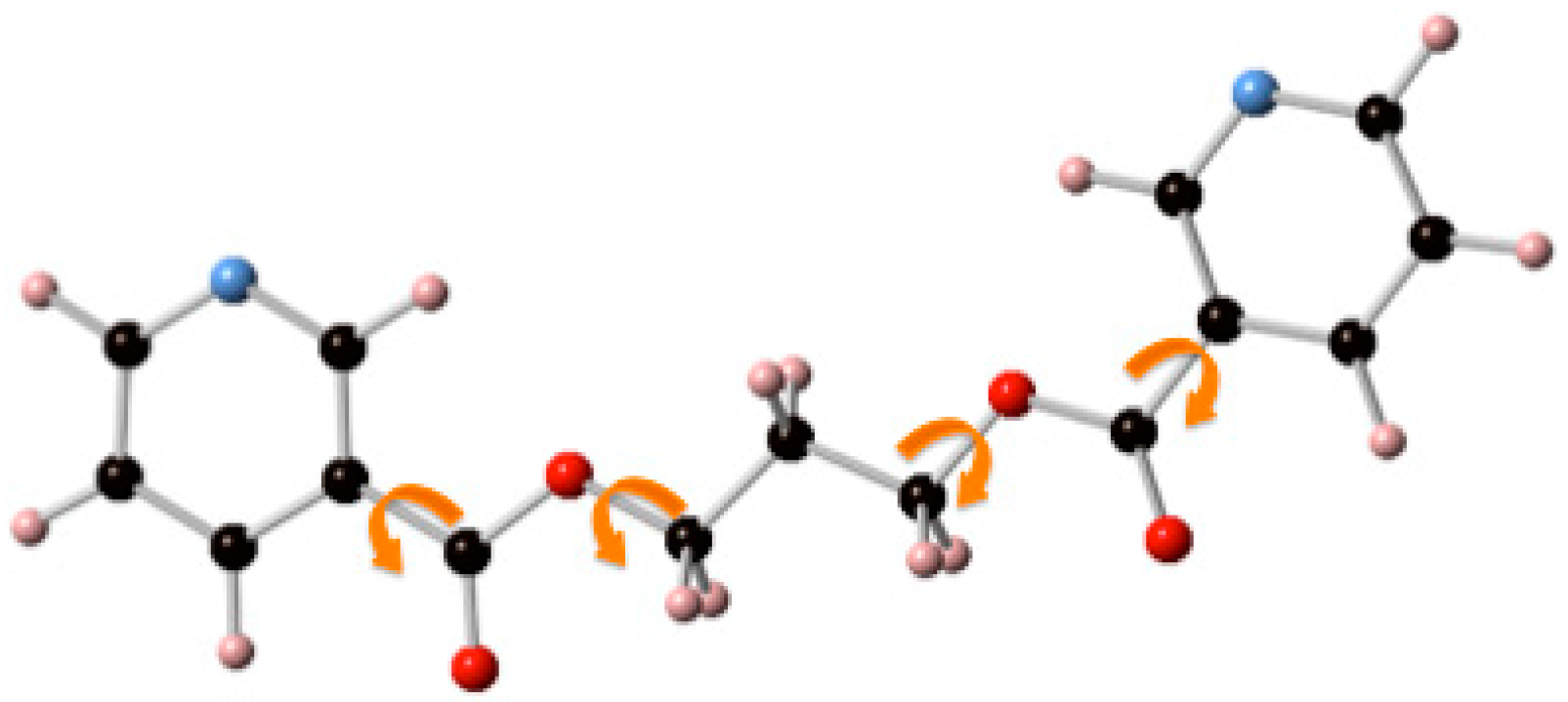
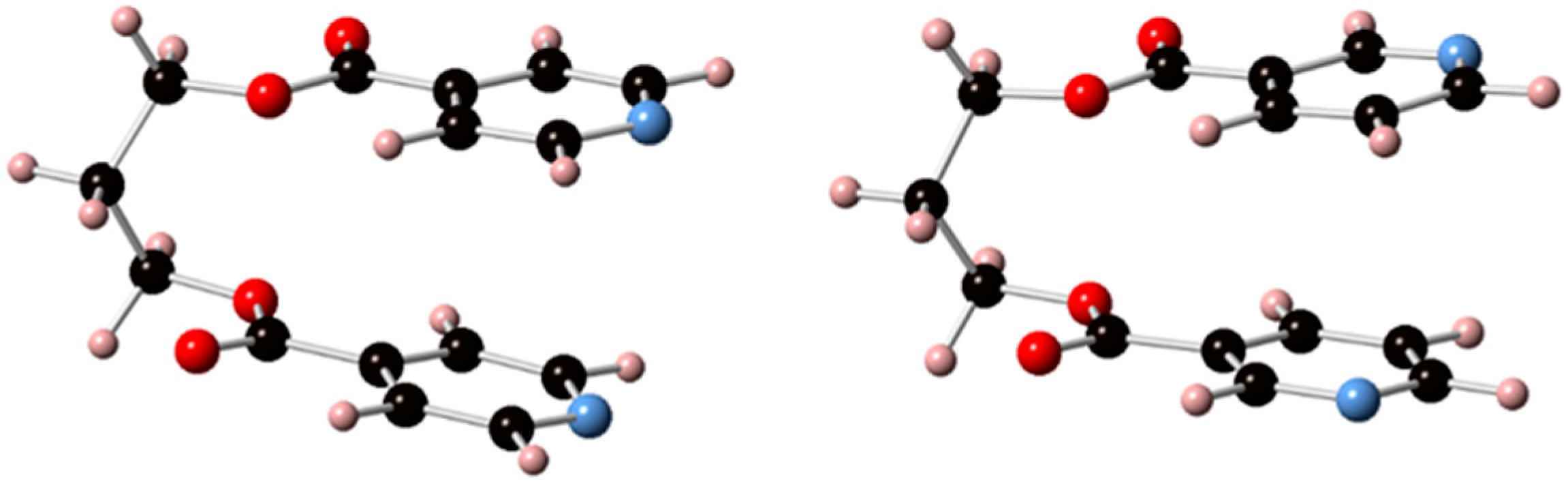
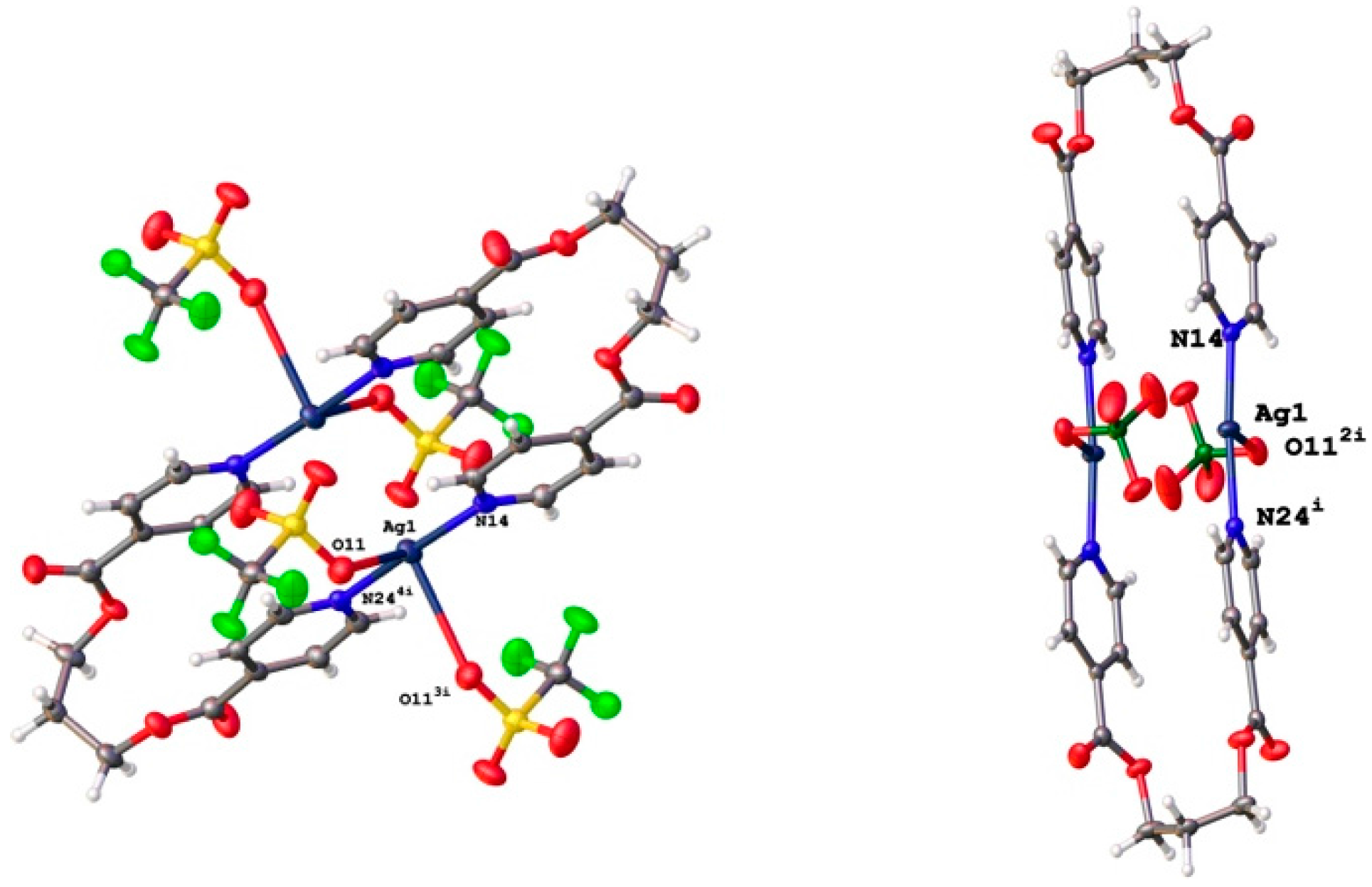
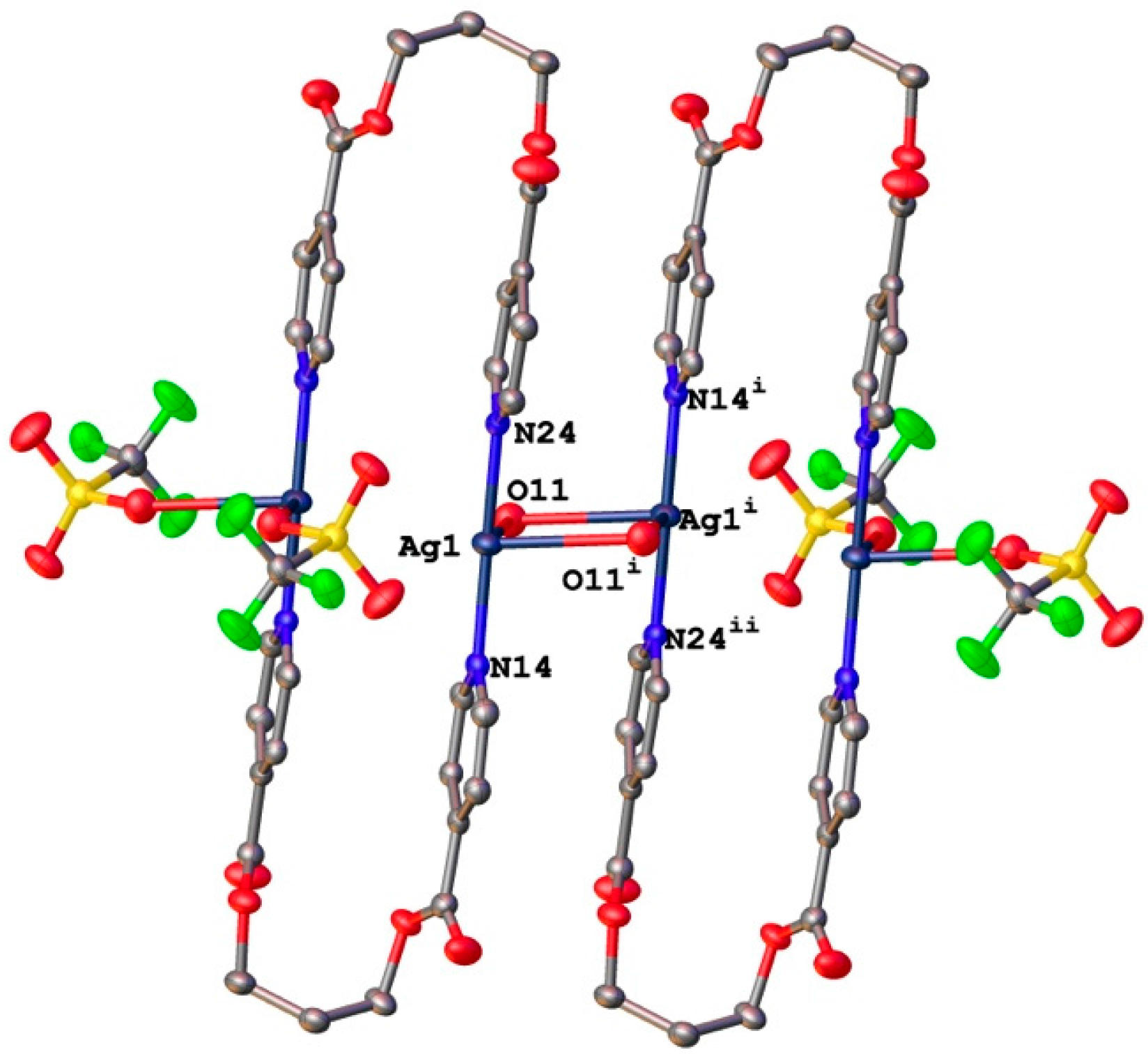
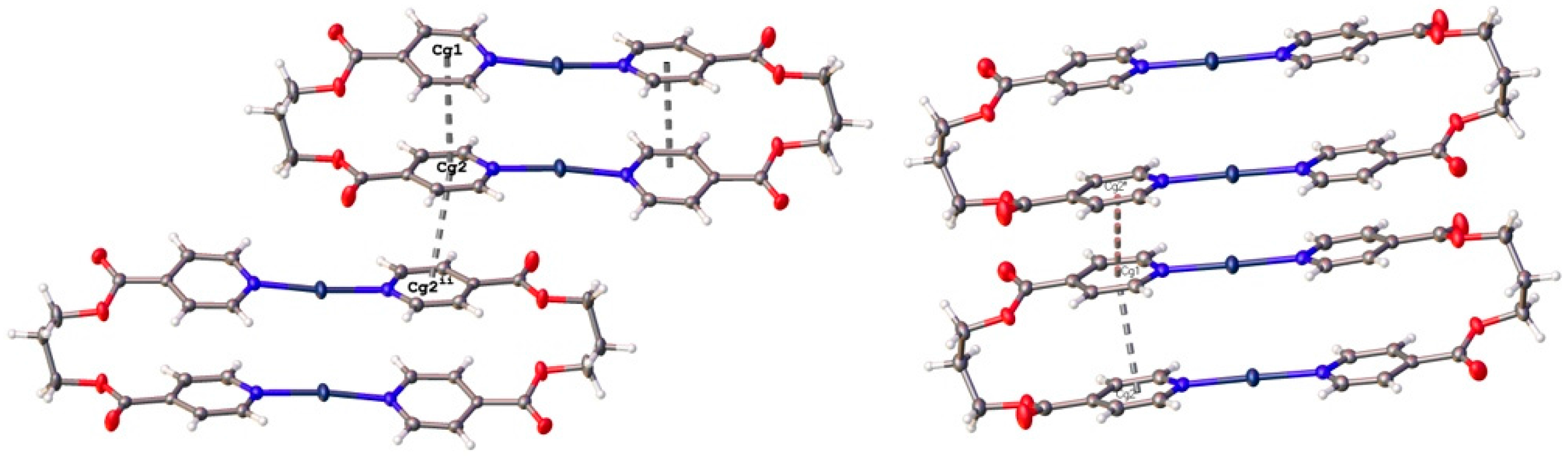
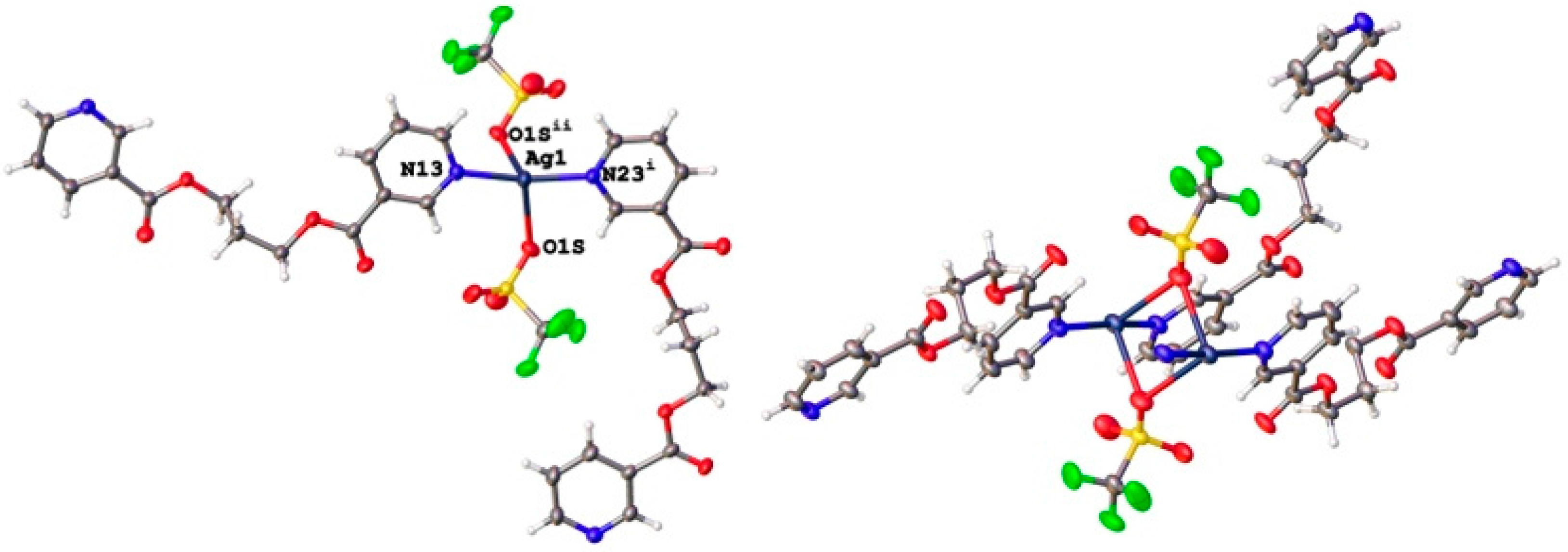
3.2. Structural Analysis
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Molecular formula | C32H28Ag2F6N4O14S2 | C30H28Ag2Cl2N4O16 | C16H14AgF3N2O7S | C30H28Cl2Cu2N4O8 |
| Formula weight | 1086.44 | 987.20 | 543.22 | 770.54 |
| Space group | P-1 | P21/c | P21/n | C2/c |
| a/Å | 6.5913(5) | 10.3551(5) | 7.9745(6) | 15.8963(11) |
| b/Å | 11.7365(10) | 7.5927(4) | 10.9040(6) | 29.2769(12) |
| c/Å | 13.2883(10) | 21.4540(9) | 21.8561(13) | 15.2635(10) |
| α/° | 65.594(6) | 90 | 90 | 90 |
| β/° | 89.733(6) | 98.996(3) | 94.865(5) | 117.882(5) |
| γ/° | 84.604(7) | 90 | 90 | 90 |
| V/Å3 | 931.30(13) | 1,666.03(14) | 1,893.6(2) | 6,278.9(7) |
| Z | 1 | 2 | 4 | 8 |
| Dc/Mg·m−3 | 1.937 | 1.968 | 1.905 | 1.630 |
| μ/MoKα/mm−1 | 1.268 | 1.421 | 1.248 | 1.582 |
| F(000) | 540 | 984 | 1080 | 3136 |
| Reflections collected | 15,998 | 25,740 | 11,865 | 52,992 |
| Unique reflections | 3,469 | 3,123 | 3,544 | 5,921 |
| No. of params | 271 | 245 | 271 | 415 |
| GOF on F2 | 1.084 | 1.044 | 0.977 | 0.982 |
| Final R indices [I ≥ 2σ(I)] | R1 = 0.0472, wR2 = 0.1168 | R1 = 0.0274, wR2 = 0.0685 | R1 = 0.0330, wR2 = 0.0848 | R1 = 0.0472, wR2 = 0.1024 |
| R int | 0.0920 | 0.0620 | 0.0731 | 0.0927 |
| Largest difference Peak, hole/e Å−3 | 0.645 and −1.537 | 0.771 and −0.624 | 0.527 and −0.998 | 0.650 and −0.433 |

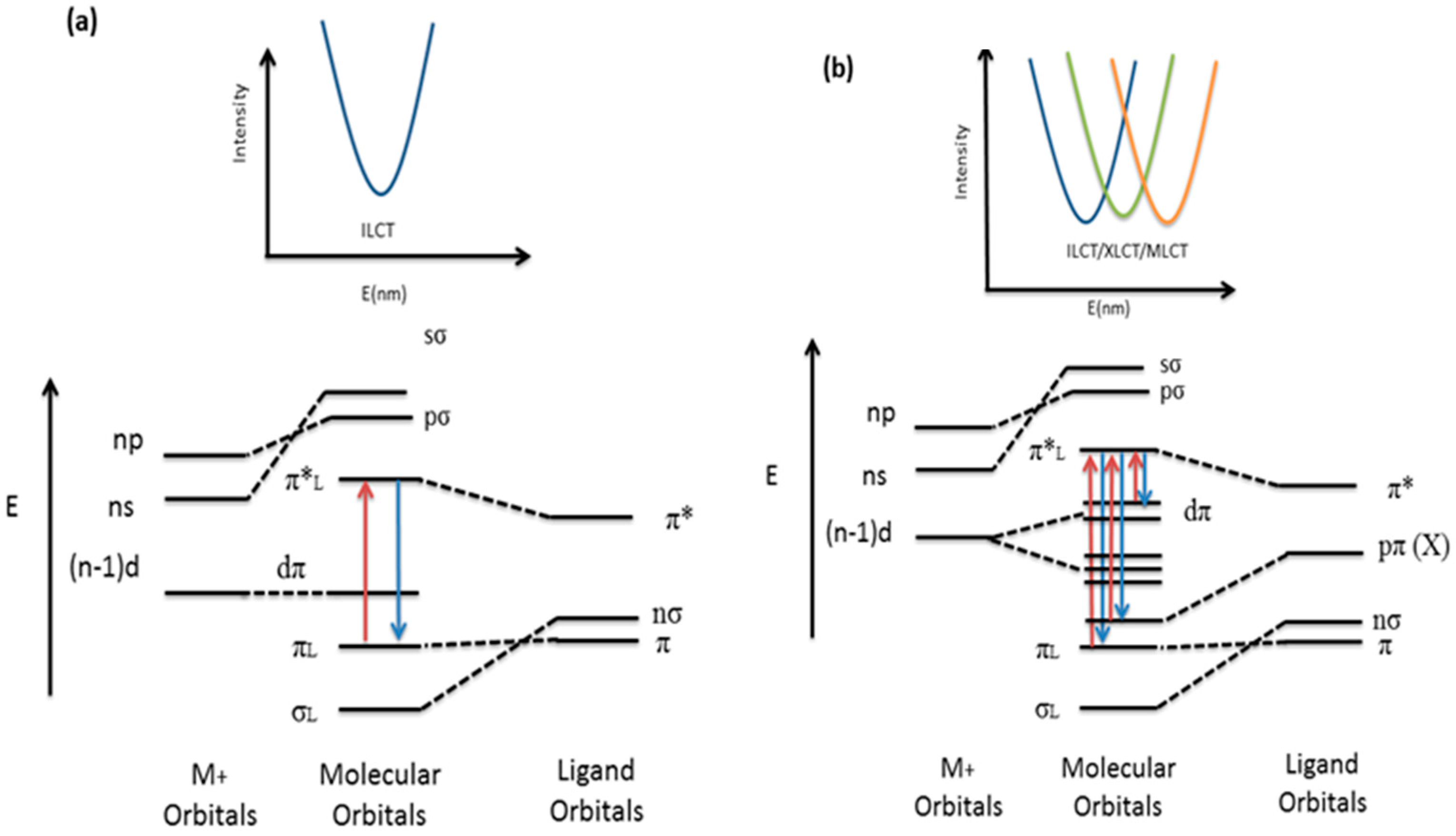
3.3. Luminescent Properties
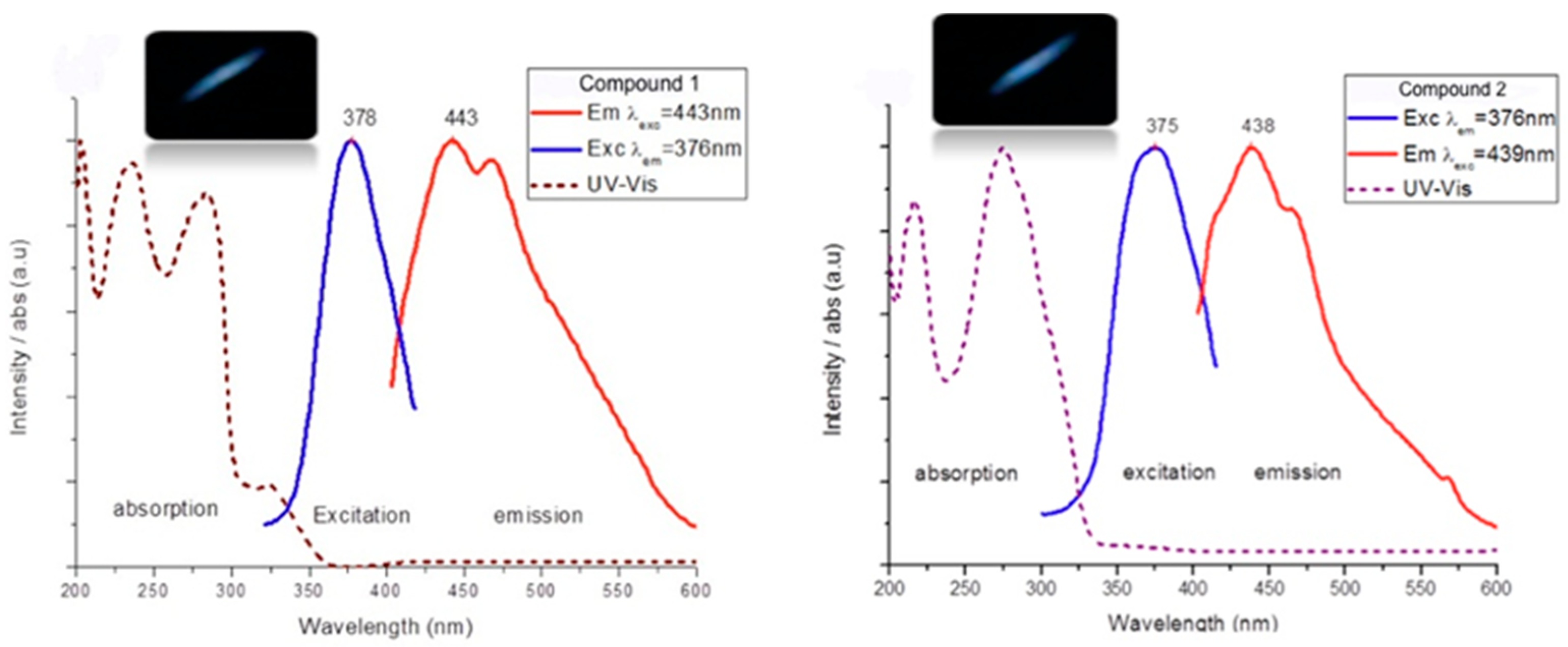
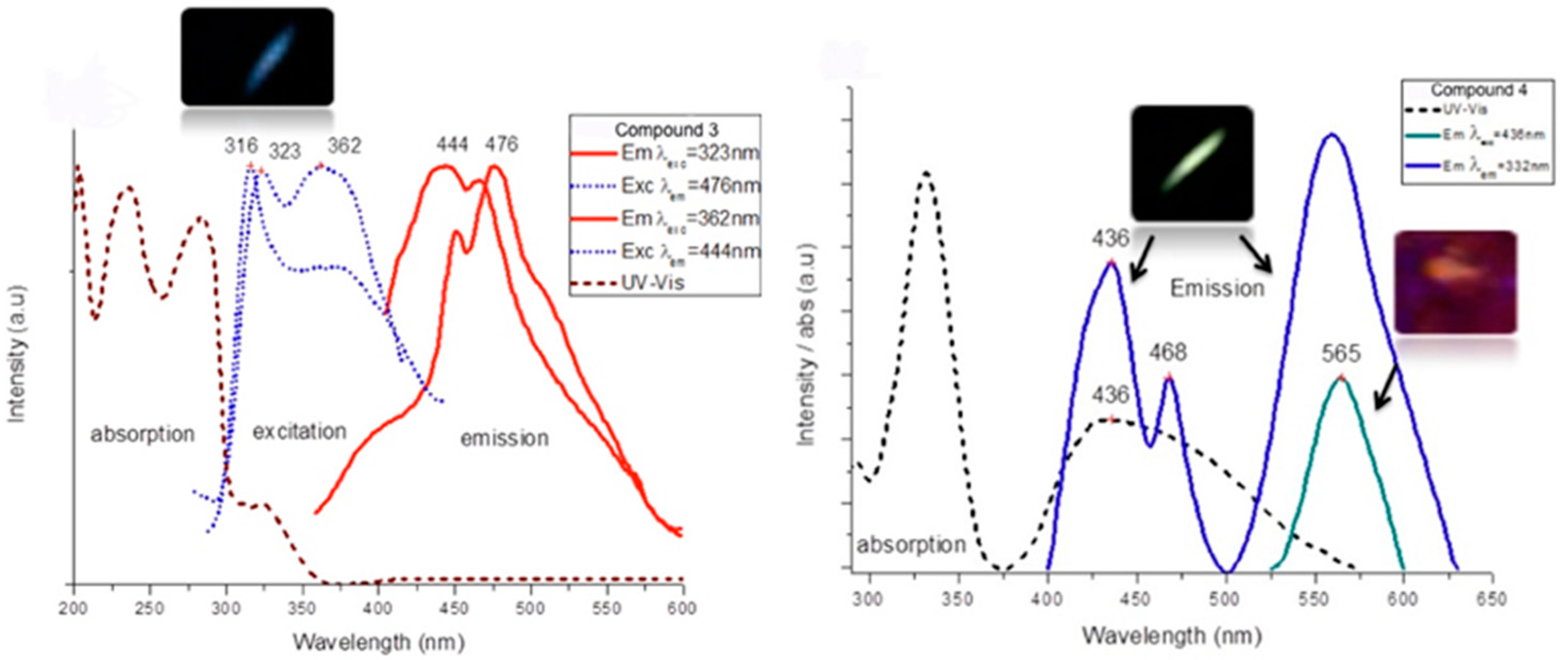
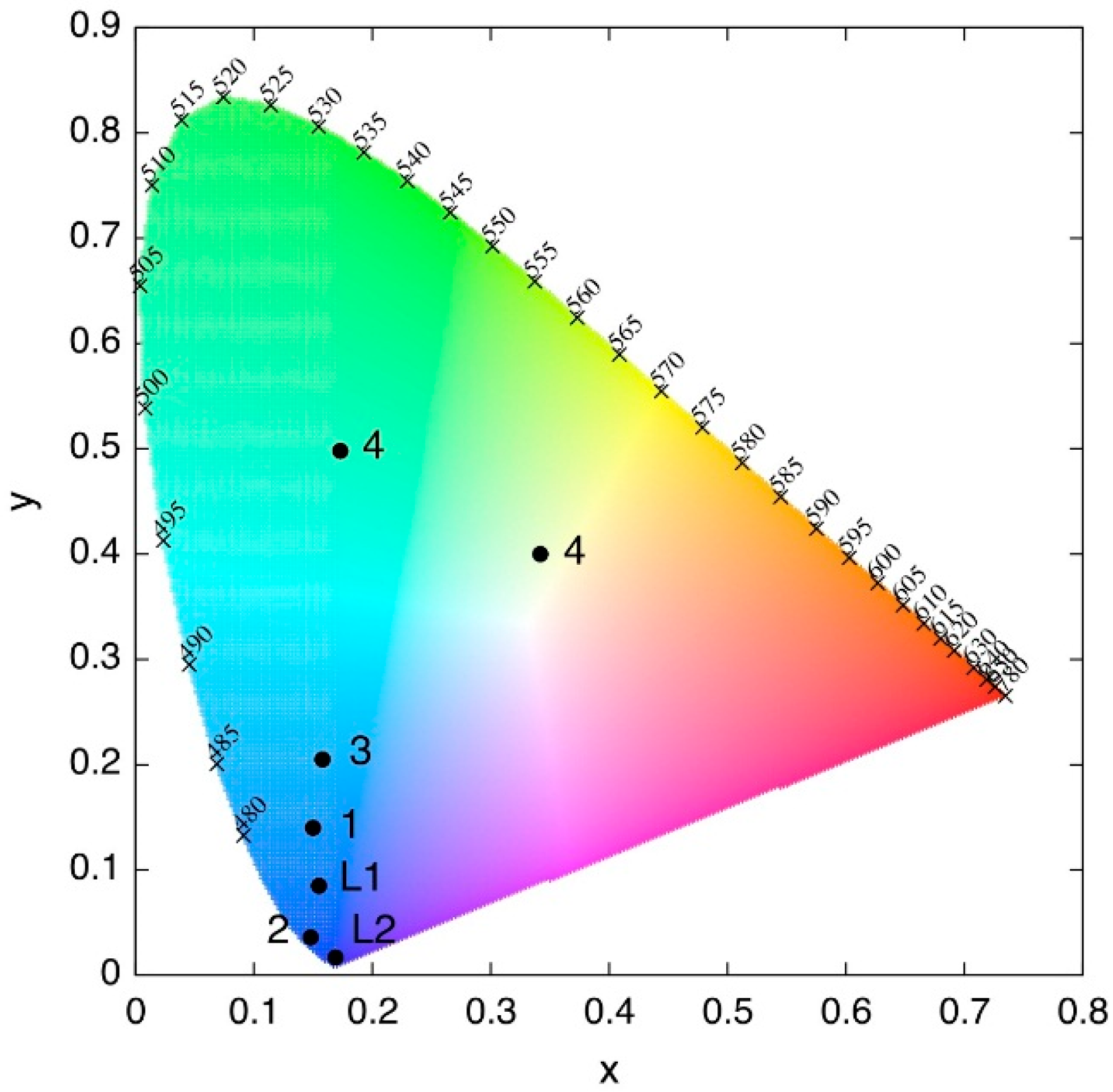
| Compound | Diffuse Reflectancy λ (nm) | Excitation λexc (nm) | Emission λem (nm) | Lifetime τ (ms) | CIE Chromaticity Coordinates |
|---|---|---|---|---|---|
| L1 | 235, 290, 329 | 349 | 438 | 0.96 | 0.155, 0.085 |
| L2 | 225, 266, 332 | 340 | 407 | 0.93 | 0.169, 0.017 |
| {Ag(L1)CF3SO3}2 (1) | 238, 270, 325 | 378 | 443/467 | 0.95/1.20 | 0.150, 0.140 |
| {Ag(L1)ClO4}n (2) | 220, 279, 318 | 376 | 439 | 1.06 | 0.148, 0.036 |
| {Ag(L2)CF3SO3}n (3) | 328, 424 | 323/362 | 444/476 | 1.07/1.21 | 0.158, 0.205 |
| {CuCl(L2)}n (4) | 332, 436 | 332 436 | 436/565 565 | 0.173, 0.498 0.342, 0.400 |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O– and N–donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Pettinari, C.; Tabacaru, A.; Gallic, S. Coordination polymers and metal–organic frameworks based on poly(pyrazole)-containing ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Almeida Paz, F.A.; Klinowski, J.; Vilela, S.M.F.; Tomé, J.P.C.; Cavaleiro, J.A.S.; Roch, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar] [CrossRef] [PubMed]
- Bellussi, G.; Carati, A.; Rizzo, C.; Millini, R. New trends in the synthesis of crystalline microporous materials. Catal. Sci. Technol. 2013, 3, 833–857. [Google Scholar] [CrossRef]
- Lalonde, M.; Bury, W.; Karagiaridi, O.; Brown, Z.; Hupp, J.T.; Farha, O.K. Transmetalation: Routes to metal exchange within metal–organic frameworks. J. Mater. Chem. A 2013, 1, 5453–5468. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–Organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.W.; Lo, K.K.-W. Luminescent polynuclear d10 metal complexes. Chem. Soc. Rev. 1999, 28, 323–334. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M. Polycatenation, polythreading and polyknotting in coordination network chemistry. Coord. Chem. Rev. 2003, 246, 247–289. [Google Scholar] [CrossRef]
- Oshio, H.; Saito, Y.; Ito, T. Cluster assembly by hydrogen bonds: Channel structure of Cu4L4 cubanes. Angew. Chem. Int. Ed. 1997, 36, 2673–2675. [Google Scholar] [CrossRef]
- Du, M.; Bu, X.H.; Guo, Y.M.; Liu, H.; Batten, S.R.; Ribas, J.; Mak, T.C.W. First CuII diamondoid net with 2-fold interpenetrating frameworks. The role of anions in the construction of the supramolecular arrays. Inorg. Chem. 2002, 41, 4904–4908. [Google Scholar] [CrossRef] [PubMed]
- Chaterjee, B.; Noveron, J.C.; Resendiz, M.J.E.; Liu, J.; Yamamoto, T.; Parker, D.; Cinke, M.; Nguyen, C.V.; Arif, A.M.; Stang, P.J. Self-assembly of flexible supramolecular metallacyclic ensembles: Structure and adsorption properties of their nanoporous crystalline frameworks. J. Am. Chem. Soc. 2004, 126, 10645–10656. [Google Scholar] [CrossRef] [PubMed]
- Song, L.C.; Zhang, W.-X.; Hu, Q.-M. Synthesis and characterization of novel one-dimensional coordination polymers self-assembled from Co(NCS)2 and flexible diester-bridged pyridine-based ligands. Chin. J. Chem. 2002, 20, 1421–1429. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Chung, S.S.; Li, W.S.; Schröder, M. In situ ligand synthesis and construction of an unprecedented three-dimensional array with silver (i): A new approach to inorganic crystal engineering. Chem. Commun. 1997. [Google Scholar] [CrossRef]
- Maekawa, M.; Konaka, H.; Suenaga, Y.; Kuroda-Sowab, T.; Munakatab, M. A variety of one-, two- and three-dimensional copper(I) and silver(I)co-ordination polymers assembled by 1,4-bis(4-pyridyl)butadiyene and 1,4-bis(2-pyridyl)butadiyne. J. Chem. Soc. Dalton Trans. 2000. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M. Three-dimensional architectures of intertwined planar coordination polymers: The first case of interpenetration involving two different bidimensional polymeric motifs. New. J. Chem. 1998, 22, 1319–1321. [Google Scholar] [CrossRef]
- Barandika, M.G.; Cortez, R.; Lezama, L.; Urtiaga, M.K.; Arriotua, M.; Rojo, T. Synthesis and magnetostructural characterization of two ferromagnetic nickel(II) dimers. J. Chem. Soc. Dalton Trans. 1999. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Brown, K.G.; Graham, D.; Kirkhouse, J.B.; Kittner, M.; Mayor, C.; McHugh, C.J.; Murdoch, P.; Smith, W.E. Chromophore containing bipyridyl ligands. Part 1: Supramolecular solid-state structure of Ag(I) complexes. New. J. Chem. 2005, 29, 826–832. [Google Scholar] [CrossRef]
- Wu, H.; Thanasekaran, P.; Tsai, S.H.; Wu, J.Y.; Huang, S.M.; Wen, Y.S.; Lu, K.L. Self-assembly, reorganization, and photophysical properties of Silver(I)−schiff-base molecular rectangle and polymeric array species. Inorg. Chem. 2006, 45, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.Y.; Min, D.; Lee, S.W. A Long 3,3‘-Bipyridine-type linking ligand and its coordination polymers: [ZnL(NO3)2], [CoL1.5(NO3)2], and [CoL2(NO3)2] X (L = py-CH=N–(CH3)C6H3−C6H3(CH3)−N=CH-py); X = Benzene, Toluene). Cryst. Growth Des. 2006, 6, 342–347. [Google Scholar] [CrossRef]
- Gao, D.Q.; Cheng, A.L.; Xu, Y.X.; Yan, C.H.; He, M.Y. New Inorganic-organic hybrid supramolecular architectures generated from 2,5-bis(3-pyridyl)-3,4-diaza-2,4-hexadiene. Cryst. Growth Des. 2005, 5, 1005–1011. [Google Scholar] [CrossRef]
- Zhu, H.F.; Li, L.; Okamura, T.; Zhao, W.; Sun, W.-Y.; Ueyamay, N. Syntheses, structures and photoluminescence properties of Ag(I), Cu(II), Zn(II) and Mn(II) complexes with N,N’-bis(3-pyridylmethyl)-1,4-benzenedimethyleneimine bull. Chem. Soc. Jpn. 2003, 76, 761–767. [Google Scholar] [CrossRef]
- Halder, G.J.; Neville, S.M.; Kepert, C.J. A highly distorted (10,3)-a coordination framework constructed from alternating T-shaped and trigonal nodes. CrystEngComm 2005, 7, 266–268. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Three-dimensional metal–organic framework with (3,4)-connected net, synthesized from anionic liquid medium. Chem. Commun. 2004. [Google Scholar] [CrossRef]
- Liu, G.F.; Zhang, W.; Chen, Y.; Liu, D.; Lang, J.P. Solvothermal synthesis and crystal structure of a luminescent 2D copper(I) coordination polymer with a (3,4)-connected net. Inorg. Chem. Commun. 2007, 10, 1049–1053. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Batten, S.R.; Hamit, H.; Hoskins, B.F.; Robson, R. A cubic (3,4)-connected net with large cavities in solvated [Cu-3(tpt)(4)](ClO4)(3) (tpt=2,4,6-tri(4-pyridyl)-1,3,5-triazine). Angew. Chem. Int. Ed. Engl. 1996, 35, 1690–1692. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Blake, A.J.; Champness, N.R.; Lemonovskii, D.A.; Majouga, A.G.; Zyk, N.V.; Schröder, M. Supramolecular design of one-dimensional coordination polymers based on silver(I) complexes of aromatic nitrogen-donor ligands. Coord. Chem. Rev. 2001, 222, 155–192. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Hubberstey, P.; Li, W.S.; Withersby, M.A.; Schröder, M. Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord. Chem. Rev. 1999, 183, 117–138. [Google Scholar] [CrossRef]
- Vallejos, J.; Brito, I.; Cárdebas, A.; Bolte, M.; López-Rodríguez, M. 4-[3-(Isonicotinoyloxy)propoxycarbonyl]pyridinium diiodidoargentate(I). Acta Crystallog. Sect. E 2011, E67, m1759–m1760. [Google Scholar]
- Blake, A.J.; Brooks, N.R.; Champness, N.R.; Crew, M.; Deveson, A.; Fenske, D.; Gregory, D.H.; Hanton, L.R.; Hubberstey, P.; Schröder, M. Topological isomerism in coordination polymers. Chem. Commun. 2001. [Google Scholar] [CrossRef]
- Kumar, C.A.; Karthikeyan, S.; Varghese, B.; Veena, V.; Sakthivel, N.; Manimaran, B.J. Self-assembly of thiolato-bridged manganese(i)-based metallarectangles: One-pot synthesis and structural characterization. Org. Chem. 2014, 766, 86–94. [Google Scholar]
- Li, F.; Li, X.; Li, T.; Su, W.; Cao, R. Design and syntheses of 1D and 2D coordination polymers resulting from flexible building blocks. J. Mol. Struct. 2006, 782, 116–121. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Rizzato, S. New polymeric networks from the self-assembly of silver(I) salts and the flexible ligand 1,3-bis(4-pyridyl)propane (bpp). A systematic investigation of the effects of the counterions and a survey of the coordination polymers based on bpp. Cryst. Eng. Commun. 2002, 4, 121–129. [Google Scholar] [CrossRef]
- Brito, I.; Vallejos, J.; Cárdenas, A.; López-Rodríguez, M.; Bolte, M.; Llanos, J. Two novel lineal d10 metal-organic frameworks with a ditopic flexible linker: Structures, blue luminescence and thermal stability. Inorg. Chem. Commun. 2011, 14, 897–901. [Google Scholar] [CrossRef]
- Vallejos, J.; Brito, I.; Cárdenas, A.; López-Rodríguez, M.; Bolte, M.; Llanos, J. A novel double-stranded staircase Cu(I)-iodide coordination polymer based on bis(4-pyridyl-carboxylate) ligand with flexible propyl spacer: Syntheses, crystal structure, luminescence properties and thermal stability. Inorg. Chem. Commun. 2012, 24, 59–62. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lai, S.W.; Che, C.M.; Fu, W.; Zhou, Z.-Y.; Zhu, N. Structural variations and spectroscopic properties of luminescent mono- and multinuclear Silver(I) and Copper(I) complexes bearing phosphine and cyanide ligands. Inorg. Chem. 2005, 44, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.W.; Fung, W.K.M.; Cheung, K.K. Synthesis, photophysics and crystal structures of hexanuclearcopper(i) and silver(I) acetylide complexes. Chem. Commun. 1997. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Lai, S.-W.; Che, C.-M.; Cheung, K.K.; Zhou, Z.-Y. Luminescent tetranuclear Silver(I) arylacetylide complexes bearing tricyclohexylphosphine ligands: Synthesis, molecular structures, and spectroscopic comparison with Gold(I) and Copper(I) arylacetylide. Organometallics 2002, 21, 2275–2282. [Google Scholar] [CrossRef]
- Omary, M.A.; Mohamed, A.A.; Rawashdeh-Omary, M.A.; Fackler, J.P. Photophysics of supramolecular binary stacks consisting of electron-rich trinuclear Au(I) complexes and organic electrophiles. Coord. Chem. Rev. 2005, 249, 1372–1381. [Google Scholar] [CrossRef]
- Balch, A.L. Photofunctional transition metal complexes. In Remarkable Luminescence Behaviors and Structural Variations of Two-Coordinate Gold(I) Complexes; Springer: Berlin, Germany, 2007; pp. 1–40. [Google Scholar]
- Graham, P.M.; Pike, R.D.; Sabat, M.; Baile, R.D.; Pennington, W.T. Coordination Polymers of Copper(I) Halides. Inorg. Chem. 2000, 39, 5121–5132. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Vallejos, J.; Bolte, M.; López-Rodríguez, M.; Cárdenas, A. Propane-1,3-diyl bis(pyridine-4-carboxylate). Acta. Crystallogr. 2010, E66, o1015. [Google Scholar]
- Brito, I.; Vallejos, J.; López-Rodríguez, M.; Cárdenas, A.; Bolte, M. Propane-1,3-diaminium bis(pyridine-4-carboxylate) monohydrate. Acta. Crystallogr. 2011, E67, o2423. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Vallejos, J.; López-Rodríguez, M.; Cárdenas, A.; Bolte, M. A monoclinic modification of propane-1,3-diyl bis(pyridine-3-carboxylate). Acta. Crystallogr. 2011, E67, o278. [Google Scholar] [CrossRef] [PubMed]
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta. Crystallogr. 1995, A51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta. Crystallogr. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. 2015, C71, 3–8. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 09, Version Revision D. 1, Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Valencia, L.; Bastida, R.; Macias, A.; Vicente, M.; Perez-Lourido, P. Synthesis and helical polymeric structure of a luminescent pendant-armed macrocyclic silver(I) complex with Ag–Ag interactions. New J. Chem. 2005, 29, 424–426. [Google Scholar] [CrossRef]
- Yeh, T.-T.; Wu, J.-Y.; Wen, Y.-S.; Liu, Y.-H.; Twu, J.; Tao, Y.-T.; Lu, K.-L. Luminescent silver metal chains with unusual μ4-bonded 2,2’-bipyrazine. Dalton Trans. 2005. [Google Scholar] [CrossRef] [PubMed]
- Yimaz, V.T.; Hamamci, S.; Kazak, C. A novel two-dimensional silver(I) saccharinato coordination polymer constructed from weak Ag⋯C interactions: Synthesis, IR spectra and X-ray structure. J. Organomet. Chem. 2008, 693, 3885–3888. [Google Scholar] [CrossRef]
- Schaltin, S.; Brooks, N.R.; Sniekers, J.; Depuydt, D.; Meervelt, L.V.; Binnemans, K.; Fransaer, J. Room-temperature silver-containing liquid metal salts with nitrate anions. Phys. Chem. Chem. Phys. 2013, 15, 18934–18943. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Yip, J.H.K.; Vitta, J.J. Coordination polymers od d10 metals and N,N’-bis(3-pyridine-carboxamide)-1,2-ethane. J. Chem. Soc. Dalton Trans. 2001. [Google Scholar] [CrossRef]
- Chesman, A.S.R.; Turner, D.R.; Ross, T.M.; Neville, S.M.; Lu, J.; Murray, K.S.; Batten, S.R. Chains, helices, sheets and unusual 3D nets: Diverse structures of the flexible, ditopic ligand 1,2-bis(3-(4-pyridyl)pyrazolyl)ethane. Polyhedron 2010, 29, 2–9. [Google Scholar] [CrossRef]
- Mascal, M.; Kerdelhue, J.L.; Blake, A.J.; Cooke, P.A.; Mortimer, R.J.; Teat, S.J. On the nature of arene η6 interactions in the solid state and the use of cylindrophanes as ligands for sandwich complexation of metals with longer-range interactions with the benzene ring. Eur. J. Inorg. Chem. 2000, 3, 485–490. [Google Scholar] [CrossRef]
- Ford, P.C.; Vogler, A. Photochemical and photophysical properties of tetranuclear and hexanuclear clusters of metals with d10 and s2 electronic configurations. ACC. Chem. Res. 1993, 26, 220–226. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence properties of multinuclear Copper(I) compounds. Chem. Rev. 1999, 99, 3625–3648. [Google Scholar] [CrossRef] [PubMed]
- Cariati, E.; Bourassa, J.; Ford, P.C. Luminescence response of the solid state polynuclear copper(I) iodide materials [CuI(4-picoline)]x to volatile organic compounds. Chem. Commun. 1998. [Google Scholar] [CrossRef]
- Sabin, F.; Ryu, C.K.; Ford, P.C.; Vogler, A. Photophysical properties of hexanuclear copper(I) and silver(I) clusters. Inorg. Chem. 1992, 31, 1941–1945. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.-K.; Guo, C.-X.; Wang, R.-J.; Mak, T.C.W.; Che, C.-M. Synthesis, crystal structure, photophysical properties and theoretical studies of [Cu3(Ph2PCH2PPh2)3(WS4)]ClO4. J. Chem. Soc. Dalton Trans. 1995. [Google Scholar] [CrossRef]
- Chan, C.-K.; Cheung, K.-K.; Che, C.-M. Structure and spectroscopic properties of a luminescent inorganic cyclophane from self-assembly of copper(I) and two ligand components. Chem. Commun. 1996. [Google Scholar] [CrossRef]
- Barrie, J.D.; Dunn, B.; Hollingsworth, G.; Zink, J.I. Optical spectroscopy of copper(I)-doped Na+-β-alumina. J. Phy. Chem. 1989, 93, 3958–3963. [Google Scholar] [CrossRef]
- Provencher, R.; Harvey, P.D. Theoretical study and luminescence properties of the cyclic Cu3(dppm)3OH2+ cluster. The first luminescent cluster host at room temperature. Inorg. Chem. 1996, 35, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Drouin, M.; Zhang, T. Crystallographic, theoretical, and spectroscopic studies of the luminescent d10–d10Binuclear Copper acetate complex Cu2(dppm)2(O2CCH3)+. Inorg. Chem. 1997, 36, 4998–5005. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Lo, K.K.-W.; Fung, W.K.-M.; Wang, C.-R. Design of luminescent polynuclear copper(I) and silver(I) complexes with chalcogenides and acetylides as the bridging ligands. Coord. Chem. Rev. 1998, 171, 17–41. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Lee, Y.-A.; Crespo, O.; Deaton, J.; Tang, C.; Gysling, H.J.; Gimeno, M.C.; Larraz, C.; Villacampa, M.D.; Laguna, M.D.; et al. Intensely luminescent Gold(I)-Silver(I) cluster complexes with tunable structural features. J. Am. Chem. Soc. 2004, 126, 9488–9489. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.V.; Douglas, P.; Winscom, C.J. Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- Cariati, E.; Bu, X.; Ford, P.C. Solvent- and vapor-induced isomerization between the luminescent solids [CuI(4-pic)]4 and [CuI(4-pic)]∞ (pic = methylpyridine). The Structural basis for the observed luminescence vapochromism. Chem. Matter 2000, 12, 3385–3391. [Google Scholar] [CrossRef]
- Wen, L.L.; Dang, D.B.; Duan, C.Y.; Li, Y.-Z.; Tian, Z.-F.; Meng, Q.-J. 1D Helix, 2D Brick-wall and herringbone, and 3D interpenetration d10metal-organic framework structures assembled from pyridine-2,6-dicarboxylic acid N-oxide. Inorg. Chem. 2005, 44, 7161–7170. [Google Scholar] [CrossRef] [PubMed]
- Schanda, J. Colourimetry: Understanding the CIE System; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wang, M.-S.; Guo, S.-P.; Cai, L.-Z.; Zou, J.-P.; Xu, G.; Zhou, W.-W.; Zheng, F.-K.; Gou, G.-C. A Direct white-light-emitting metal-organic framework with tunable yellow-to-white photoluminescence by variation of excitation light. J. Am. Chem. Soc. 2009, 131, 13572–13573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Qi, X.-L.; Liu, Z.-J.; Zhu, A.-X.; Chen, Y.; Wang, J.; Chen, X.-M. Syntheses, structures, and porous/luminescent properties of silver 3-alkyl-1,2,4-triazolate frameworks with rare 3-connected topologies. Cryst. Growth Des. 2011, 11, 796–802. [Google Scholar] [CrossRef]
- Fortin, D.; Drouin, M.; Turcotte, M.; Hervey, P.D. Quasi-unidimensional {[M(dmb)2]Y}n organometallic polymers (M = Cu(I), Ag(I); dmb = 1,8-Diisocyano-p-menthane; Y = BF4−, PF6−, NO3−, ClO4−, CH3CO2−).1a structural, calorimetric, and luminescence properties. J. Am. Chem. Soc. 1997, 119, 531–541. [Google Scholar] [CrossRef]
- Fang, Z.-L.; He, J.-G.; Zhang, Q.-S.; Zhang, Q.-K.; Wu, X.-Y.; Yu, R.-M.; Lu, C.Z. pH-Controlled construction of Cu(I) coordination polymers: In situ transformation of ligand, network topologies, and luminescence and UV–Vis–NIR absorption Properties. Inorg. Chem. 2011, 50, 11403–11411. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejos, J.; Brito, I.; Cárdenas, A.; Bolte, M.; Conejeros, S.; Alemany, P.; Llanos, J. Self-Assembly of Discrete Metallocycles versus Coordination Polymers Based on Cu(I) and Ag(I) Ions and Flexible Ligands: Structural Diversification and Luminescent Properties. Polymers 2016, 8, 46. https://doi.org/10.3390/polym8020046
Vallejos J, Brito I, Cárdenas A, Bolte M, Conejeros S, Alemany P, Llanos J. Self-Assembly of Discrete Metallocycles versus Coordination Polymers Based on Cu(I) and Ag(I) Ions and Flexible Ligands: Structural Diversification and Luminescent Properties. Polymers. 2016; 8(2):46. https://doi.org/10.3390/polym8020046
Chicago/Turabian StyleVallejos, Javier, Iván Brito, Alejandro Cárdenas, Michael Bolte, Sergio Conejeros, Pere Alemany, and Jaime Llanos. 2016. "Self-Assembly of Discrete Metallocycles versus Coordination Polymers Based on Cu(I) and Ag(I) Ions and Flexible Ligands: Structural Diversification and Luminescent Properties" Polymers 8, no. 2: 46. https://doi.org/10.3390/polym8020046