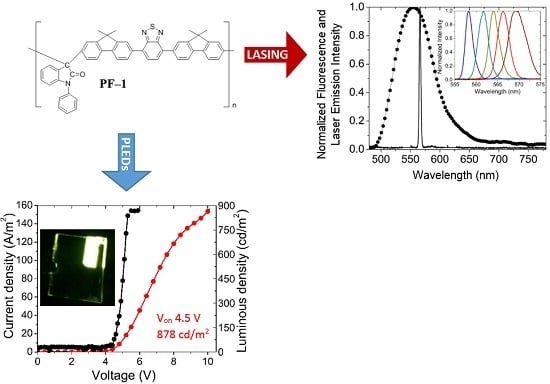
Light Emission Properties of a Cross-Conjugated Fluorene Polymer: Demonstration of Its Use in Electro-Luminescence and Lasing Devices
Abstract
:1. Introduction
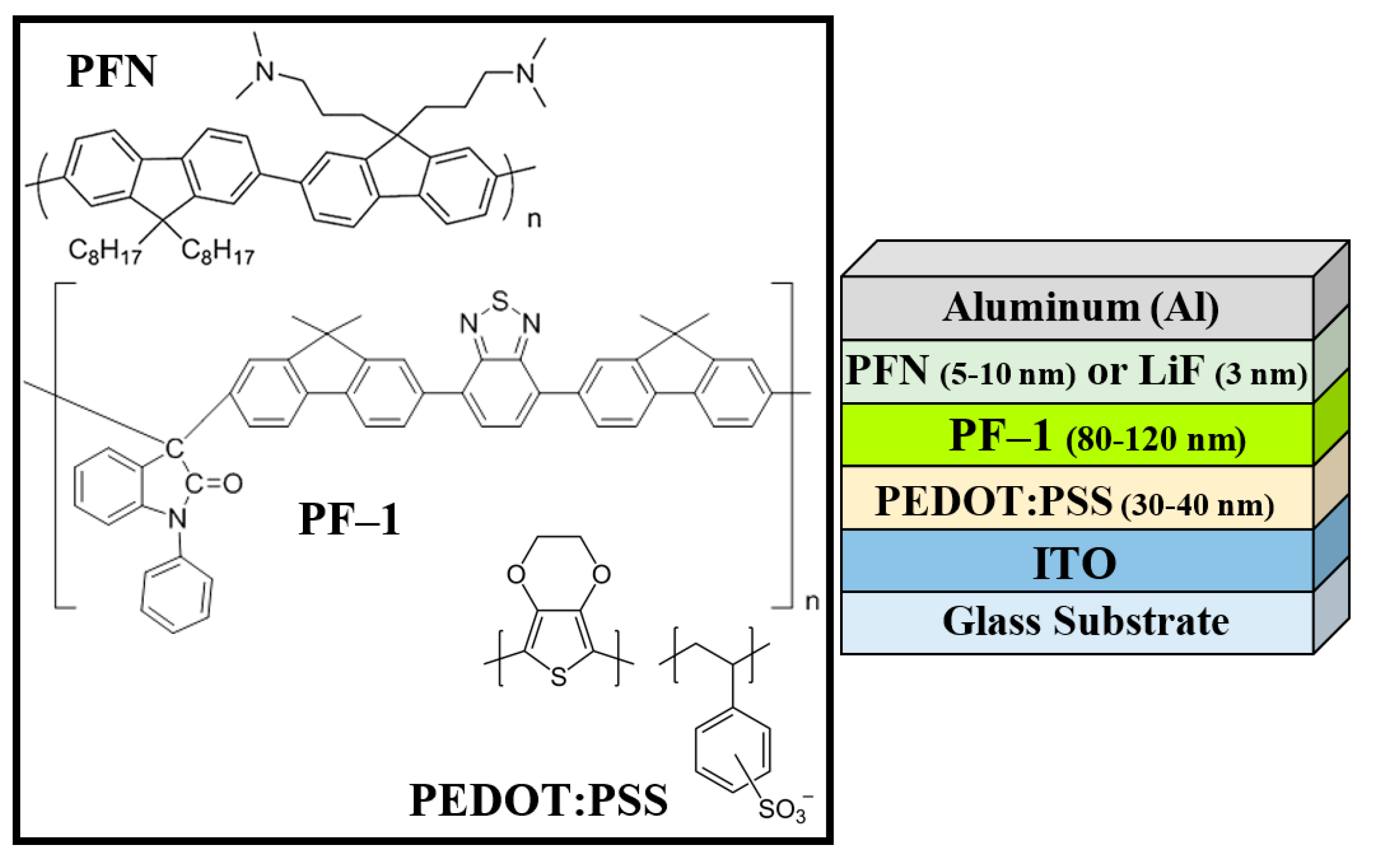
2. Materials and Methods
2.1. Materials
2.2. Experimental
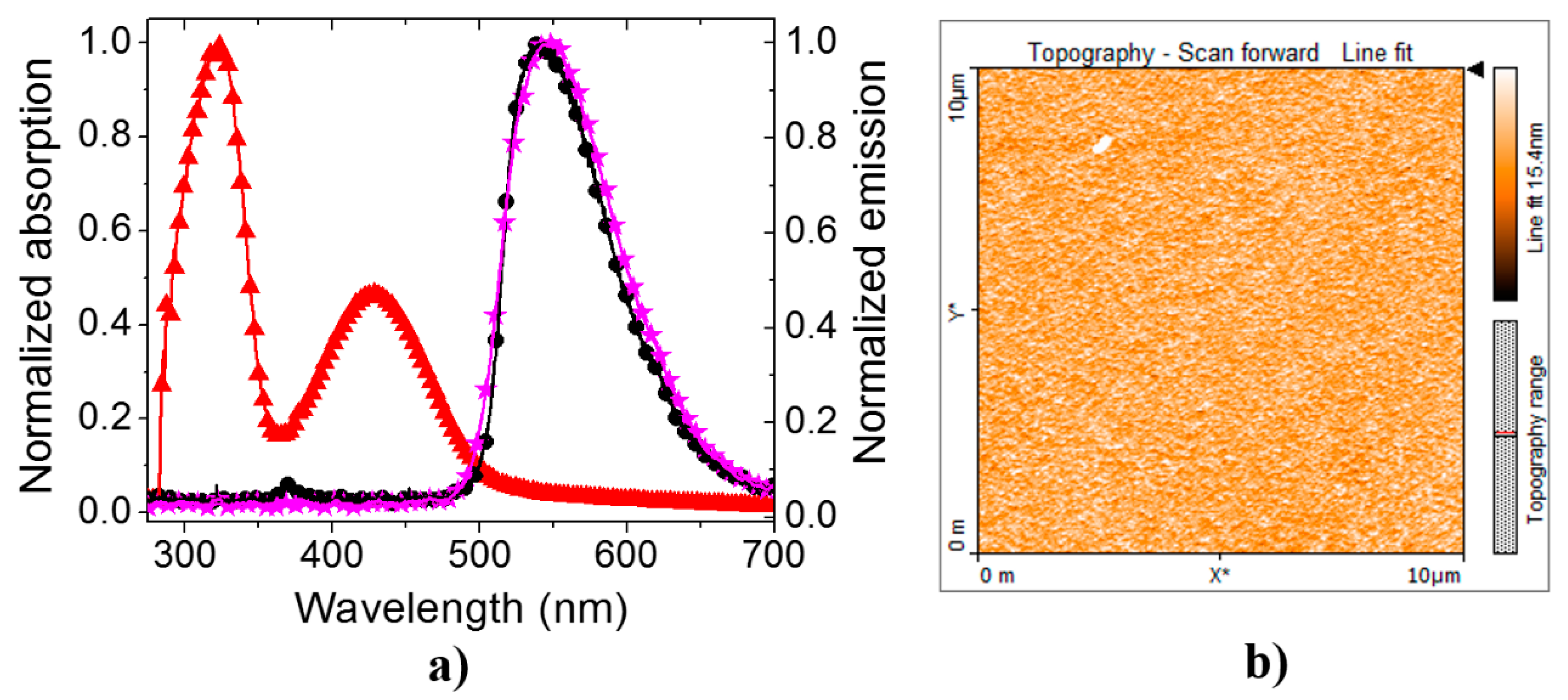
3. Results and Discussion
3.1. PLEDs
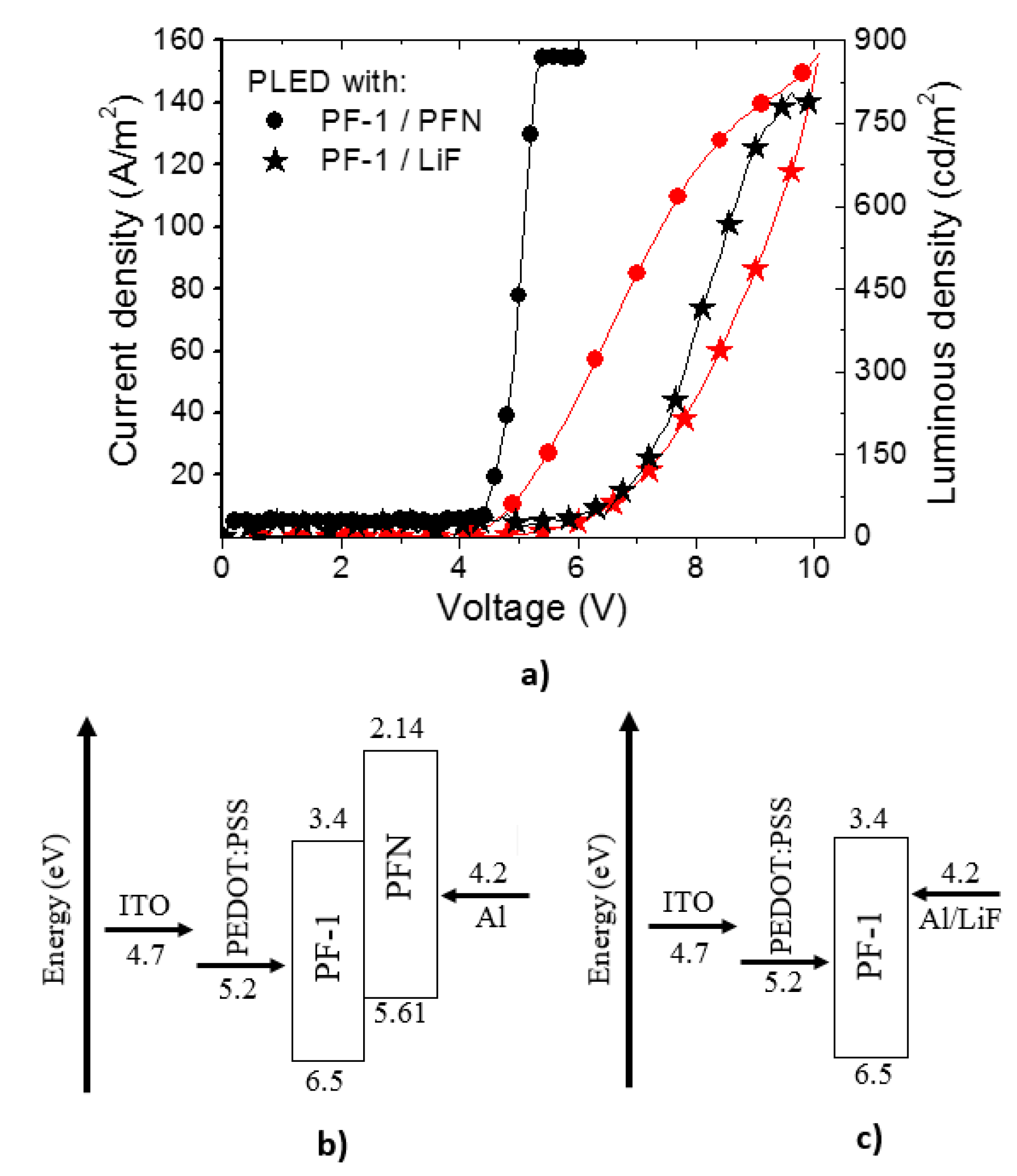
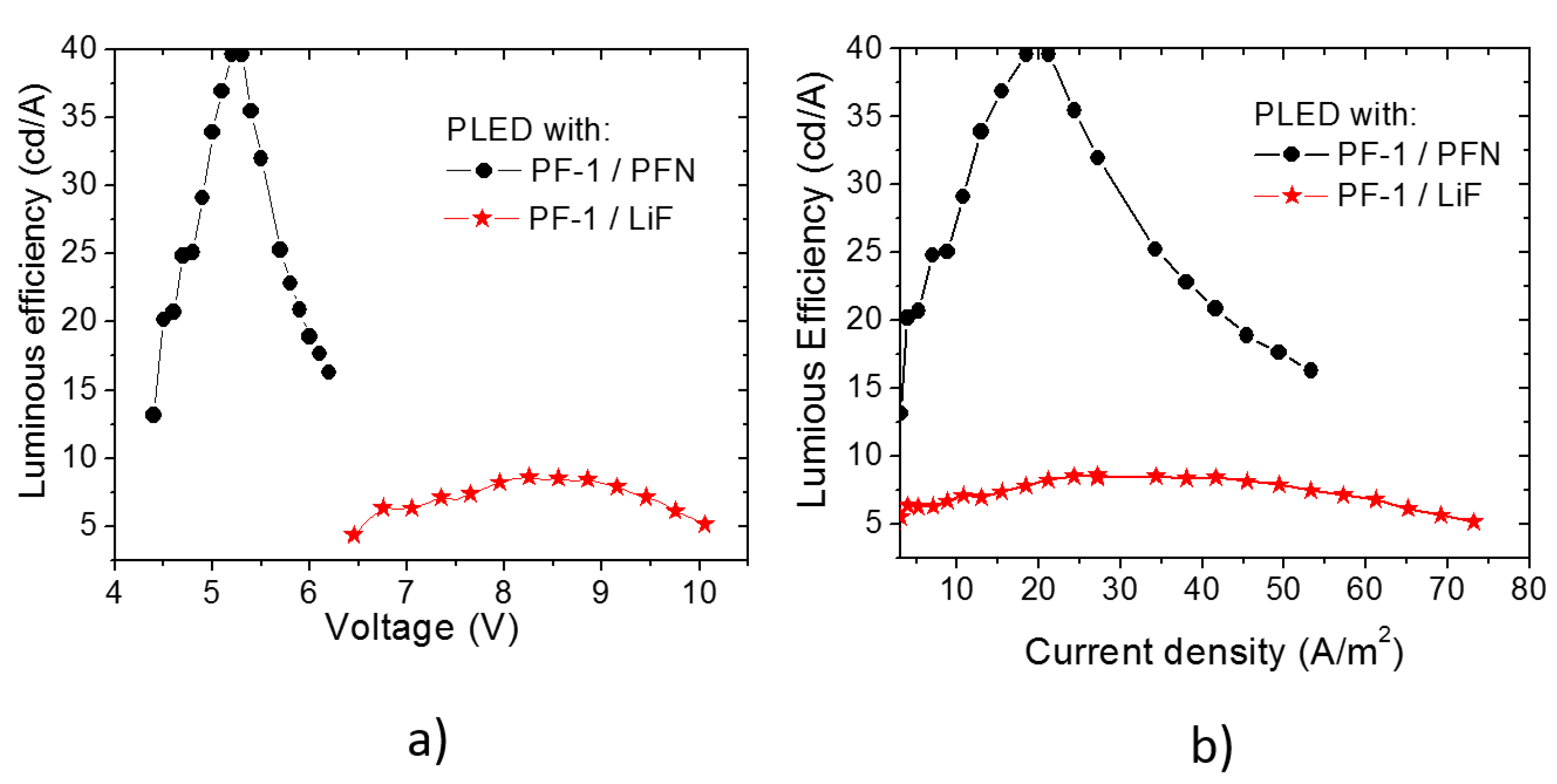
| Material | λ of Emission (nm) | Turn on voltage | Luminous density (cd/m2) | Efficiency (cd/A) | Ref. |
|---|---|---|---|---|---|
| PF–1 /PFN | 551 | 4.5 | 878 | 40 | This work |
| PF–1/LiF | 551 | 6.5 | 805 | 9 | This work |
| DDH | 555 | 8 | 600 | NA | [41] |
| FPtppND | 545 | 9 | 1000 | 25.9 | [42] |
| PIM-1 | 515 | 10 | 10 | 1.71 | [43] |
| Carbazole homopolymer | 527a | 5 | NA | 0.45–15, 23b | [44] |
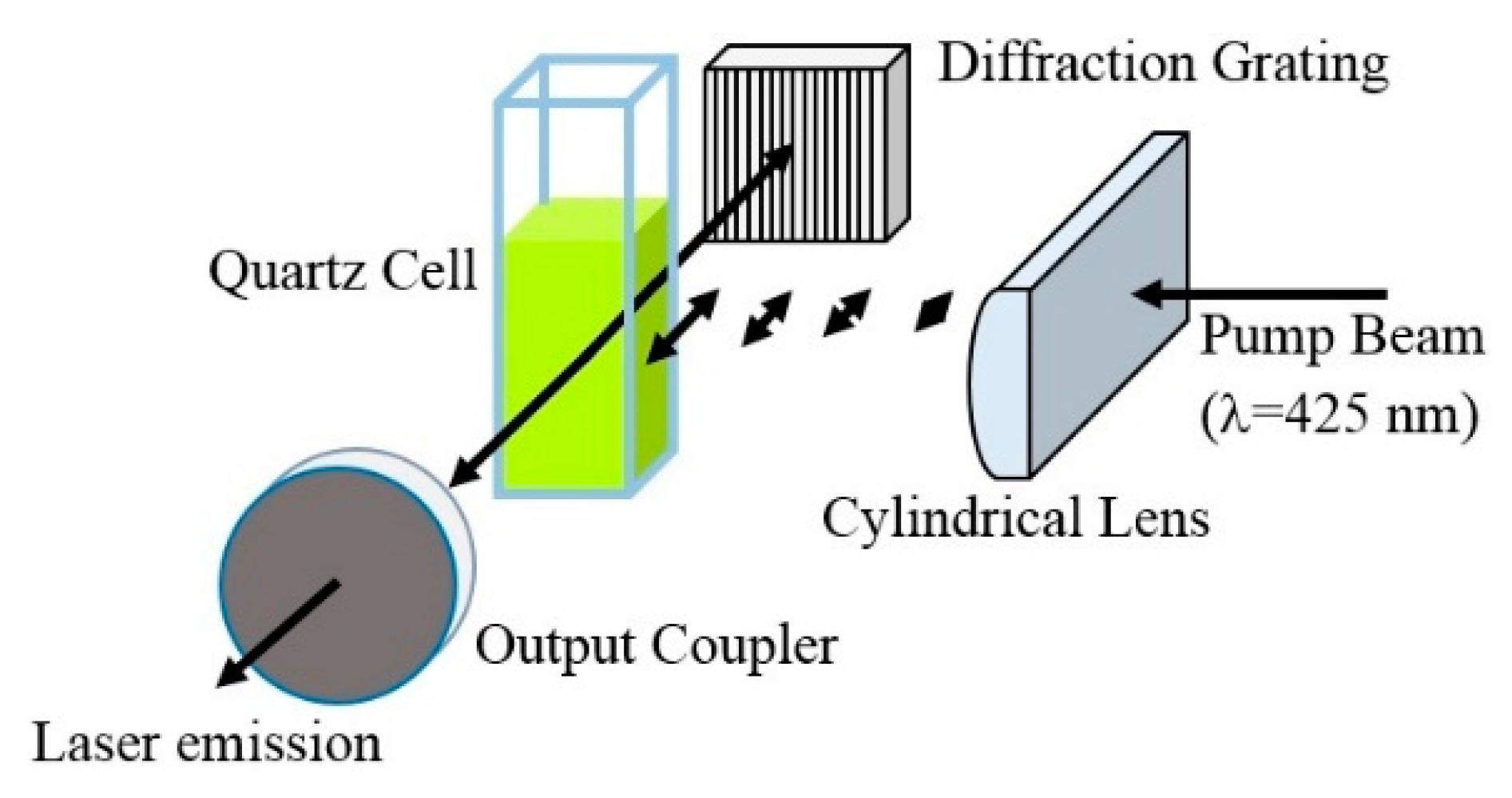
3.2. Lasing of PF–1 Compound
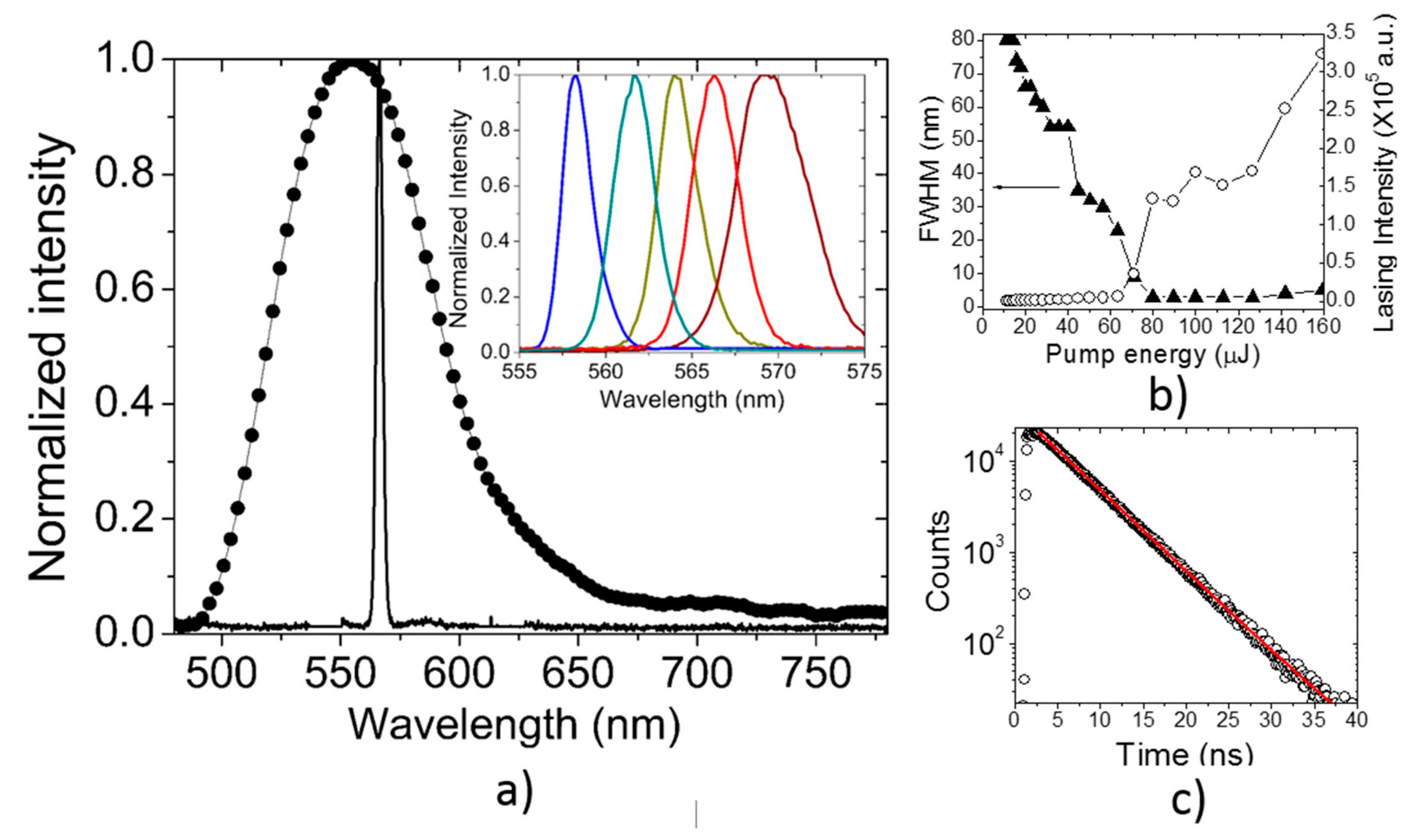
| Material | Solvent | λ of maximum lasing (nm) | σe × 10−16 cm2 | τf (ns) | Fluorescence range (nm) | Ref. |
|---|---|---|---|---|---|---|
| PF1 | Clorobnezene | 566 | 4.24 | 4.9 | 520–600 | This work |
| Rhodamine 6G | Ethanol | 566 | 4.17 | 3.9a | 530–590 | [46,47] |
| POPOP | Methanol | 555 | 2.82 | 0.93 | 400–460 | [36] |
| DDFP | DMSO | 515 | 3.23 | 2.3 | 470–535 | [37] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Mackay, K.; Friend, R.H.; Burns, P.L. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lussem, B. White organic light-emitting diodes with fluorescent tube efficiency. Nature 2009, 459, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Kalyani, N.; Dhoble, S. Organic light emitting diodes: Energy saving lighting technology—A review. Renew. Sust. Energ. Rev. 2012, 16, 2696–2723. [Google Scholar] [CrossRef]
- Kalyani, N.; Dhoble, S. Novel approaches for energy efficient solid state lighting by RGB organic light emitting diodes—A review. Renew. Sust. Energ. Rev. 2014, 32, 448–467. [Google Scholar]
- Chuang, C.; Chang, C.; Chang, C.; Wang, Y.; Lin, Y.; Leung, M. Poly(9,9-dialkylfluorene-co-triphenylamine)s—A family of photo-curable hole-transport polymers for polymer light-emitting diodes applications. Eur. Polym. J. 2014, 56, 33–44. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Zhang, J.; Tang, W.; Tang, A.; Peng, H.; Xu, Z.; Teng, F.; Wang, Y. Key issues and recent progress of light efficient organic light-emitting diodes. J. Photochem. Photobiol. C 2013, 17, 69–104. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, J.; Han, X.; Yin, J.; Yu, G.; Liu, S. Fluorene-based novel highly emissive fluorescent molecules with aggregate fluorescence change or aggregation-induced emission enhancement characteristics. Dyes Pigments 2015, 112, 59–66. [Google Scholar] [CrossRef]
- Pu, Y.; Chiba, T.; Ideta, K.; Takahashi, S.; Aizawa, N.; Hikichi, T.; Kido, J. Fabrication of organic light-emitting devices comprising stacked light-emitting units by solution-based processes. Adv. Mater. 2015, 27, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Peng, Z.; Zhang, X.; Wang, P.; Lee, C.-S.; Lee, S.-T. Highly efficient non-doped blue organic light-emitting diodes based on fluorene derivatives with high thermal stablity. Adv. Funct. Mater. 2005, 15, 1716–1721. [Google Scholar] [CrossRef]
- Scherf, U.; List, E. Semiconducting polyfluorenes–Towards reliable structure–property relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Klaerner, G.; Miller, R.D. Polyfluorene derivatives: Effective conjugation lengths from well-defined oligomers. Macromolecules 1998, 31, 2007–2009. [Google Scholar] [CrossRef]
- Feng, X.J.; Chen, S.; Ni, Y.; Wong, M.; Lam, M.; Cheah, K.; Lai, G. Fluorene derivatives for highly efficient non-doped single-layer blue organic light-emitting diodes. Org. Electron. 2014, 15, 57–64. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, C.; Bie, H.; Chen, Z. Synthesis and photoluminescent properties of some novel fluorene derivatives. Dyes Pigments 2005, 64, 31–34. [Google Scholar] [CrossRef]
- Beaupre, S.; Boudreault, P.; Leclerc, M. Solar-energy production and energy-efficient lighting: Photovoltaic devices and white-light-emitting diodes using poly(2,7-fluorene), poly(2,7-carbazole), and poly(2,7-dibenzosilole) derivatives. Adv. Mater. 2010, 22, E6–E27. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ke, L.; Tan, L.; Lin, T.; Kietzke, T.; Chen, Z. Conjugated copolymers based on fluorine-thieno [3,2-b]thiophene for light-emitting diodes and photovoltaic cells. Macromolecules 2007, 40, 6164–6171. [Google Scholar] [CrossRef]
- Morales, A.R.; Belfield, K.D.; Hales, J.M.; van Stryland, E.W.; Hagan, D.J. Synthesis of two-photon absorbing unsymmetrical fluorenyl–based chromophores. Chem. Mater. 2006, 18, 4972–4980. [Google Scholar] [CrossRef]
- Cohanoschi, I.; Garcia, M.; Toro, C.; Belfield, K.D.; Hernandez, F.E. Three-photon absorption of a new series of halogenated fluorene derivatives. Chem. Phys. Lett. 2006, 430, 133–138. [Google Scholar]
- Moore, A.; Chesney, A.; Bryce, M.; Batsanov, A.; Kelly, J.; Howard, J.; Perepichka, I.; Perepichka, D.; Meshulam, G.; Berkovic, G.; et al. Synthesis, structures and nonlinear optical properties of novel D-π-A chromophores: Intramolecular charge transfer from 1,3-dithiole of ferrocene moieties to polynitrofluorene or dicyanomethylene moieties through conjugated linkers. Eur. J. Org. Chem. 2001, 14, 2671–2687. [Google Scholar] [CrossRef]
- Kim, H.; Cho, B. Two-photon materials with large two-photon cross sections. Structure–Property relationship. Chem. Commun. 2009, 153–164. [Google Scholar] [CrossRef]
- Schafer, K.; Belfield, K.; Yao, S.; Frederiksen, P.; Hales, J.; Kolattukudy, P. Fluorene-based fluorescent probes with high two-photon action cross-sections for biological multiphoton imaging applications. J. Biomed. Opt. 2005, 10, 051402. [Google Scholar] [CrossRef] [PubMed]
- Belfield, K.D.; Schafer, K.; Liu, Y.; Liu, J.; Ren, X.; van Stryland, E. Multiphoton-absorbing organic materials for microfabrication, emerging optical applications and non-destructive three-dimensional imaging. J. Phys. Org. Chem. 2000, 13, 837–849. [Google Scholar] [CrossRef]
- Belfield, K.; Ren, X.; van Stryland, E.; Hagan, D.; Dubikovski, V.; Meisak, E. Near-IR two photoinitiated polymerization using a fluorene/amine initiating system. J. Am. Chem. Soc. 2000, 122, 1217–1218. [Google Scholar] [CrossRef]
- Charlot, M.; Izard, N.; Mongin, O.; Riehl, D.; Blanchard-Desce, M. Optical limiting with soluble two-photon absorbing quadrupoles: Structure-property relationships. Chem. Phys. Lett. 2006, 417, 297–302. [Google Scholar] [CrossRef]
- Lin, T.; He, G.; Zheng, Q.; Prasad, P. Degenerate two-/three-photon absorption and optical power limiting properties in femtosecond regime of a multi-branched chromophore. J. Mater. Chem. 2006, 16, 2490–2498. [Google Scholar] [CrossRef]
- Makarov, N.; Rebane, A.; Drobizhev, M.; Wolleb, H.; Spahni, H. Optimizing two-photon absorption for volumetric optical data storage. J. Opt. Soc. Am. B 2007, 24, 1874–1885. [Google Scholar] [CrossRef]
- Gholami, M.; Tykwinski, R. Oligomeric and polymeric systems with a cross-conjugated π-framework. Chem. Rev. 2006, 106, 4997–5027. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.C.G.; Zolotukhin, M.G.; Maldonado, J.L.; Rehmann, N.; Meerholz, K.; Fröhlich, N.; Kudla, C.J.; Scherf, U. A high molecular weight aromatic PhOLED matrix polymer obtained by metal-free, superacid-catlyze polyhydroxyalkylation. Maromolecules 2009, 42, 9225–9230. [Google Scholar] [CrossRef]
- Ricks, A.; Solomon, G.; Colvin, M.; Scott, A.; Chen, K.; Ratner, M.; Wasielewski, M. Controlling electron transfer in donor–bridge–acceptor molecules using cross-conjugated bridges. J. Am. Chem. Soc. 2010, 132, 15427–15434. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Tian, Y.; Chen, C.-Y.; Cheng, Y.-J.; Young, A.C.; Jen, A.K.-Y. Cross-conjugated polymers with large two-photon absorption cross-sections for metal ion sensing. J. Phys. Chem. C 2007, 111, 10673–10681. [Google Scholar] [CrossRef]
- Aparicio-Ixta, L.; Ramos-Ortiz, G.; Pichardo-Molina, J.L.; Maldonado, J.L.; Tellez-Lopez, V.M.; Martinez-Fong, D.; Zolotukhin, M.G.; Formine, S.; Meneses-Nava, M.A.; Barbosa-Garcia, O. Two-photon excited fluorescence of silica nanoparticles loaded with a fluorene-based monomer and its cross-conjugated polymer: Their application to cell imaging. Nanoscale 2012, 4, 7751–7759. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ortız, G.; Maldonado, J.L.; Hernandez, M.C.G.; Zolotukhin, M.G.; Fomine, S.; Fröhlich, N.; Scherf, U.; Galbrechtc, F.; Preis, E.; Salmon, M.; et al. Synthesis, characterization and third-order non-linear optical properties of novel fluorene monomers and their cross-conjugated polymers. Polymer 2010, 51, 2351–2359. [Google Scholar] [CrossRef]
- Brabec, C.J.; Shapen, S.E.; Winder, C.W.; Sariciffci, N.S.; Denk, P. Effect of LiF/metal electrodes on the performance of plastic solar cells. Appl. Phys. Lett. 2001, 80, 1288–1290. [Google Scholar] [CrossRef]
- StöBel, M.; Staudigel, J.; Steuber, F.; Blässing, J.; Simmerer, J.; Winnacker, A.; Neuner, H.; Metzdorf, D.; Johannes, H.; Kowalsky, W. Electron injection and transport in 8-hydroxyquinoline aluminum. Synth. Met. 2000, 111–112, 19–24. [Google Scholar]
- Truong, Q.; Truong, M.; Park, C.; Jung, J. Study of MEH-PPV/PCBM active layer morphology and its application for hybrid solar cell performance. Bull. Mater. Sci. 2012, 35, 277–281. [Google Scholar] [CrossRef]
- El-Daly, S.A.; Asiri, A.M.; Alamry, K.; Khan, S.A. Spectroscopic studies and laser activity of 3-(4-dimethylamino-phenyl)-1-(2,5-dimethyl-furan-3-yl)-propenone (DDFP): A new green laser dye. J. Photolum. 2013, 137, 6–14. [Google Scholar] [CrossRef]
- El-Daly, S.A.; El-Azim, S.A.; Elmekawey, F.M.; Elbaradei, B.Y.; Shama, S.A.; Asiri, A.M. Photophysical parameters, excitation energy transfer, and photoreactivity of 1,4-bis(5-phenyl-2-oxazolyl)benzene (popop) laser dye. Int. J. Photoenergy 2012, 1–10. [Google Scholar] [CrossRef]
- Raoufi, D.; Hosseinpanahi, F. Surface morphology dynamics in ITO thin films. J. Mod. Phys. 2012, 3, 645–651. [Google Scholar] [CrossRef]
- Wu, H.; Huang, F.; Mo, Y.; Yang, W.; Wang, D.; Peng, J.; Cao, Y. Efficient electron Injection from a bilayer cathode consisting of aluminum and alcohol-/water-soluble conjugated polymers. Adv. Mater. 2004, 16, 1826–1830. [Google Scholar] [CrossRef]
- Hung, L.; Tang, C.; Mason, M. Enhanced electron injection in organic electroluminescence devices using an Al/LiF electrode. Appl. Phys. Lett. 1997, 70, 152–154. [Google Scholar] [CrossRef]
- Szlachcic, P.; Danel, K.; Gryl, M.; Stadnicka, K.; Usatenko, Z.; Nosidlak, N.; Lewinska, G.; Sanetra, J. Organic light emitting diodes (OLED) based on helical structures containing 7-membered fused rings. Dyes Pigments 2015, 114, 184–195. [Google Scholar] [CrossRef]
- Poloek, A.; Lin, C.; Chen, C.; Chen, C. High colour rendering index and colour stable hybrid white efficient OLEDs with a double emitting layer structure using a single phosphorescence dopant of heteroleptic platinum complexes. J. Mater. Chem. C. 2014, 2, 10343–10356. [Google Scholar] [CrossRef]
- Gupta, B.; Kedawat, G.; Kumar, P.; Rafiee, M.; Tyagi, P.; Srivastava, R.; Ajayan, P. An n-type, new emerging luminescent polybenzodioxane polymer for application in solution-processed green emitting OLEDs. J. Mater. Chem. C. 2015, 3, 2568–2574. [Google Scholar] [CrossRef]
- Dijken, A.; Bastiaansen, J.; Kiggen, N.; Langeveld, B.; Rothe, C.; Monkman, A.; Bach, I.; Stössel, P.; Brunner, K. Carbazole compounds host materials for triplet emitters in organic light–emitting diodes: Polymer host for high-efficiency light–emitting diodes. J. Am. Chem. Soc. 2004, 126, 7718–7727. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Muto, J. Concentration dependent laser threshold of rhodamine 6G in methanol solution. Opt. Comm. 1986, 59, 199–201. [Google Scholar] [CrossRef]
- Peterson, O.; Webb, J.; McColgin, W. Organic dye threshold. J. Appl. Phys. 1971, 42, 1917–1928. [Google Scholar] [CrossRef]
- Wiedmann, J.; Penzokofer, A. Excited-state absorption cross-sections in rhodamine dyes determined after molecular reorientation. Il Nuovo Cimento 1981, 63B, 459–469. [Google Scholar] [CrossRef]
- Penzokofer, A.; Leupacher, W. Fluorescence behavior of highly concentrated Rhodamine 6G solutions. J. Lumin. 1987, 37, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Pauck, T.; Hennig, R.; Perner, M.; Lemmer, U.; Siegner, U.; Mahrt, R.F.; Scherf, U.; Müllen, K.; Bässler, H.E.; Göbel, O. Femtosecond dynamics of stimulated emission and photoinduced absorption in a PPP-type ladder polymer. Chem. Phys. Lett. 1995, 244, 171–176. [Google Scholar] [CrossRef]
- Yan, M.; Rothberg, L.J.; Kworck, E.W.; Miller, T.M. Interchain excitations in conjugated polymers. Phys. Rev. Lett. 1995, 75, 1992–1995. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, L.J.; Yan, M.; Ppdimitrakopoulos, F.; Galvin, M.E.; Kworck, E.W.; Miller, T.M. Photophysics of phenylenevinylene polymers. Synth. Met. 1996, 80, 41–58. [Google Scholar] [CrossRef]
- Hide, F.; Diaz-García, M.A.; Schwartz, B.J.; Andersson, M.R.; Pei, Q.; Heeger, A.J. Semiconducting polymers: A new class of solid–state laser materials. Science 1996, 273, 1833. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Servin, S.; Lozano-Hernández, L.-A.; Maldonado, J.-L.; Carriles, R.; Ramos-Ortíz, G.; Pérez-Gutiérrez, E.; Scherf, U.; Zolotukhin, M.G. Light Emission Properties of a Cross-Conjugated Fluorene Polymer: Demonstration of Its Use in Electro-Luminescence and Lasing Devices. Polymers 2016, 8, 43. https://doi.org/10.3390/polym8020043
Romero-Servin S, Lozano-Hernández L-A, Maldonado J-L, Carriles R, Ramos-Ortíz G, Pérez-Gutiérrez E, Scherf U, Zolotukhin MG. Light Emission Properties of a Cross-Conjugated Fluorene Polymer: Demonstration of Its Use in Electro-Luminescence and Lasing Devices. Polymers. 2016; 8(2):43. https://doi.org/10.3390/polym8020043
Chicago/Turabian StyleRomero-Servin, Sergio, Luis-Abraham Lozano-Hernández, José-Luis Maldonado, Ramón Carriles, Gabriel Ramos-Ortíz, Enrique Pérez-Gutiérrez, Ullrich Scherf, and Mikhail G. Zolotukhin. 2016. "Light Emission Properties of a Cross-Conjugated Fluorene Polymer: Demonstration of Its Use in Electro-Luminescence and Lasing Devices" Polymers 8, no. 2: 43. https://doi.org/10.3390/polym8020043