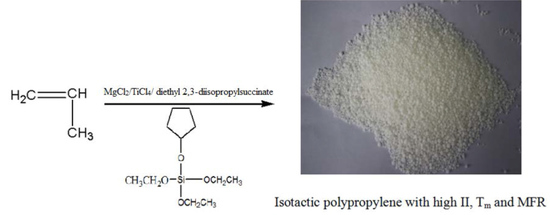
Study on Hydrogen Sensitivity of Ziegler–Natta Catalysts with Novel Cycloalkoxy Silane Compounds as External Electron Donor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Propylene Bulk Polymerization
2.3. Characterization
2.4. Preparation of Electron Donors
2.5. Preparation of the Catalysts
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Locatelli, P.; Morrini, G.; Noristi, L.; Albizzati, E. Polymerization stereochemistry with Ziegler–Natta catalysts containing dialkylpropane diethers: A tool for understanding internal/external donor relationships. Macromolecules 1996, 29, 3341–3345. [Google Scholar] [CrossRef]
- Morrini, G.; Albizzati, E.; Balbontin, G.; Mingozzi, I.; Sacchi, M.C.; Forlini, F.; Tritto, I. Microstructure distribution of polypropylenes obtained in the presence of traditional phthalate/silane and novel diether donors: A tool for understanding the role of electron donors in MgCl2-supported Ziegler–Natta catalysts. Macromolecules 1996, 29, 5770–5776. [Google Scholar] [CrossRef]
- Soga, K.; Shiono, T. Ziegler–Natta catalysts for olefin polymerizations. Prog. Polym. Sci. 1997, 22, 1503–1546. [Google Scholar] [CrossRef]
- Dusseault, J.J.A.; Hsu, C.C. MgCl2-supported Ziegler–Natta catalysts for olefin polymerization: Basic structure, mechanism, and kinetic behavior. Polym. Rev. 1993, 2, 103–145. [Google Scholar]
- Tanase, S.; Katayama, K.; Yabunouchi, N.; Sadashima, T.; Tomotsu, N. Design of novel malonates as internal donors for MgCl2-supported TiCl4 type polypropylene catalysts and their mechanistic aspects, Part 1. J. Mol. Catal. A Chem. 2007, 273, 211–217. [Google Scholar] [CrossRef]
- Vestberg, T.; Denifl, P.; Parkinson, M.; Wilén, C.E. Effects of external donors and hydrogen concentration on oligomer formation and chain end distribution in propylene polymerization with Ziegler–Natta catalysts. J. Polym. Sci. Pol. Chem. 2010, 48, 351–358. [Google Scholar] [CrossRef]
- Nikolaeva, M.; Mikenas, T.; Matsko, M.; Zakharov, V. Effect of AlEt3 and an external donor on the distribution of active sites according to their stereospecificity in propylene polymerization over TiCl4/MgCl2 catalysts with different titanium content. Macromol. Chem. Phys. 2016, 217, 1384–1395. [Google Scholar] [CrossRef]
- Kaushik, V.K.; Gupta, V.K.; Patil, H.; Naik, D.G. Role of electron donor in polymer catalyst synthesis: An XPS study. Catal. Lett. 2008, 121, 58–62. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lee, Y.J.; Park, J.R.; Lee, D.H.; Yoon, K.B. Control of molecular weight distribution for polypropylene obtained by commercial Ziegler-Natta catalyst: Effect of electron donor. Macromolecules 2011, 19, 622–628. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Locatelli, P. Stereochemistry of the initiation step in Ziegler–Natta catalysts containing dialkyl propane diethers: A tool for distinguishing the role of internal and external donors. Macromol. Symp. 1995, 89, 91–100. [Google Scholar] [CrossRef]
- Batt-Coutrot, D.; Wolf, V.; Malinge, J.; Saudemont, T.; Grison, C. Study of dimethoxysilacycloalkanes as external donors in Ziegler–Natta stereospecific propylene polymerisation. Polym. Bull. 2005, 54, 377–385. [Google Scholar] [CrossRef]
- Alshaiban, A.; Soares, J.B.P. Effect of varying hydrogen concentration, external donor concentration, and temperature on propylene polymerization kinetics and microstructure of polypropylene made with a 4th generation Ziegler–Natta Catalyst. Macromol. React. Eng. 2014, 8, 723–735. [Google Scholar] [CrossRef]
- Alshaiban, A.; Soares, J.B.P. Effect of hydrogen and external donor on propylene polymerization kinetics with a 4th-generation Ziegler–Natta catalyst. Macromol. React. Eng. 2012, 6, 265–274. [Google Scholar] [CrossRef]
- Massimiliano, T.; Morini, G.; Guerra, G.; Corradini, P.; Cavallo, L. Influence of 1,3-diethers on the stereospecificity of propene polymerization by supported Ziegler–Natta catalysts. A theoretical investigation on their adsorption on (110) and (100) lateral cuts of MgCl2 platelets. Macromolecules 2000, 33, 1134–1140. [Google Scholar]
- Thorman, J.L.; Blackmon, K.P. Novel Combinations of Silane Electron Donors for Use in Catalyst Compositions. U.S. Patent 20060252894, 9 November 2006. [Google Scholar]
- Resconi, L.; Cavallo, L.; Fait, A.; Peimontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Elisa, P.; Mauro, A.; Francesco, C. Control of degradation reactions during radical functionalization of polypropylene in the melt. Macromolecules 2004, 37, 8414–8423. [Google Scholar]
- Blackmon, K.P. Production of Ultra High Melt Flow Polypropylene Resins. Eur. Patent 1,223,181, 17 July 2002. [Google Scholar]
- Musgrave, M.W. Pelletized Polyolefin Having Ultra-High Melt Flow and Its Articles of Manufacture. U.S. Patent 6,423,800, 23 July 2002. [Google Scholar]
- Takefumi, Y.; Motoki, H.; Maki, S. Catalyst Component and Catalyst for Olefin Polymerization and Method for Producing Olefin Polymer Using Those. U.S. Patent 8,247,504, 15 February 2007. [Google Scholar]
- Bauch, C.G. Polymerization of Olefins Using a Ziegler-Natta Catalyst System Having an External Electron Donor. U.S. Patent 6,818,711, 1 July 2004. [Google Scholar]
- Liu, Z.; Zhang, X.L.; Huang, H.B.; Yi, J.J.; Huang, Q.G.; Yang, W.T. Synthesis of (co-)polyethylene with broad molecular weight distribution by the heterogeneous Ziegler–Natta catalysts via one-pot strategy. J. Ind. Eng. Chem. 2012, 18, 2217–2224. [Google Scholar] [CrossRef]
- Endo, M.; Takamizawa, M.; Ishihara, T. A Method for the Preparation of a Cycloalkyl Silane Compound. U.S. Patent 4,957,607, 17 August 1988. [Google Scholar]
- Huang, Q.G.; Liu, W.J.; Dou, X.L.; Zhang, X.L.; Kong, Y.; Yang, W.T. Preparation Method and Application of Spherical Magnesium Halide Carrier. CN Patent 101,955,556, 26 January 2010. [Google Scholar]
- Garoff, T.; Virkkunen, V.; Jaaskelainen, P.; Vestberg, T. A qualitative model for polymerisation of propylene with a MgCl2-supported TiCl4 Ziegler–Natta catalyst. Eur. Polym. J. 2003, 39, 1679–1685. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Morini, G.; Albizzati, E.; Balbontin, G.; Mingozzi, I.; Cristofori, A.; Sudmeijer, O.; Van Kessel, G.M.M. Aspects of hydrogen activation in propene polymerization using MgCl2/TiCl4/diether catalysts. Macromol. Chem. Phys. 1996, 197, 2501–2510. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Morini, G. Aspects of hydrogen activation in propene polymerization using MgCl2/TiCl4/diether catalysts. Macromol. Symp. 2001, 173, 21–36. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Morini, G.; Balbontin, G.; Camurati, I.; Heere, J.J.R.; Mingozzi, R.; Testoni, F. Effects of internal and external donors on the regio- and stereoselectivity of active species in MgCl2-supported catalysts for propene polymerization. Macromol. Chem. Phys. 2001, 202, 1995–2002. [Google Scholar] [CrossRef]
- Seth, M.; Margl, P.M.; Ziegler, T. A density functional embedded cluster study of proposed active sites in heterogeneous Ziegler–Natta catalysts. Macromolecules 2002, 35, 7815–7829. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Li, T. Effect of external donors on performance of N-catalyst in propylene polymerization. Petrochem. Technol. 2004, 34, 615–618. [Google Scholar]
- Chadwick, J.C.; Kessel, G.; Sudmeijer, O. Regio- and stereospecificity in propene polymerization with MgCl2-supported Ziegler–Natta catalysts: Effects of hydrogen and the external donor. Macromol. Chem. Phys. 2003, 196, 1431–1437. [Google Scholar] [CrossRef]
- Gui, Q.D.; Xin, Z.; Zhu, W.P.; Dai, Z. Effects of an organic phosphorus nucleating agent on crystallization behaviors and mechanical properties of poly(propylene). J. Appl. Polym. Sci. 2003, 88, 297–301. [Google Scholar] [CrossRef]
- Burfield, D.R.; Loi, P.S.T. The use of infrared spectroscopy for determination of polypropylene stereoregularity. J. Appl. Polym. Sci. 1988, 36, 279–293. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Zhou, Q.; Li, H.; Zhang, L. Active centers in propylene polymerization catalysts of the fourth generation. J. Catal. 2015, 332, 156–163. [Google Scholar] [CrossRef]
- Proto, A.; Oliva, L.; Pellecchia, C.; Sivak, A.J.; Cullo, L. Isotactic-specific polymerization of propene with supported catalysts in the presence of different modifiers. Macromolecules 1990, 23, 2904–2907. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Mendichi, R.; Zannoni, G.; Noristi, L. Activation effect of alkoxysilanes as external donors in magnesium chloride-supported Ziegler–Natta catalysts. Macromolecules 1992, 25, 5914–5918. [Google Scholar] [CrossRef]
- Kashiwa, N.; Yoahitake, J. Kinetic study on propylene polymerization with MgCl2/TiCl4-AlEt3/PhCO2Et System—The role of ethyl benzoate. Polym. Bull. 1984, 12, 99–104. [Google Scholar] [CrossRef]
- Soga, K.; Shiono, T.; Doi, Y. Influence of internal and external donors on activity and stereospecificity of Ziegler-Natta catalysts. Makromol. Chem. 1988, 189, 1531–1541. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tritto, I.; Locatelli, P. Stereochemical investigation of the effect of Lewis bases in heterogeneous Ziegler–Natta initiator systems. Prog. Polym. Sci. 1991, 16, 331–360. [Google Scholar] [CrossRef]
- Harkkonen, M.; Seppala, J.V.; Chujo, R.; Kogure, Y. External silane donors in Ziegler–Natta catalysis: A two-site model simulation of the effects of various alkoxysilane compounds. Polymer 1995, 36, 1499–1505. [Google Scholar] [CrossRef]


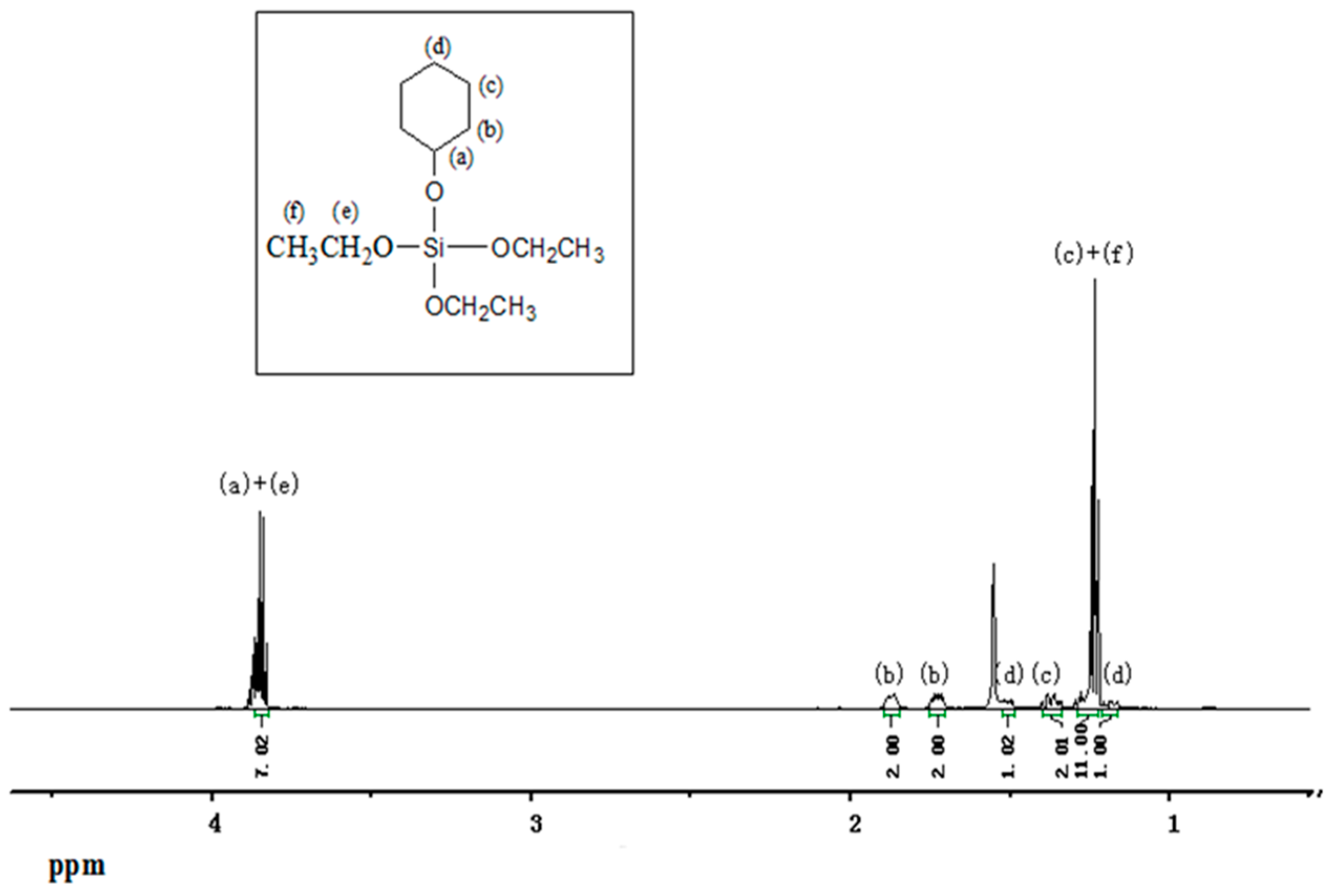
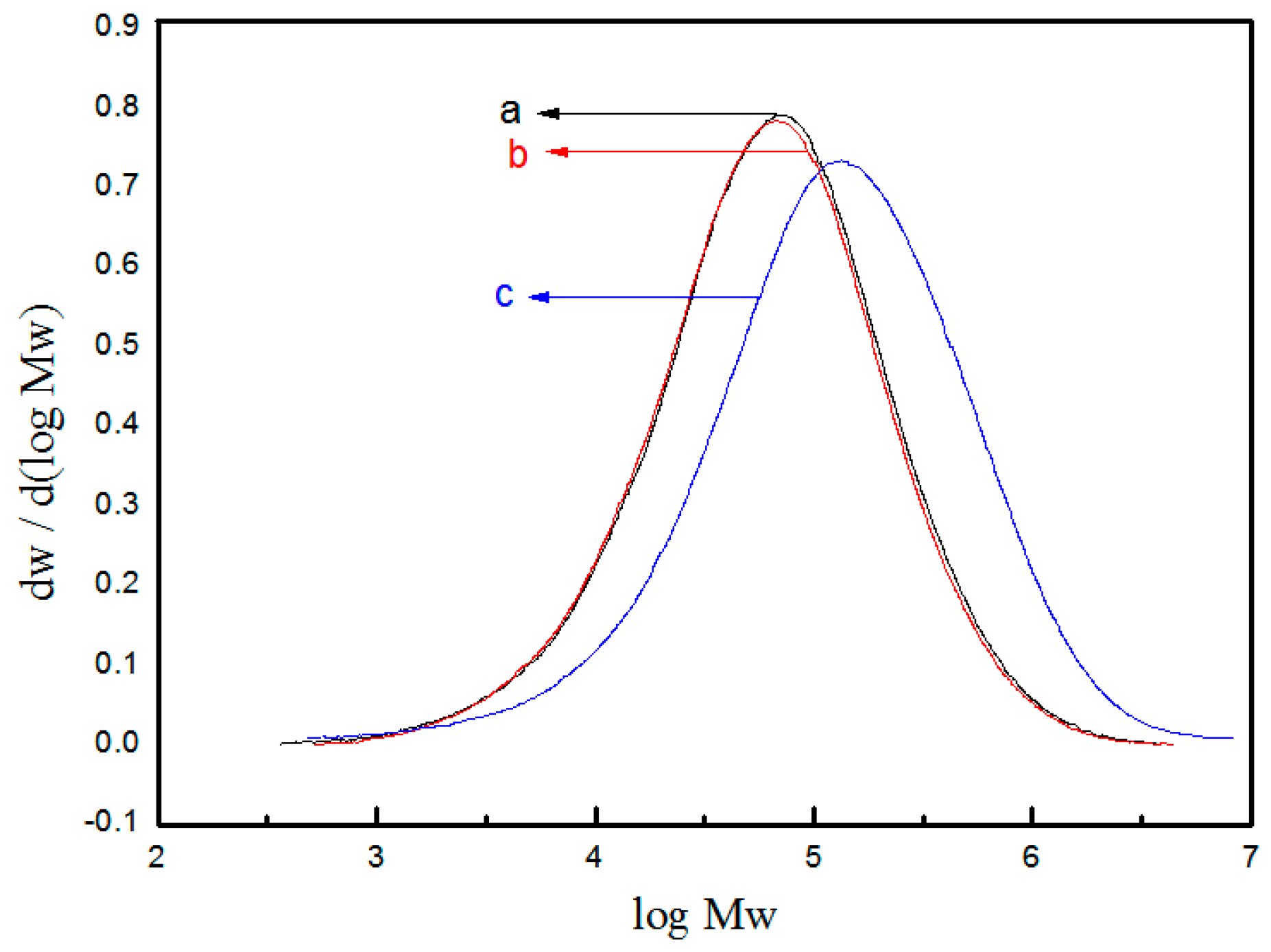
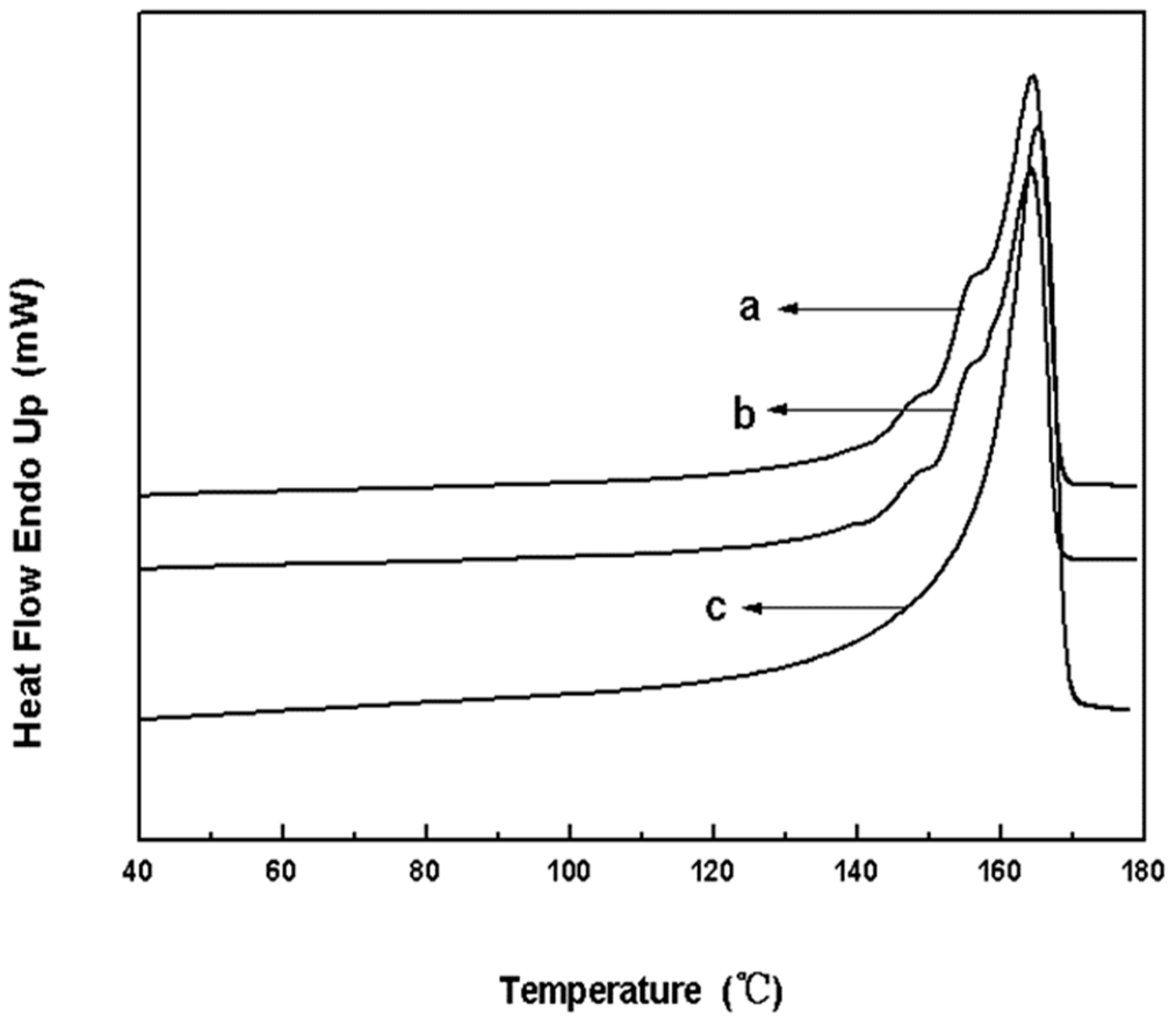
| Run | EED | H2 (MPa) | A a ×10−3 | II b (%) | A998/A973 | II c (%) | MFR d (g/10 min) |
|---|---|---|---|---|---|---|---|
| 1 | ED1 | 0.1 | 10.4 | 98.6 | 0.916 | 98.7 | 25 |
| 2 | 0.2 | 12.5 | 98.3 | 0.911 | 98.2 | 120 | |
| 3 | ED2 | 0.1 | 10.9 | 97.6 | 0.908 | 98.0 | 29 |
| 4 | 0.2 | 12.7 | 98.2 | 0.904 | 97.6 | 121 | |
| 5 | ED3 | 0.1 | 10.1 | 97.8 | 0.909 | 98.1 | 7 |
| 6 | 0.2 | 12.2 | 98.3 | 0.907 | 97.9 | 12 | |
| 7 | — | 0.1 | 12.4 | 81.1 | 0.727 | 81.2 | 9 |
| 8 | 0.2 | 13.8 | 78.7 | 0.703 | 79.0 | 17 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, J.; He, L.; Nan, F.; Wang, F.; Yang, W.; Zhang, M.; Sun, T.; Huang, Q.; Yi, J. Study on Hydrogen Sensitivity of Ziegler–Natta Catalysts with Novel Cycloalkoxy Silane Compounds as External Electron Donor. Polymers 2016, 8, 433. https://doi.org/10.3390/polym8120433
Li H, Wang J, He L, Nan F, Wang F, Yang W, Zhang M, Sun T, Huang Q, Yi J. Study on Hydrogen Sensitivity of Ziegler–Natta Catalysts with Novel Cycloalkoxy Silane Compounds as External Electron Donor. Polymers. 2016; 8(12):433. https://doi.org/10.3390/polym8120433
Chicago/Turabian StyleLi, Hongming, Jing Wang, Lei He, Feng Nan, Fan Wang, Wantai Yang, Mingge Zhang, Tianxu Sun, Qigu Huang, and Jianjun Yi. 2016. "Study on Hydrogen Sensitivity of Ziegler–Natta Catalysts with Novel Cycloalkoxy Silane Compounds as External Electron Donor" Polymers 8, no. 12: 433. https://doi.org/10.3390/polym8120433