The silicone patch was prepared as a double-layer film, with an outer nonadhesive layer. The nonadhesive layer acts as a backing layer and prevents sticking of the patch to the clothing while applied to the skin. The inner adhesive layer must assure stable contact between the patch and the skin in order to hold the dressing in place. To ensure the comfort of the patient, both layers as a whole must be flexible and soft to eliminate discomfort and the possibility of adhesion failure during application due to muscles and joints movements.
3.1. Silicone Films Morphology
Structure examination was performed using images obtained by optical and scanning electron microscopes. The adhesive layer and nonadhesive layer (with or without silicone oil), separately or combined into a double-layer patch, were examined.
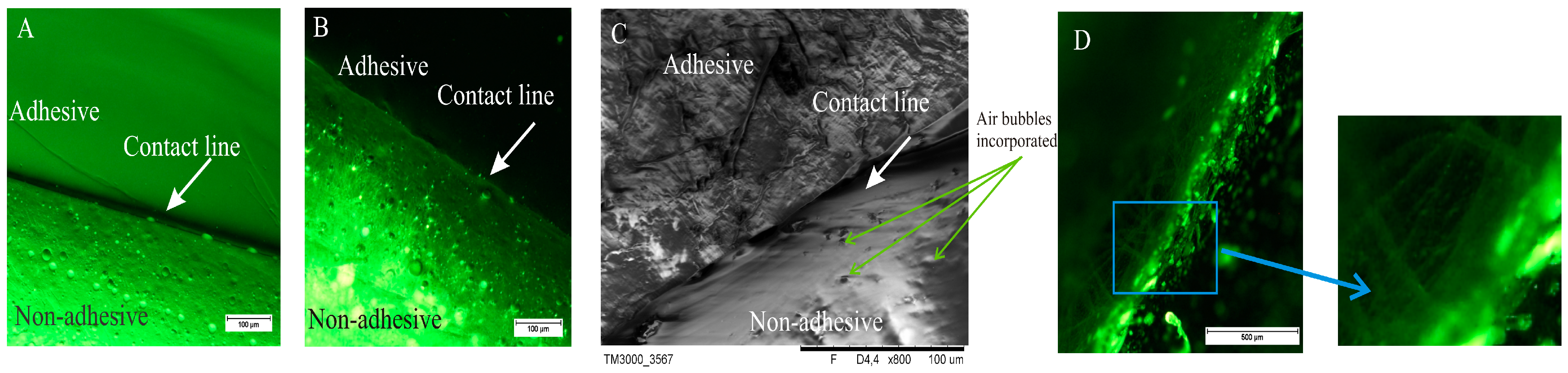
Figure 2 shows microscopic image of DLASil with the border line between both layers. In the two first images (
Figure 2A,B) the contact line is straight and condensed, while the third image (
Figure 2C), obtained by SEM, provides some details confirming an irregular and mobile structure of the silicone polymer. The matrix layer, consisting of nonadhesive elastomer, forms a thick structure, and air bubbles are visible. It should be emphasised that great attention must be paid during the mixing of the two components of Gumosil elastomer because of a tendency to entrap the air bubbles in a crosslinking structure. The adhesive layer, which directly contacts the skin, is characterised in the optical image (
Figure 2A) by a very smooth and homogeneous structure, while the SEM image shows an irregular structure, resembling a jelly (
Figure 2C). The silicone oil incorporated into the nonadhesive layer is visible as the lighter dots in the image (
Figure 2B). In the case of the Cica-Care patch, its diverse structure on the border of the layers and some “thread” structures are observed (
Figure 2D).
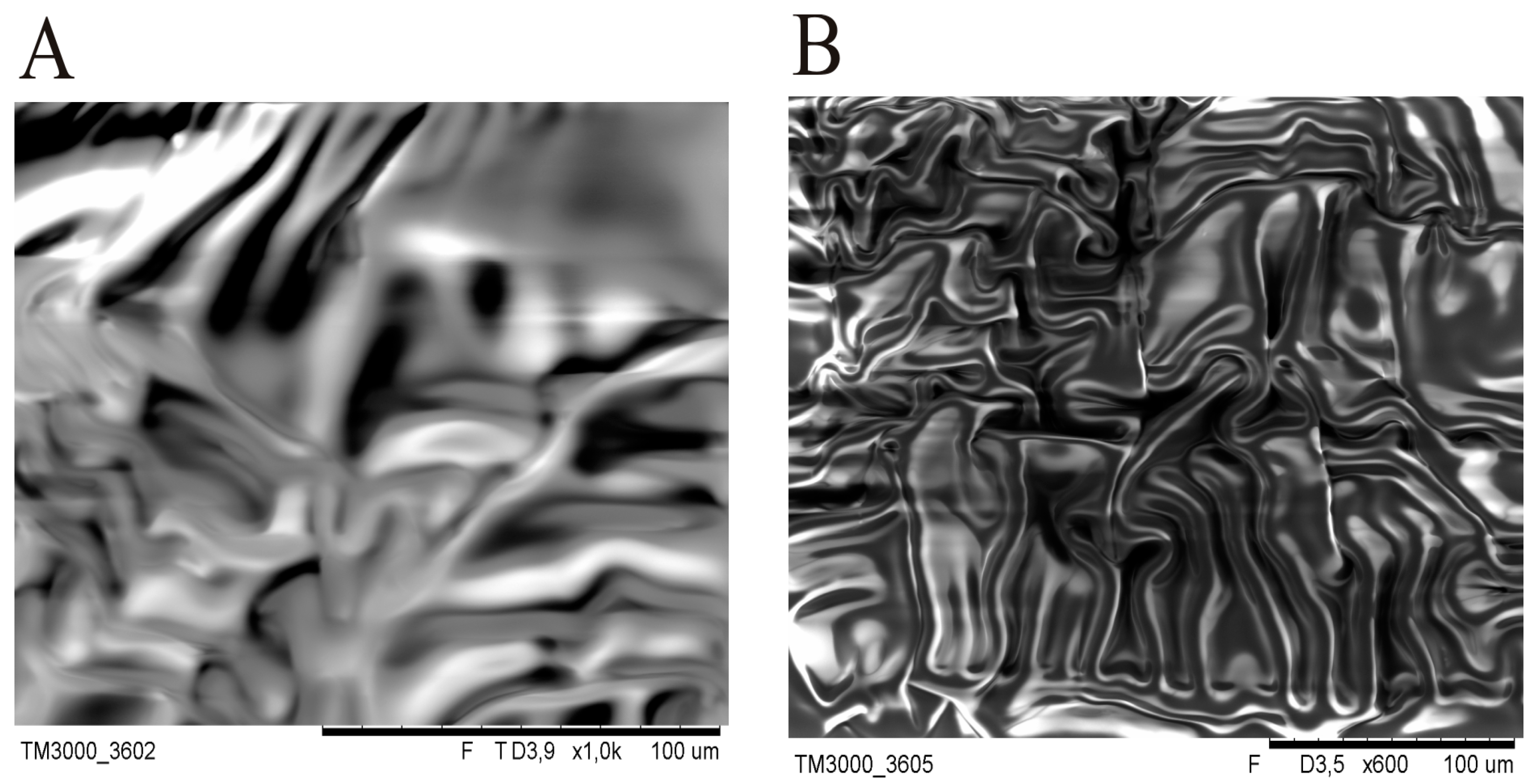
At the early stage of the experiment, SEM images of silicone patches without gold sputtering of the samples were recorded. However, during SEM testing with electron bombardment, a significant difference in DLASil’s behaviour was noticed. The adhesive layer is very sensitive (
Figure 3), while the nonadhesive layer is more resistant to high electrical potentials.
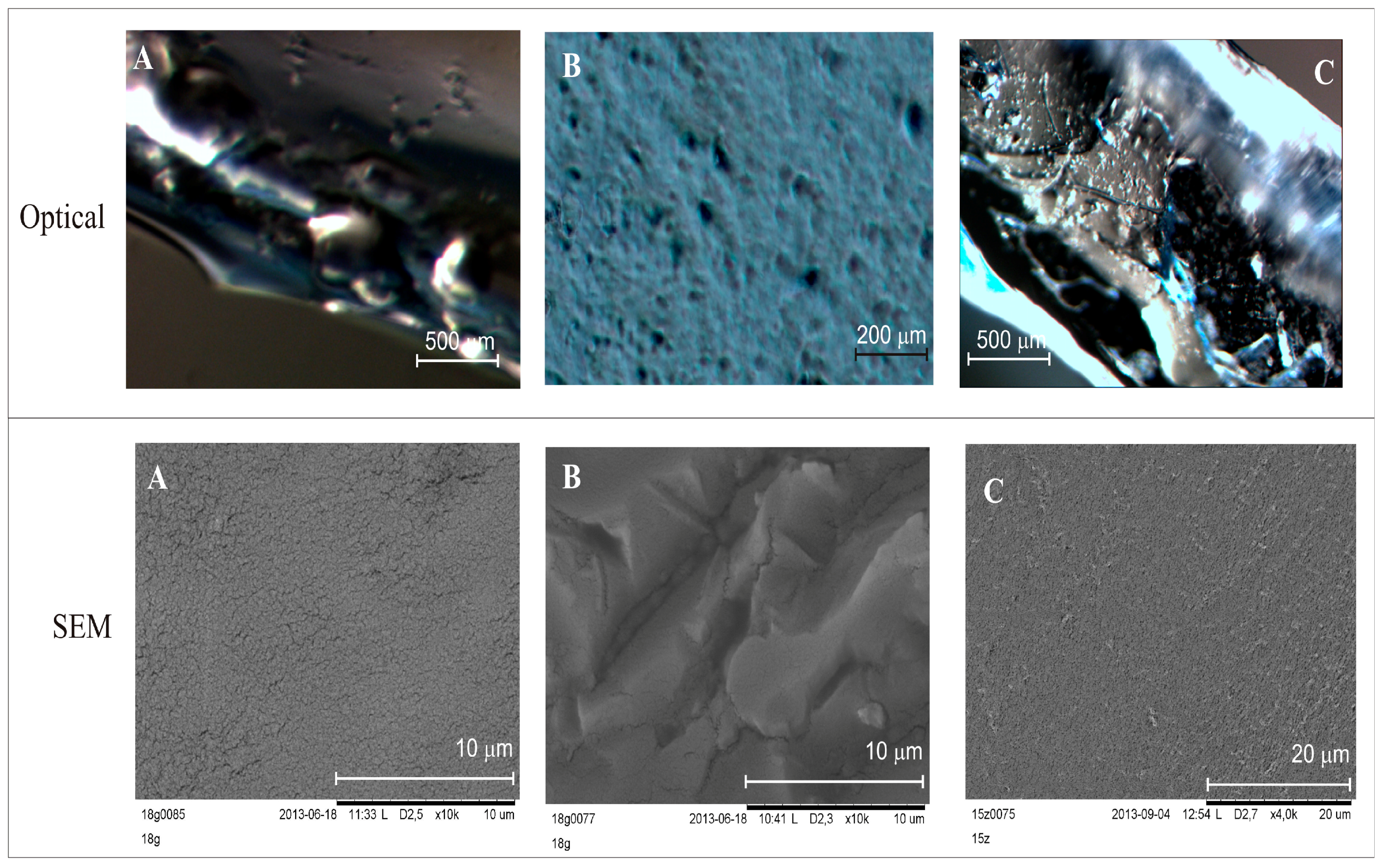
The differences in the layers are clearly noticeable in the images of DLASil and in commercially available patches (
Figure 4). Images obtained by the optical microscope correspond to the organoleptic appearance of silicone elastomers. A loose, gel-like structure of tacky, adhesive layer of DLASil and Cica-Care (
Figure 4A,C) can be identified without difficulties in the microscopic images. This layer is homogenous. It provides the effective cohesion with the non-adhesive layer (
Figure 4B).
In the SEM image (
Figure 4B), the tight structure of matrix-reservoir can be noticed. In comparison to adhesive layer this layer is irregular and rigid. Silicone film DLASil, compared with other commercially available preparation (e.g., Cica-Care), possesses a rougher structure (
Figure 4C). The adhesive surface of Cica-Care is considered the smoothest, which may partially explain that the lowest adhesion force values were observed for this product [
15].
DLASil silicone film consists of two types of elastomers with a three-dimensional network structure. The structure is formed during the crosslinking by addition reaction, which occurs between two reactive silicone polymers containing different functional groups (our publications, 2011, 2015). Components of Gumosil Part A and DC Part B contain a polydimethylsiloxane (PDMS) with a SiH siloxane crosslinker, while Gumosil Part B and DC Part A carry vinyl-enblocked polymer with a reactive dimethylvinyl CH
2=CH
2 group with a platinum catalyst (Pt-Karstedt). The addition reaction (hydrosilylation) is initiated by mixing Parts A and B. In the process of crosslinking, binding of linear polymer molecules into spatial macromolecule occurs, resulting in so-called “bridges” being formed with no byproducts. In the course of the reaction, disruption of the multiple C=C bonds of the vinyl group occurs, and a Si–C bond is formed as a result. Consequently, multiple addition reaction of Si–H with vinyl groups form numerous bridges connecting hydrocarbon chains of polysiloxane. In the previous work [
17], the molecular weights of the individual components, used to prepare the silicone elastomer, were determined. The increase in molecular weight of the polymer causes a change in its macroscopic properties, especially in physical properties of the material (e.g., increase of the melting point, strength, elasticity, and hardness).
3.2. Mechanical Characteristics of Silicone Patches
Mechanical properties of silicone DLASil were compared with commercially available silicone patches, Codosil™ and Cica-Care, which were considered as applicable and possessing desirable elastic features for medical purposes (
Table 1,
Figure 5). Liquid polydimethylsiloxane, SO (kinetic viscosity 350 cSt), was chosen as an additive, which can be used as a levigating agent, substantial for obtaining a homogenous distribution of the active substances incorporated into the transdermal patch. Matrices formed with PDMS are highly hydrophobic with a relatively high density in comparison to other commonly used polymeric matrix-formers [
18]. When excipients are added, significant changes in mechanical properties of the matrices are always reported. In some cases, hydrophobic solids (e.g., fumed silica) are combined with PDMS, causing increased hardness and durability of the silicone matrix material with simultaneously decreased diffusivity and modifying its mechanical behaviour (e.g., elasticity) [
18].
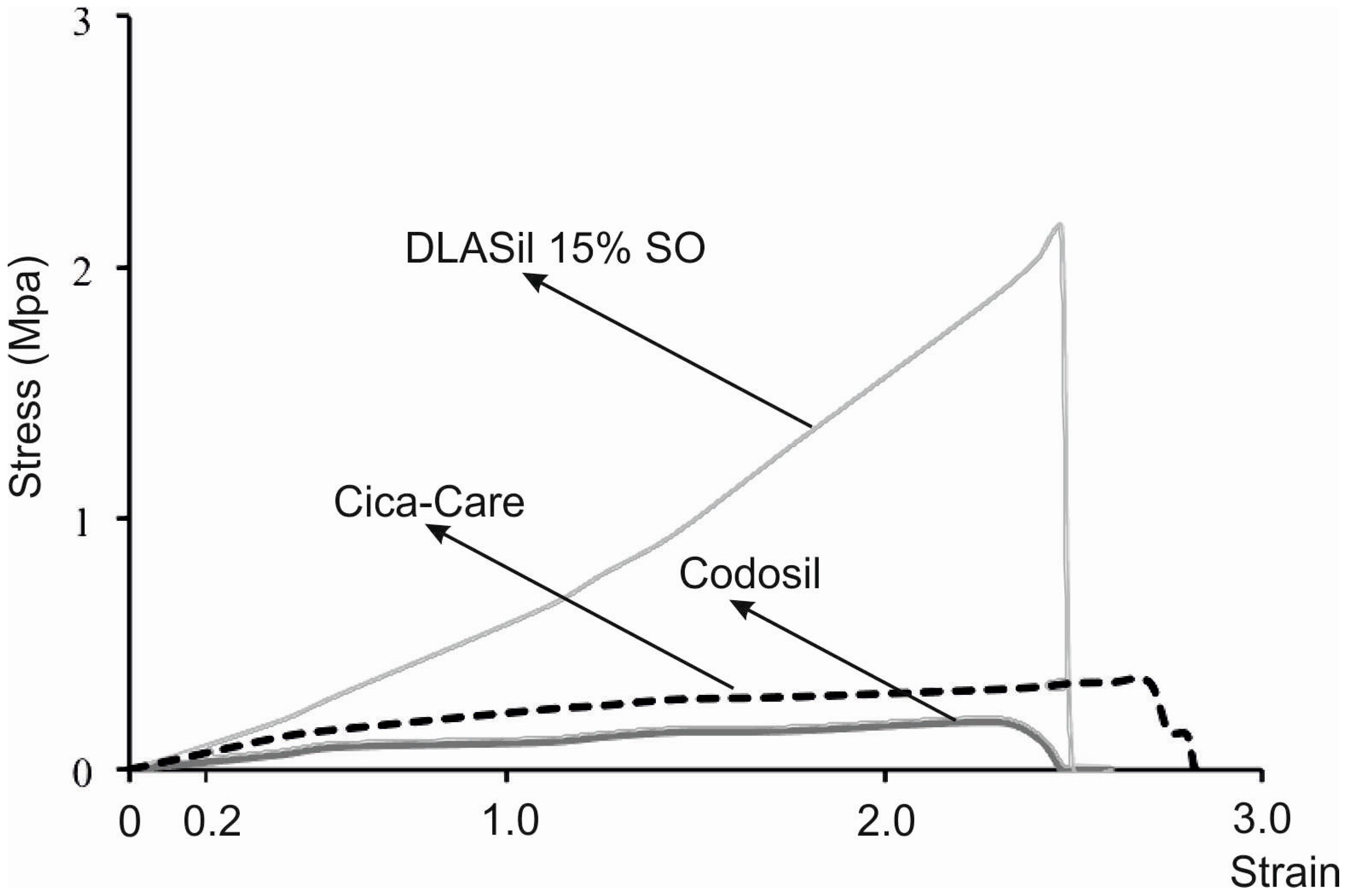
Elastic behaviour of the prepared silicone films was described by material deformation under stress condition of the assay. A typical plot of the strain–stress curve obtained for DLASil, Cica-Care, and Codosil™ patches is presented in
Figure 5. The examined materials showed both elastic and plastic deformation regions on the curve. Linear slope of the initial part of the curve indicates that Hook’s law holds for the elastic deformation part of the curve, hence modulus E (Young’s modulus) was determined [
15]. Linearity in the strain region (from 0 to 0.2) was noted for both DLASil formulations and commercially available patches (Cica-Care and Codosil™), thereby defining modulus E as ratio of tensile stress to strain, expressed as a fraction of original length (deformation over original length). Increasing SO content was found to cause significant loss in modulus E values, which corresponds to increasing elasticity of modified DLASil films (
Table 1). Modulus E values for Codosil™ patch were comparable to those determined for DLASil films without SO, which indicates similar elastic features. On the contrary, elasticity established for the Cica-Care patch indicated significantly lower stiffness, although in comparison to formulations of DLASil, modification with increasing SO content allows achievement of the patch’s elasticity at a comparable level.
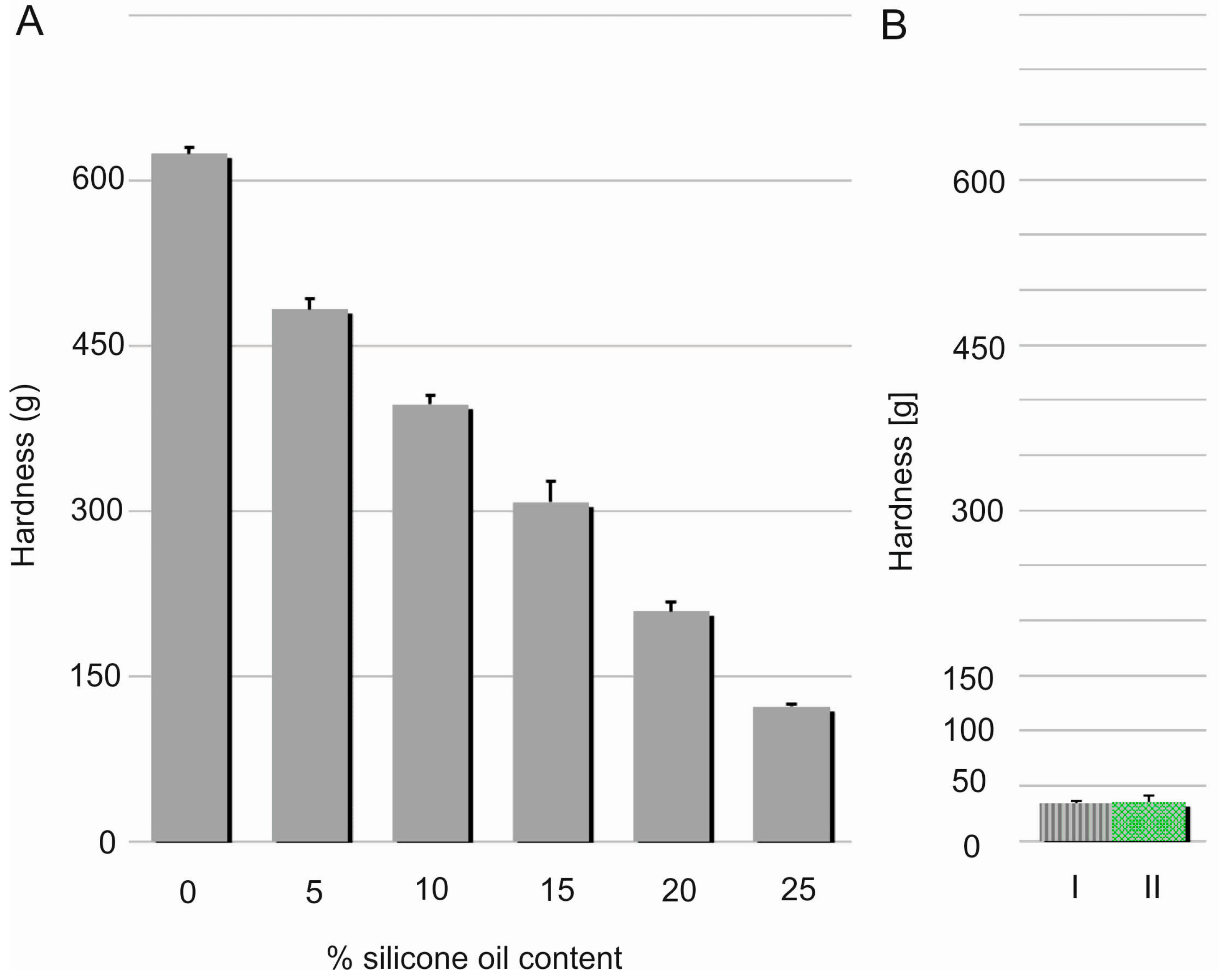
Hardness, considered as material resistance to local deformation, is technically examined by penetration studies with a hard object [
19]. Linear decrease (R
2 = 0.9933) in the films’ hardness was noted with increasing content of the silicone oil (
Figure 6). This effect can be probably explained by relaxation of a dense chain structure which is plasticised with SO. This assumption seems to be proven by simultaneous elasticity improvement expressed by modulus E values. Nevertheless, hardness of DLASil films containing 25%
w/
w of SO was found to be significantly higher in comparison to Codosil™ and Cica-Care patches. Though, it was still considered as approvable in terms of applicability of the newly formulated matrices. On the other hand, higher durability of DLASil allows its application without an additional supporting layer, while a very soft Codosil™ requires a backing layer.
Tensile strength (TS), which corresponds to material ability to withstand stress in tension mode, was calculated by dividing maximum force (N detected at break point by the cross-section area (mm
2) of the sample [
20]. The results are presented in
Table 1. Under the same stress conditions, both Codosil™ and Cica-Care patches were proven to be considerably more fragile than DLASil film, which confirms the observation of the structures using SEM. Although durability of DLASil films constantly decreased as a function of increasing SO content in the matrix, even an additional 25% of the liquid component did not result in the same low tensile strength. Due to the oil sweating effect, it was impossible to further increase SO content.
Percentage elongation (%EB) was calculated by the equation
EB =
∆l/
l0·100, where
∆l is maximum elongation and
l0 is initial length of sample [
21]. The percentage elongation (%EB) parameter describes the capability of the material to resist permanent deformation. The effect of SO present in DLASil films was found to be insignificant, which indicates that basic polymeric structure—formed during the crosslinking reaction and responsible for extensibility of the films—appears to remain intact. Still, DLASil formulations were found in an acceptable range of endurance to withstand tensile strain, established on values noted for both commercially available products examined (
Table 1).
Finally, mechanical characteristic of DLASil was found altered by SO addition into DLASil. In the case of the examined silicone elastomers, variation of tensile parameters of the films may be attributed to disturbance of the crosslinking reaction caused by the presence of silicone oil used as levigating agent (during incorporation of an active substance or a filler). Generally, the degree of crosslinking of the polymer depends on the length of the chain, molecular weight, and viscosity [
17,
22,
23]. Therefore, insignificant reduction of the %EB value parameter suggests that the addition of SO does not affect the crosslinking reaction, so the basic polymeric DLASil structure is maintained. Considering flexibility and durability of SO-modified films, the observed alterations may be explained by the presence of free channels filled by silicone oil in submicroscopic structure of the polymers. Consequently, the relation between the compressive strength, the stiffness and the tensile strength will be affected [
24].
Therefore, combined mechanical characterisation with morphology studies of polymer materials for medical use is beneficial, mostly for evaluation and optimisation during formulation of pharmaceutical product.
In the case of a dense polymer network, the lower ability to achieve maximum elongation can be seen, with a tendency to increase for DLASil, when the structure was relaxed by the addition of silicone oil. For properties such as tensile strength, elongation to break, and hardness of silicone elastomers, observed changes differ slightly. The possibility of adding silicone oil to the silicone matrix and assessing its optimum amount, which neither influences the crosslinking reaction nor the mechanical properties adversely, was evaluated. It should be emphasised that the addition of liquid components to silicone elastomers is not a standard practice. The study was planned to optimise the amount of silicone oil in the matrix, so as not to alter the mechanical properties (elongation, elasticity, and flexibility). It may happen that the relation between the compressive strength, the stiffness, and the tensile strength will change due to existing or emerging defects and cracks in the submicroscopic image of polymer structure [
25]. Hence, it is important to study the morphology of polymer materials for medical use, since such observations may provide additional data to verify the mechanical properties of these materials.
3.3. Stability Studies
Further research was focused on investigation of the common environmental factors that may influence the stability of DLASil. When chemical stability of the formulation is taken into consideration, the effects of temperature, increased humidity, light, or oxidation—regarded as storage or stress conditions—are observed. The structure and morphology of silicone films were examined by means of optical and electron microscopy as well as spectroscopically by ATR–FTIR. Adhesiveness assessment was carried out to examine applicability maintenance of DLASil.
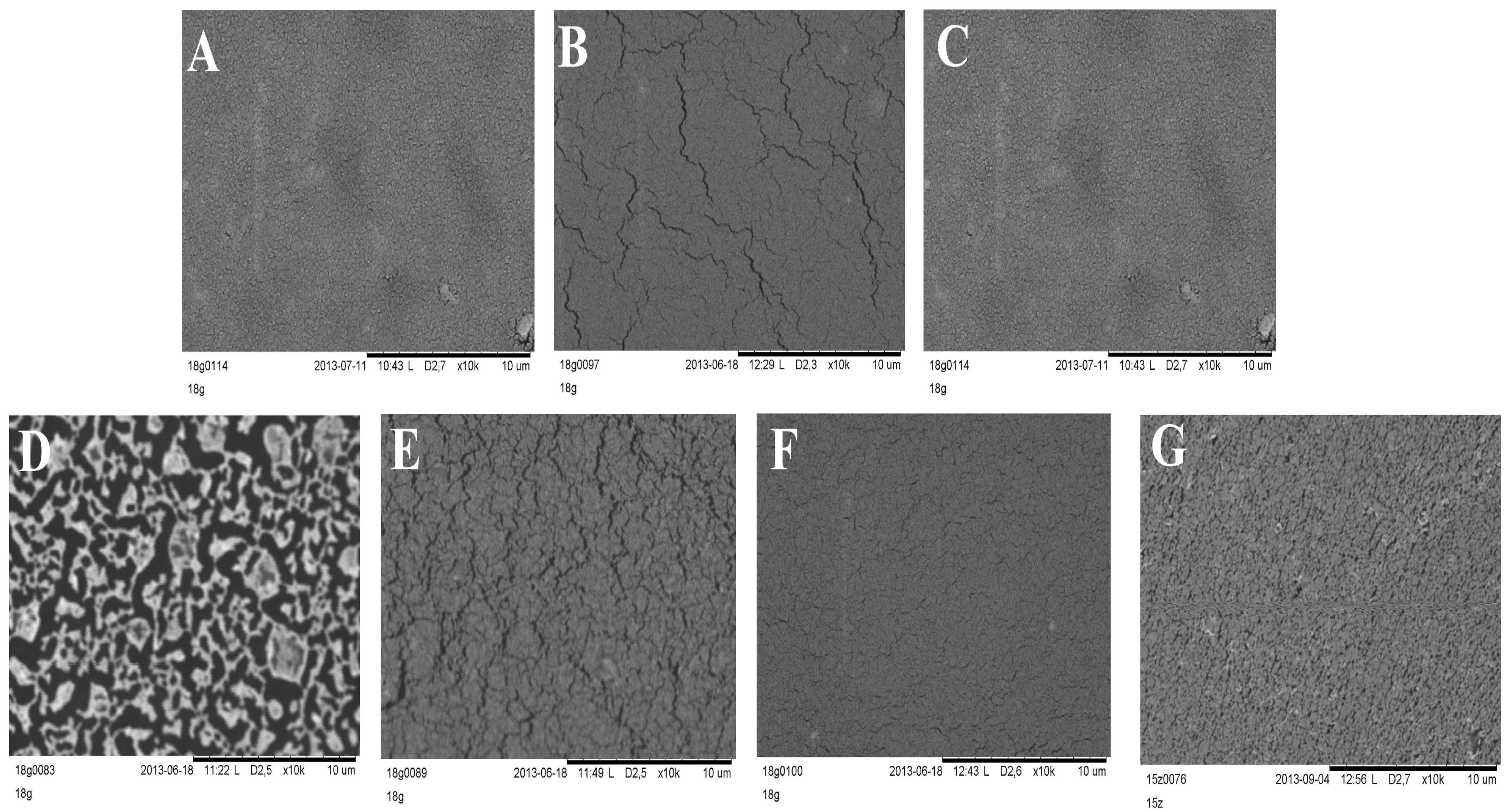
Silicone films exposed to storage or stress conditions show no noticeable changes in their appearance. It was stated that the optical microscope technique was sufficient for general screening of morphology, but SEM allowed observation of detailed changes that occurred in layers during the stability test. SEM images presented in
Figure 7 clearly show changes in the morphology of DLASil after exposition to test conditions. The most spectacular degradation in structure is observed for a sample which underwent 10-fold washing procedure. The pattern of the reference DLASil (
Figure 7A) is presented as a smooth surface, however, this changed after washing the film: severe surface fragmentation indicates disturbed structure of silicone polymer (
Figure 7D).
After irradiation of the DLASil samples in the Suntest chamber as well as after storage under stress conditions the change in the appearance of DLASil film in SEM images can be described as microcracking of the polymer structure.
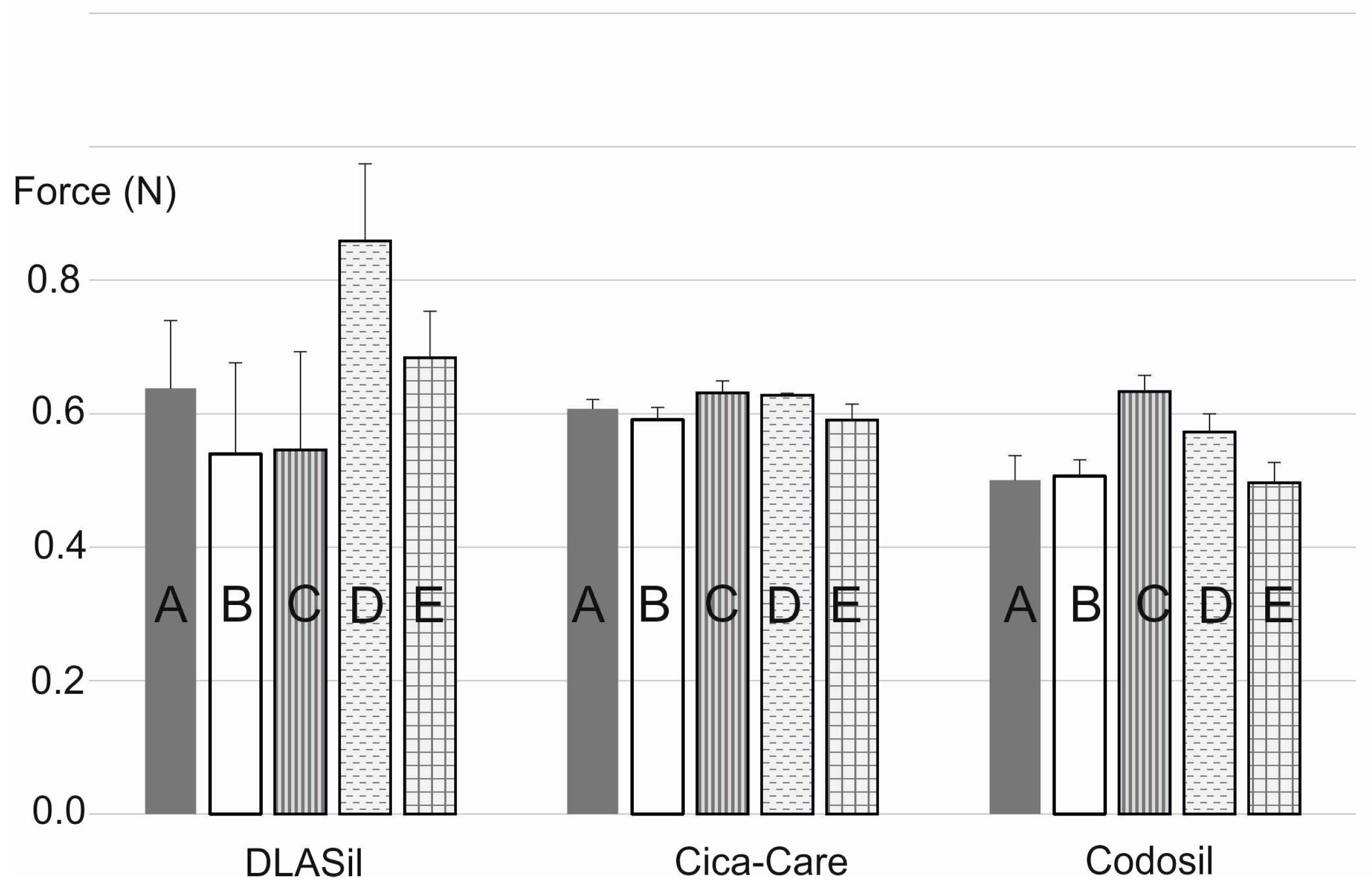
In
Figure 8, the effect of storage and stress conditions on adhesiveness of the films is presented. Storage conditions (temperature and humidity) had no statistically significant effect on either DLASil or commercially available products. Only for Cica-Care and Codosil was a slight increase of the adhesive force noted after storage at 4–6 °C with increased humidity. The effect of stress factors such as UV light exposition and 10-fold wash, observed by SEM images analysis, was also confirmed by adhesion force measurement. The increase of adhesiveness was linked to the change of the adhesive layer surface and also in the structure of the silicone polymer, which occurred due to observed crack formation.
DLASil patches were designed for a maximum of 3–4 weeks of application; it was concluded that for the considered period, stability of the films was confirmed.
Additionally, DLASil samples were characterised by means of ATR–FTIR spectroscopy after stability testing. The crosslinking process of silicone components depends on the presence of components with similar structures but possessing different functional groups at the ends of the molecule, as was shown by Pieńkowska, et al. [
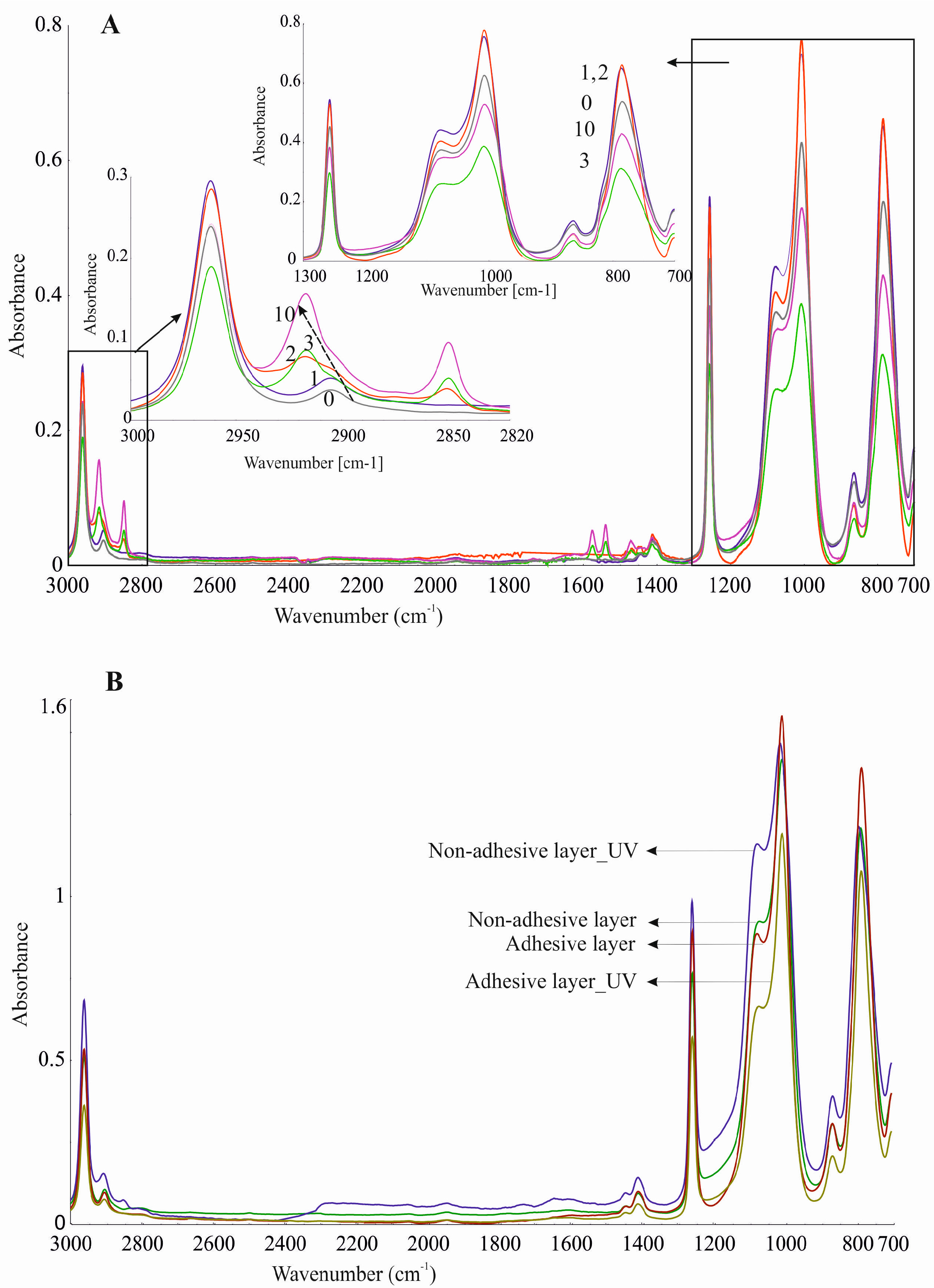
17]. When analysing the FTIR spectra (
Figure 9), differences between silicone elastomers and primary components before crosslinking could be seen. The noticeable differences between the spectra are visible at 1092, 1021–1007, and 800–786 cm
−1. This is due to the differences in the proportion of functional groups at the ends of the polymer chains before and after cure reaction. Bands signed as 3 and 4 in
Figure 9 are identical, but they have lower intensities than the bands obtained for the silicone components before crosslinking. The clear separation of Si–O–Si bands at wavenumbers 1092 and 1021 cm
−1 vanished as a consequence of crosslinking. The ATR–FTIR spectrum of the adhesive layer of DLASil has a higher intensity of bands compared to the bands of the nonadhesive elastomer. A sharp band at a wavenumber 1008 cm
−1 can be a significant band, which indicates the characteristic arrangement of groups Si–O–Si and determines the adhesion properties of the elastomer [
26].
Silicone components used to form the silicone elastomer, DLASil, do not differ significantly in the infrared spectrum (
Figure 9(1–2)). After curing, the characteristic bands of silicones are displaced and have little difference in shape, especially in the case of adhesive and nonadhesive layers’ spectrum. However, they are observed in the same region of wavenumbers in the infrared spectrum. ATR–FTIR spectra were also recorded after stability testing of each layer. Spectra presented in
Figure 10A may indicate that the adhesive layer of DLASil is a little changed during washing. Bands in the spectra retained their shape and specific wavenumber. The only change in spectrum is observed in the intensity of bands. A clear increase of intensity is observed near wavenumber 2920 and 2850 cm
−1. This region is responsible for asymmetrical and symmetrical valence (stretching) vibrations (C–H of the CH
3 group). This situation may be caused by the residual soap after washing in the surface structure of the adhesive layer of DLASil. Interactions of the silicone patch with the soap during washing are so negligible that it retains the same appearance and unchanged chemical structure. Nevertheless, there are observed diverse and irregular changes of bands, but mostly due to higher intensity, not causing any wavenumber changes. Because an identical ATR–FTIR spectrum was achieved for stored DLASil at the higher temperature, its stability was confirmed.
Additional research performed on photostability testing of DLASil has shown that UV irradiation does not influence its structure. No difference in ATR–FTIR spectrum of the DLASil film before and after UV irradiation was observed (
Figure 10B).