Synthesis and Characterization of Two-Dimensional Conjugated Polymers Incorporating Electron-Deficient Moieties for Application in Organic Photovoltaics
Abstract
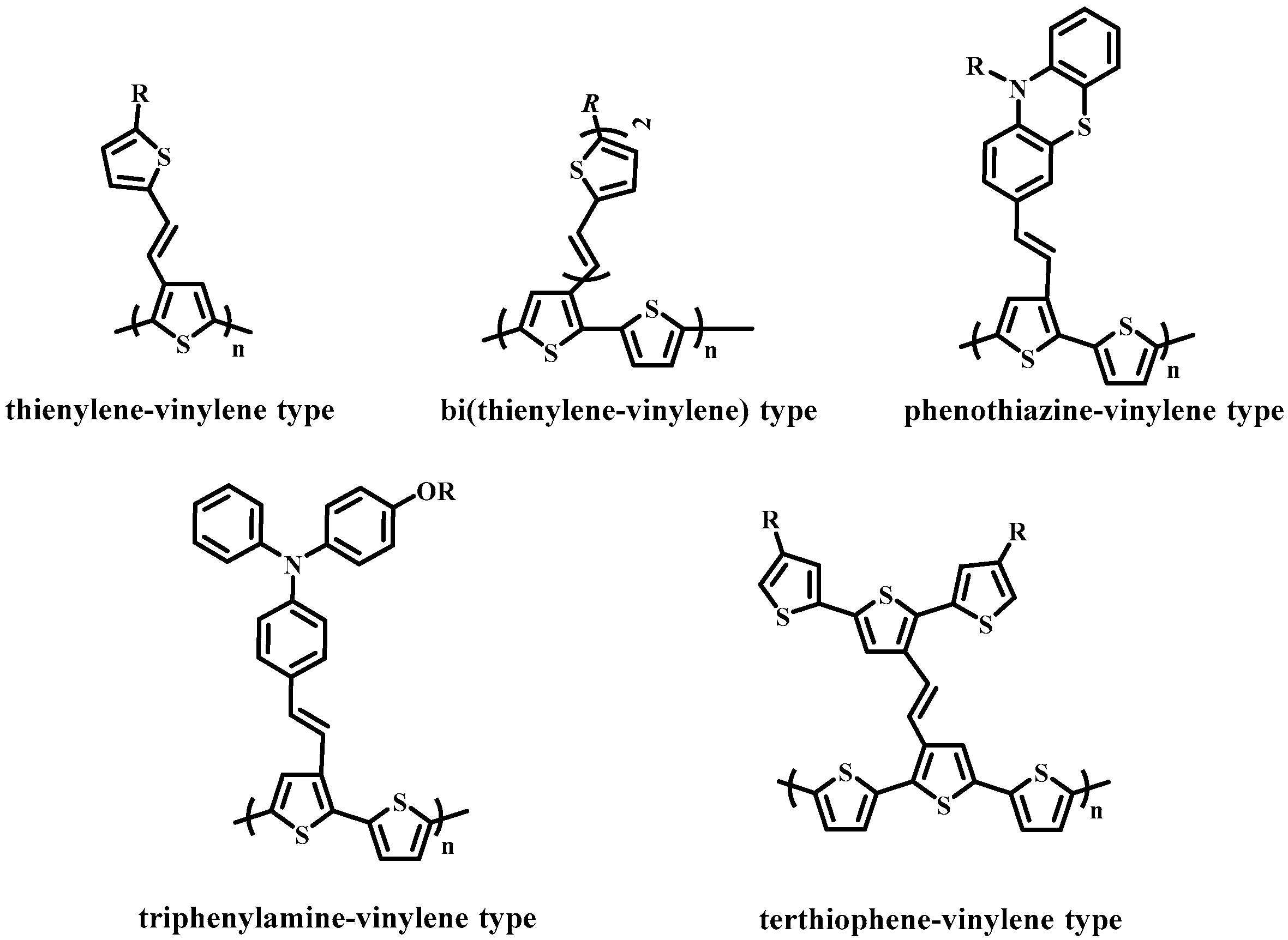
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrument and Characterization
2.3. Device Fabrication and Characterization
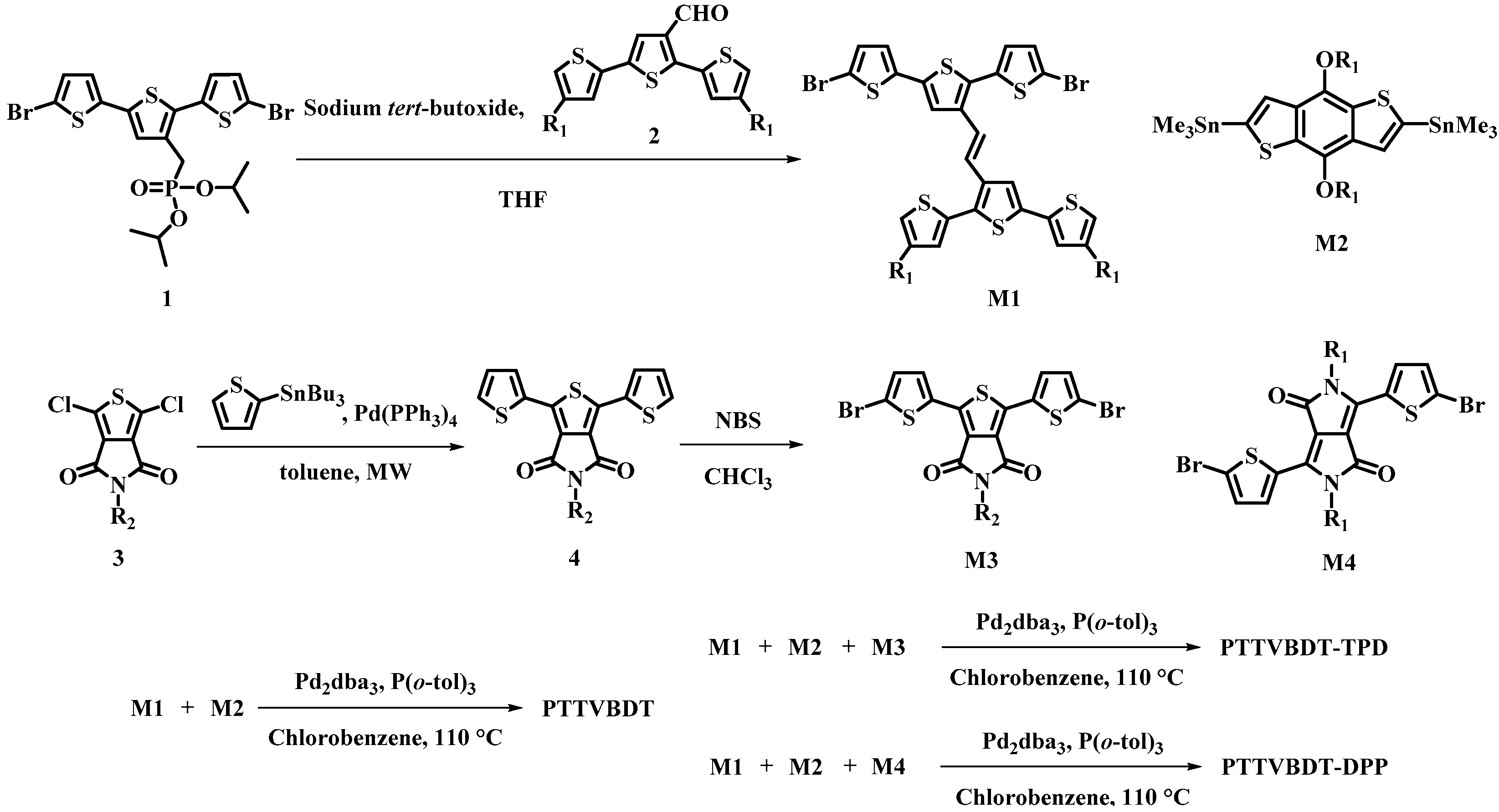
2.4. Synthesis of Monomers and Polymers
2.4.1. (E)-3′-(2-(5,5″-dibromo-[2,2′:5′,2″-terthiophen]-3′-yl)vinyl)-4,4″-bis(2-ethylhexyl)-2,2′:5′,2″-terthiophene (M1)
2.4.2. 5-Octyl-1,3-di(thiophen-2-yl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (4)
2.4.3. 1,3-Bis(5-bromothiophen-2-yl)-5-octyl-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (M3)
2.4.4. Standard Procedure of Polymerization and Purification
2.4.5. Synthesis of Polymers
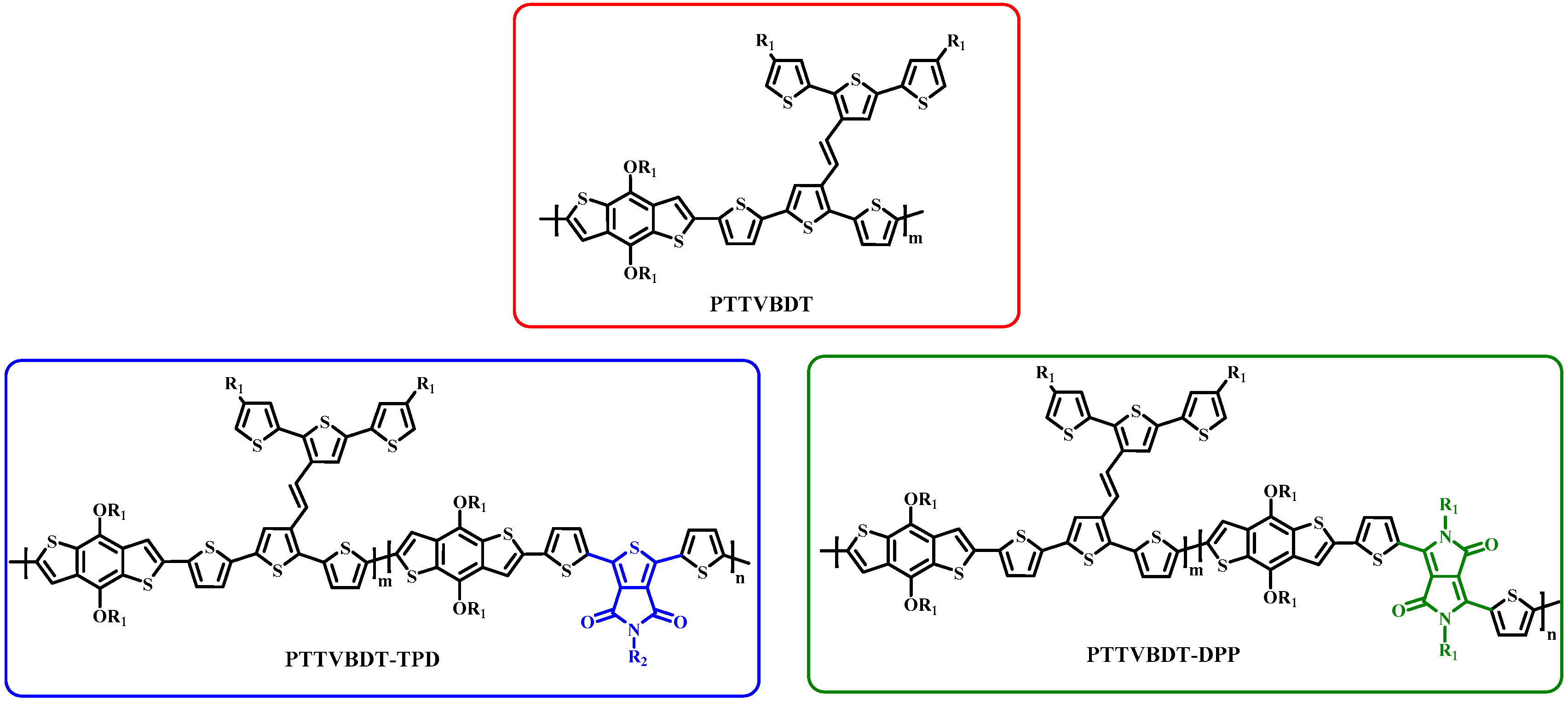
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Facchetti, A. Semiconductors for organic transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, X.; Zhang, F.; Lu, J.; Zhai, Z.; Di, C.; Jiang, Z.; Ma, W. Design of benzodithiophene-diketopyrrolopyrrole based donor-acceptor copolymers for efficient organic field effect transistors and polymer solar cells. J. Mater. Chem. 2012, 22, 22734–22742. [Google Scholar] [CrossRef]
- Qian, G.; Qi, J.; Wang, Z.Y. Synthesis and study of low-bandgap polymers containing the diazapentalene and diketopyrrolopyrrole chromophores for potential use in solar cells and near-infrared photodetectors. J. Mater. Chem. 2012, 22, 12867–12873. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Leok Chan, K.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef] [PubMed]
- Usta, H.; Sheets, W.C.; Denti, M.; Generali, G.; Capelli, R.; Lu, S.; Yu, X.; Muccini, M.; Facchetti, A. Perfluoroalkyl-functionalized thiazole–thiophene oligomers as n-channel semiconductors in organic field-effect and light-emitting transistors. Chem. Mater. 2014, 26, 6542–6556. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Hou, J. Breaking the 10% efficiency barrier in organic photovoltaics: Morphology and device optimization of well-known pbdttt polymers. Adv. Energy Mater. 2016, 6, 1502529. [Google Scholar] [CrossRef]
- Son, H.J.; Carsten, B.; Jung, I.H.; Yu, L. Overcoming efficiency challenges in organic solar cells: Rational development of conjugated polymers. Energy Environ. Sci. 2012, 5, 8158–8170. [Google Scholar] [CrossRef]
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, P.-L.T.; Najari, A.; Leclerc, M. Processable low-bandgap polymers for photovoltaic applications. Chem. Mater. 2010, 23, 456–469. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Nie, W.; Tsai, H.; Yen, H.-J.; Mohite, A.D.; Gupta, G.; Dattelbaum, A.M.; William, D.J.; Cha, K.C.; Yang, Y.; et al. Structural design of benzo[1,2-b:4,5-b′]dithiophene-based 2d conjugated polymers with bithienyl and terthienyl substituents toward photovoltaic applications. Macromolecules 2014, 47, 1008–1020. [Google Scholar] [CrossRef]
- Liao, S.-H.; Jhuo, H.-J.; Cheng, Y.-S.; Chen, S.-A. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, W.; Shen, W.; Duan, L.; Qiu, M.; Wang, J.; Yang, C.; Du, Z.; Yang, R. Novel donor-acceptor polymers containing o-fluoro-p-alkoxyphenyl-substituted benzo[1,2-b:4,5-b′]dithiophene units for polymer solar cells with power conversion efficiency exceeding 9%. J. Mater. Chem. A 2016, 4, 10212–10222. [Google Scholar] [CrossRef]
- Huang, J.; Carpenter, J.H.; Li, C.-Z.; Yu, J.-S.; Ade, H.; Jen, A.K.Y. Highly efficient organic solar cells with improved vertical donor–acceptor compositional gradient via an inverted off-center spinning method. Adv. Mater. 2016, 28, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, C.; He, C.; Li, Y. Poly[3-(5-octyl-thienylene-vinyl)-thiophene]: A side-chain conjugated polymer with very broad absorption band. Chem. Commun. 2006, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tan, Z.A.; Yan, Y.; He, Y.; Yang, C.; Li, Y. Synthesis and photovoltaic properties of two-dimensional conjugated polythiophenes with bi(thienylenevinylene) side chains. J. Am. Chem. Soc. 2006, 128, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wu, W.; Sang, G.; Yang, Y.; Liu, Y.; Li, Y. Polythiophene derivative with phenothiazine−vinylene conjugated side chain: Synthesis and its application in field-effect transistors. Macromolecules 2007, 40, 7231–7237. [Google Scholar] [CrossRef]
- Zou, Y.; Sang, G.; Wu, W.; Liu, Y.; Li, Y. A polythiophene derivative with octyloxyl triphenylamine-vinylene conjugated side chain: Synthesis and its applications in field-effect transistor and polymer solar cell. Synth. Metals 2009, 159, 182–187. [Google Scholar] [CrossRef]
- Hsiow, C.-Y.; Raja, R.; Wang, C.-Y.; Lin, Y.-H.; Yang, Y.-W.; Hsieh, Y.-J.; Rwei, S.-P.; Chiu, W.-Y.; Huang, C.-I.; Wang, L. Impact of constitution of the terthiophene-vinylene conjugated side chain on the optical and photovoltaic properties of two-dimensional polythiophenes. Phys. Chem. Chem. Phys. 2014, 16, 25111–25120. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Ye, L.; Guo, X.; Huang, Y.; Wang, P.; Zhang, S.; Zhang, J.; Huo, L.; Hou, J. Application of two-dimensional conjugated benzo[1,2-b:4,5-b′]dithiophene in quinoxaline-based photovoltaic polymers. Macromolecules 2012, 45, 3032–3038. [Google Scholar] [CrossRef]
- Gu, Z.; Shen, P.; Tsang, S.-W.; Tao, Y.; Zhao, B.; Tang, P.; Nie, Y.; Fang, Y.; Tan, S. Development of a new benzo(1,2-b:4,5-b′)dithiophene-based copolymer with conjugated dithienylbenzothiadiazole-vinylene side chains for efficient solar cells. Chem. Commun. 2011, 47, 9381–9383. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, B.; Cao, Z.; Shen, P.; Tan, Z.; Li, X.; Tan, S. Enhanced power conversion efficiencies in bulk heterojunction solar cells based on conjugated polymer with isoindigo side chain. Chem. Commun. 2013, 49, 3857–3859. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Bin, H.; Xiao, L.; Li, Y. Enhancing photovoltaic performance of copolymers containing thiophene unit with D–A conjugated side chain by rational molecular design. Macromolecules 2013, 46, 9575–9586. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Huang, Y.-C.; Hsiow, C.-Y.; Yang, Y.-W.; Huang, C.-I.; Rwei, S.-P.; Wang, H.-L.; Wang, L. Effect of side-chain architecture on the optical and crystalline properties of two-dimensional polythiophenes. Macromolecules 2013, 46, 5985–5997. [Google Scholar] [CrossRef]
- Hsiow, C.-Y.; Lin, Y.-H.; Raja, R.; Rwei, S.-P.; Chiu, W.-Y.; Dai, C.-A.; Wang, L. Modified structure of two-dimensional polythiophene derivatives by incorporating electron-deficient units into terthiophene-vinylene conjugated side chains and the polymer backbone: Synthesis, optoelectronic and self-assembly properties, and photovoltaic application. RSC Adv. 2016, 6, 67976–67985. [Google Scholar]
- Cabanetos, C.; El Labban, A.; Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; Fréchet, J.M.J.; McGehee, M.D.; Beaujuge, P.M. Linear side chains in benzo[1,2-b:4,5-b′]dithiophene–thieno[3,4-c]pyrrole-4,6-dione polymers direct self-assembly and solar cell performance. J. Am. Chem. Soc. 2013, 135, 4656–4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-H.; Park, S.; Yu, H.; Kang, H.; Song, I.; Oh, J.H.; Kim, B.J. Determining optimal crystallinity of diketopyrrolopyrrole-based terpolymers for highly efficient polymer solar cells and transistors. Chem. Mater. 2014, 26, 6963–6970. [Google Scholar] [CrossRef]
- Shi, H.; Fu, W.; Shi, M.; Ling, J.; Chen, H. A solution-processable bipolar diketopyrrolopyrrole molecule used as both electron donor and acceptor for efficient organic solar cells. J. Mater. Chem. A 2015, 3, 1902–1905. [Google Scholar] [CrossRef]
- Cheon, Y.R.; Kim, Y.J.; Ha, J.-J.; Kim, M.-J.; Park, C.E.; Kim, Y.-H. Tpd-based copolymers with strong interchain aggregation and high hole mobility for efficient bulk heterojunction solar cells. Macromolecules 2014, 47, 8570–8577. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Hou, J.; Chen, H.-Y.; Zhang, S.; Jiang, Y.; Chen, T.L.; Yang, Y. Bandgap and molecular level control of the low-bandgap polymers based on 3,6-dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione toward highly efficient polymer solar cells. Macromolecules 2009, 42, 6564–6571. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Greenblatt, M.; Dismukes, G.C. Water oxidation by λ-mno2: Catalysis by the cubical Mn4O4 subcluster obtained by delithiation of spinel LiMn2O4. J. Am. Chem. Soc. 2010, 132, 11467–11469. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, Y.; Feng, D.; Tsai, S.-T.; Son, H.-J.; Li, G.; Yu, L. Development of new semiconducting polymers for high performance solar cells. J. Am. Chem. Soc. 2009, 131, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Deng, X.; Yu, J.; Zhao, B.; Wang, Y.; Liu, Y.; Zhu, W.; Wu, H.; Cao, Y. A novel benzo[1,2-b:4,5-b′]dithiophene-based conjugated polymer with a pendant diketopyrrolopyrrole unit for high-performance solar cells. Macromolecules 2012, 46, 113–118. [Google Scholar] [CrossRef]
- Ayres, B.E.; Longworth, S.W.; McOmie, J.F.W. Synthesis of derivatives of cyclobuteno[c]thiophen and attempts to synthesise thiophen analogues of biphenylene. Tetrahedron 1975, 31, 1755–1760. [Google Scholar] [CrossRef]
- Yuan, M.-C.; Chiu, M.-Y.; Liu, S.-P.; Chen, C.-M.; Wei, K.-H. A thieno[3,4-c]pyrrole-4,6-dione-based donor−acceptor polymer exhibiting high crystallinity for photovoltaic applications. Macromolecules 2010, 43, 6936–6938. [Google Scholar] [CrossRef]
- Patil, A.V.; Lee, W.-H.; Lee, E.; Kim, K.; Kang, I.-N.; Lee, S.-H. Synthesis and photovoltaic properties of a low-band-gap copolymer of dithieno[3,2-b:2′,3′-d]thiophene and dithienylquinoxaline. Macromolecules 2011, 44, 1238–1241. [Google Scholar] [CrossRef]
- Khlyabich, P.P.; Burkhart, B.; Rudenko, A.E.; Thompson, B.C. Optimization and simplification of polymer–fullerene solar cells through polymer and active layer design. Polymer 2013, 54, 5267–5298. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Wang, J. Structures and properties of conjugated donor-acceptor copolymers for solar cell applications. J. Mater. Chem. 2012, 22, 4178–4187. [Google Scholar] [CrossRef]
- Najari, A.; Beaupré, S.; Berrouard, P.; Zou, Y.; Pouliot, J.-R.; Lepage-Pérusse, C.; Leclerc, M. Synthesis and characterization of new thieno[3,4-c]pyrrole-4,6-dione derivatives for photovoltaic applications. Adv. Funct. Mater. 2011, 21, 718–728. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Wang, L.; Su, W.-F. Polymer solar cells with poly(3,4-ethylenedioxythiophene) as transparent anode. Org. Electron. 2008, 9, 968–973. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.-C.; Hong, Z.; Gao, J.; Yang, Y.; Zhou, H.; Dou, L.; Li, G.; Yang, Y. Solution-processed small-molecule solar cells: Breaking the 10% power conversion efficiency. Sci. Rep. 2013, 3, 3356. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Mw a (kDa) | Mn a (kDa) | PDI a | m/n b | Td c (°C) |
|---|---|---|---|---|---|
| PTTVBDT | 124.6 | 47.1 | 2.64 | - | 357 |
| PTTVBDT-TPD | 309.4 | 110.3 | 2.81 | 5.9 | 358 |
| PTTVBDT-DPP | 300.6 | 120.8 | 2.49 | 7.0 | 368 |
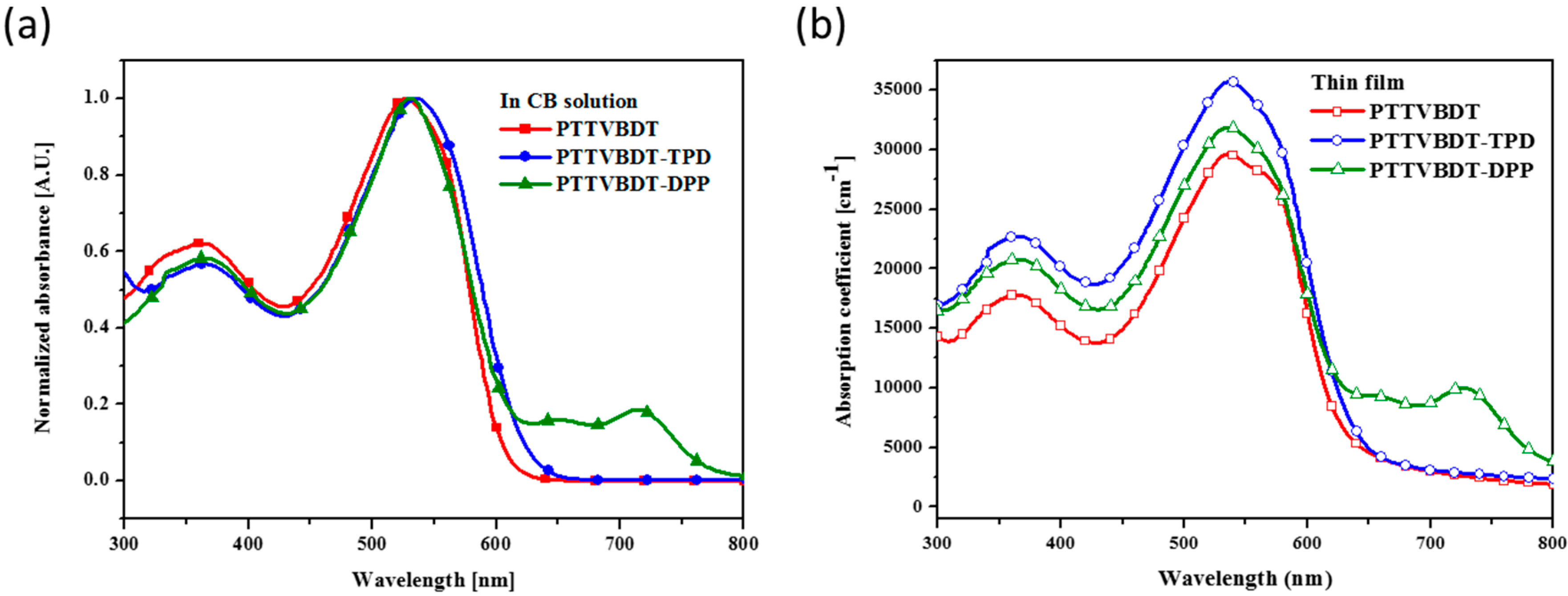
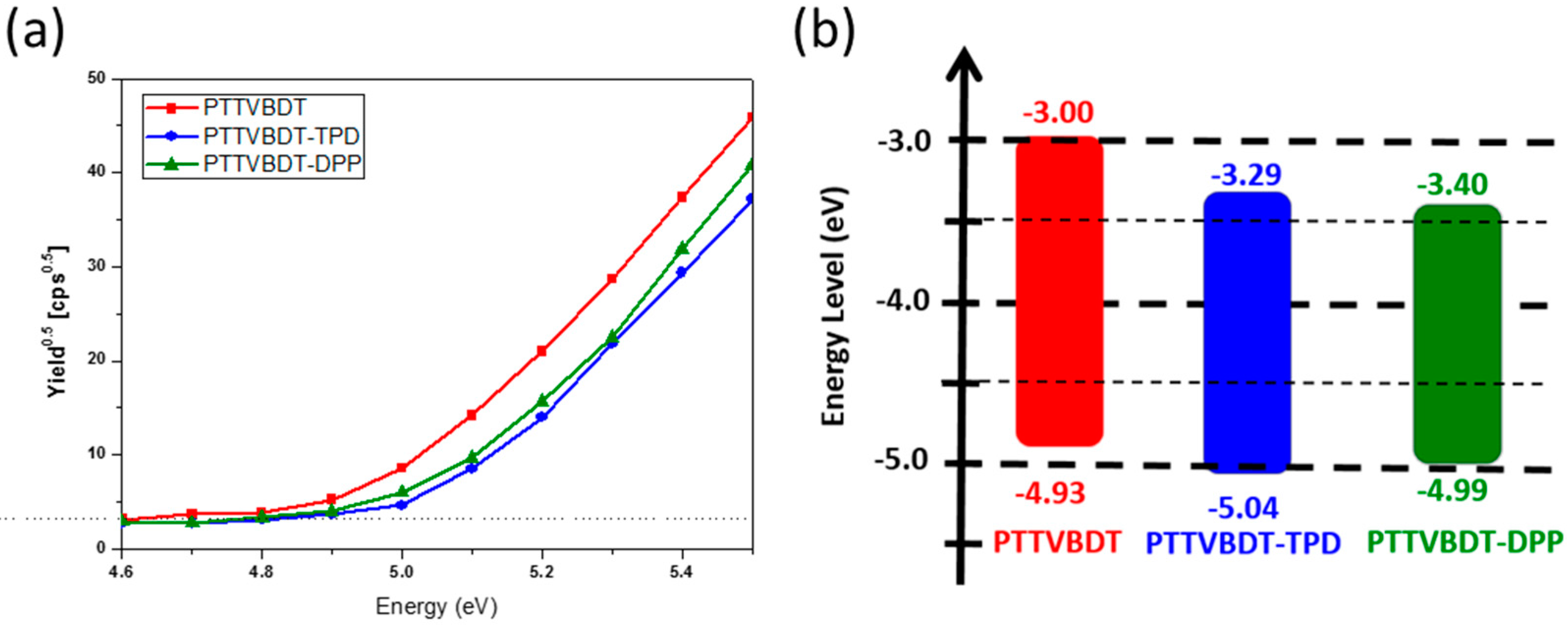
| Polymer | Solution, λmax (nm) a | Film, λmax (nm) b | λedge (nm) b | Eg, opt. (eV) c | EHOMO,AC2 (eV) d | ELUMO (eV) e |
|---|---|---|---|---|---|---|
| PTTVBDT | 526 | 536 | 635 | 1.95 | −4.93 | −2.98 |
| PTTVBDT-TPD | 534 | 537 | 647 | 1.91 | −5.04 | −3.13 |
| PTTVBDT-DPP | 530 | 536 | 791 | 1.56 | −4.99 | −3.43 |
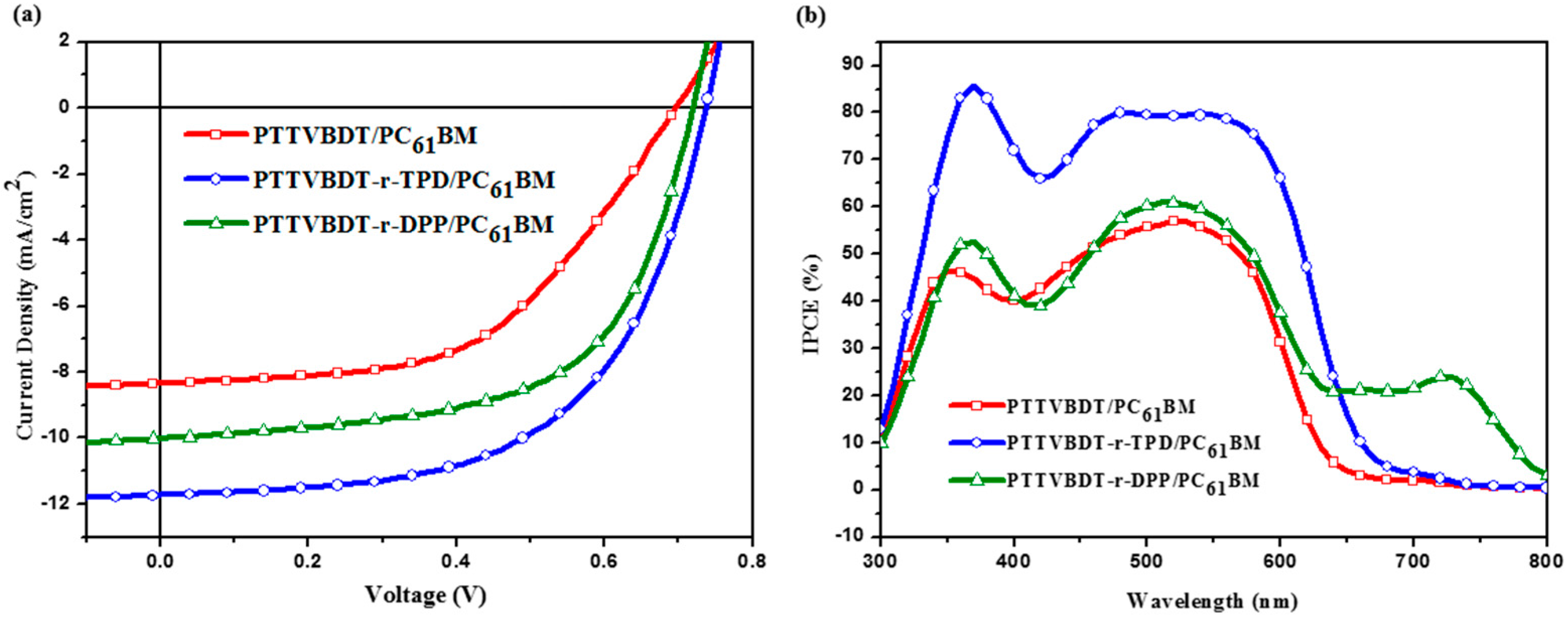
| Polymer/fullerene | Voc (mV) | Jsc (mA/cm2) | FF (%) | PCE (%) | Rsh (kΩ·cm2) | Rs (Ω·cm2) | |
|---|---|---|---|---|---|---|---|
| Avg. | Best | ||||||
| PTTVBDT/PC61BM a | 703 ± 13 | 8.10 ± 0.06 | 52.24 ± 1.08 | 2.98 | 3.10 | 0.89 | 29.17 |
| PTTVBDT-TPD/PC61BM b | 740 ± 0 | 11.65 ± 0.08 | 57.63 ± 0.03 | 4.97 | 5.01 | 1.05 | 9.78 |
| PTTVBDT-DPP/PC61BM c | 723 ± 4 | 9.96 ± 0.21 | 59.5 ± 0.4 | 4.29 | 4.39 | 0.84 | 8.56 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiow, C.-Y.; Wang, H.-Y.; Lin, Y.-H.; Raja, R.; Rwei, S.-P.; Chiu, W.-Y.; Dai, C.-A.; Wang, L. Synthesis and Characterization of Two-Dimensional Conjugated Polymers Incorporating Electron-Deficient Moieties for Application in Organic Photovoltaics. Polymers 2016, 8, 382. https://doi.org/10.3390/polym8110382
Hsiow C-Y, Wang H-Y, Lin Y-H, Raja R, Rwei S-P, Chiu W-Y, Dai C-A, Wang L. Synthesis and Characterization of Two-Dimensional Conjugated Polymers Incorporating Electron-Deficient Moieties for Application in Organic Photovoltaics. Polymers. 2016; 8(11):382. https://doi.org/10.3390/polym8110382
Chicago/Turabian StyleHsiow, Chuen-Yo, Han-Ying Wang, Yu-Hsiang Lin, Rathinam Raja, Syang-Peng Rwei, Wen-Yen Chiu, Chi-An Dai, and Leeyih Wang. 2016. "Synthesis and Characterization of Two-Dimensional Conjugated Polymers Incorporating Electron-Deficient Moieties for Application in Organic Photovoltaics" Polymers 8, no. 11: 382. https://doi.org/10.3390/polym8110382








