Conjugation of Lectin to Poly(ε-caprolactone)-block-glycopolymer Micelles for In Vitro Intravesical Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
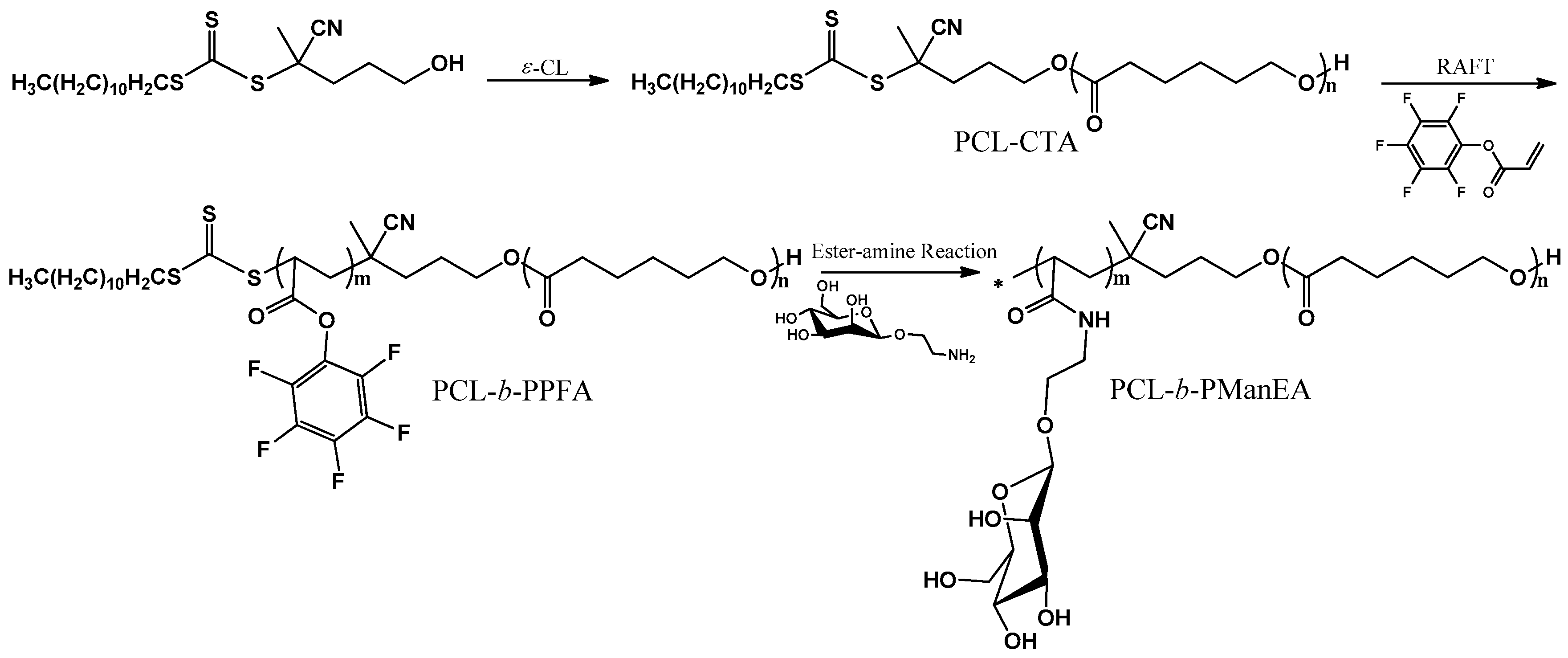
2.2. Synthesis of Poly(ε-caprolactone) (PCL) Macro-Chain Transfer Agent (Macro-CTA)
2.3. Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization of PFA Using PCL as the Macro-CTA
2.4. Synthesis of PCL-block-poly[2-(α-d-mannopyranosyloxy) ethyl acrylamide] (PCL-b-PManEA) Block Copolymers
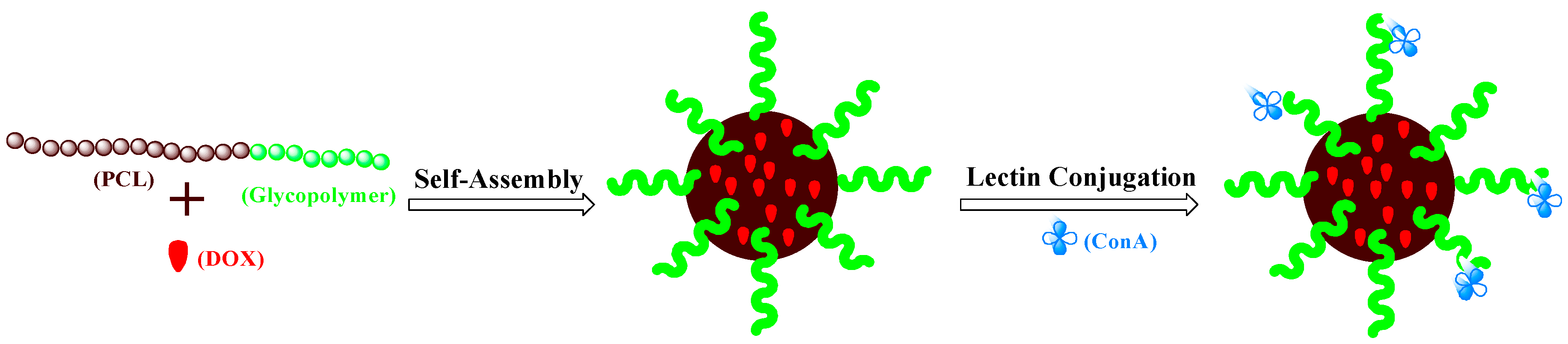
2.5. Preparation of DOX-Loaded PCL-b-PManEA Micelles
2.6. Preparation of DOX-Loaded PCL-b-PManEA Micelles-ConA Conjugates (DOX-Loaded PCL-b-PManEA@ConA Micelles)
2.7. In Vitro Drug Release
2.8. Cell Culture and Incubation
2.9. Cell Viability
2.10. Cellular Uptake
2.11. Mucoadhesive Property of DOX-loaded PCL1-b-PManEA@ConA Micelles
2.12. Characterization
3. Results and Discussion
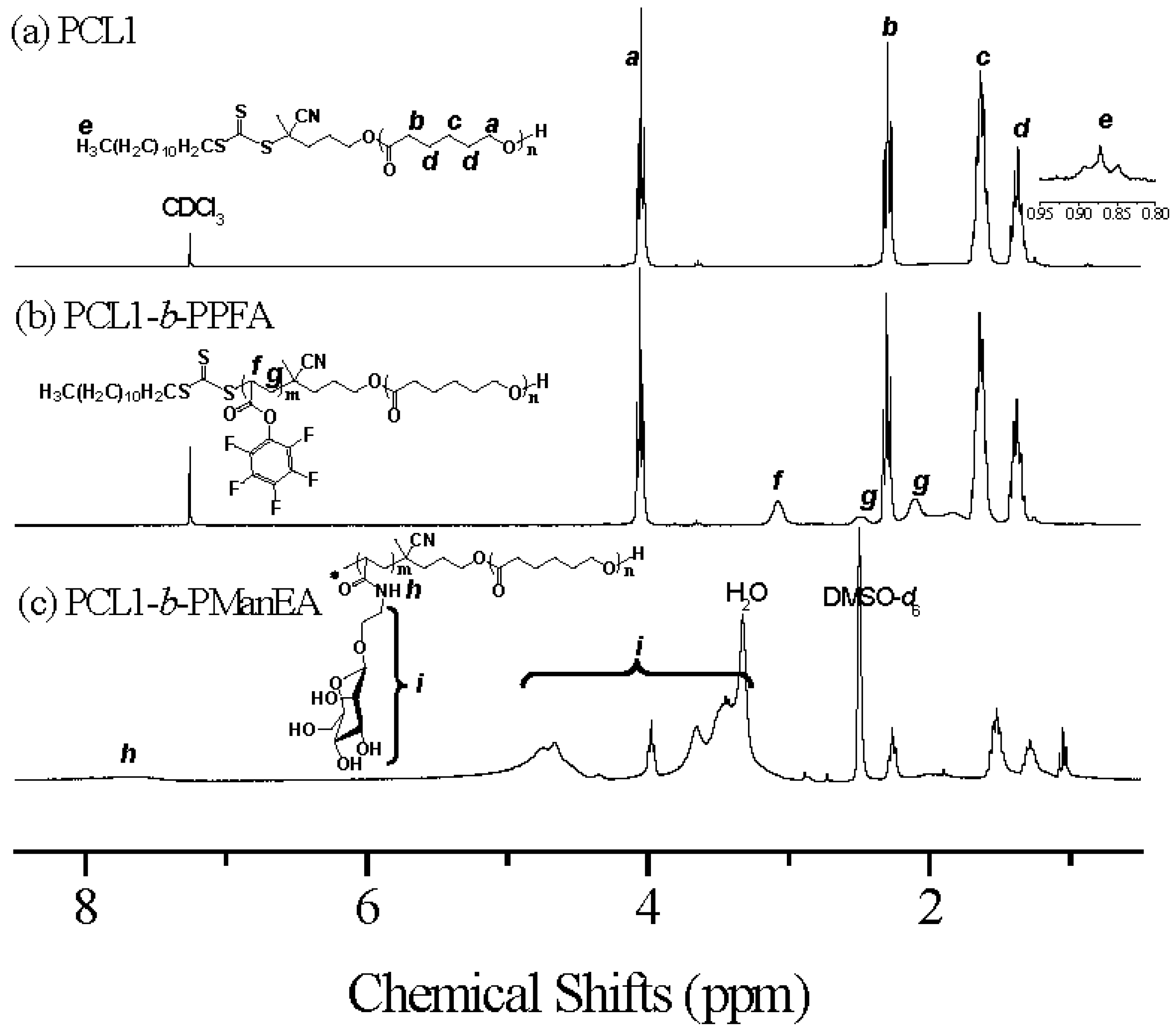
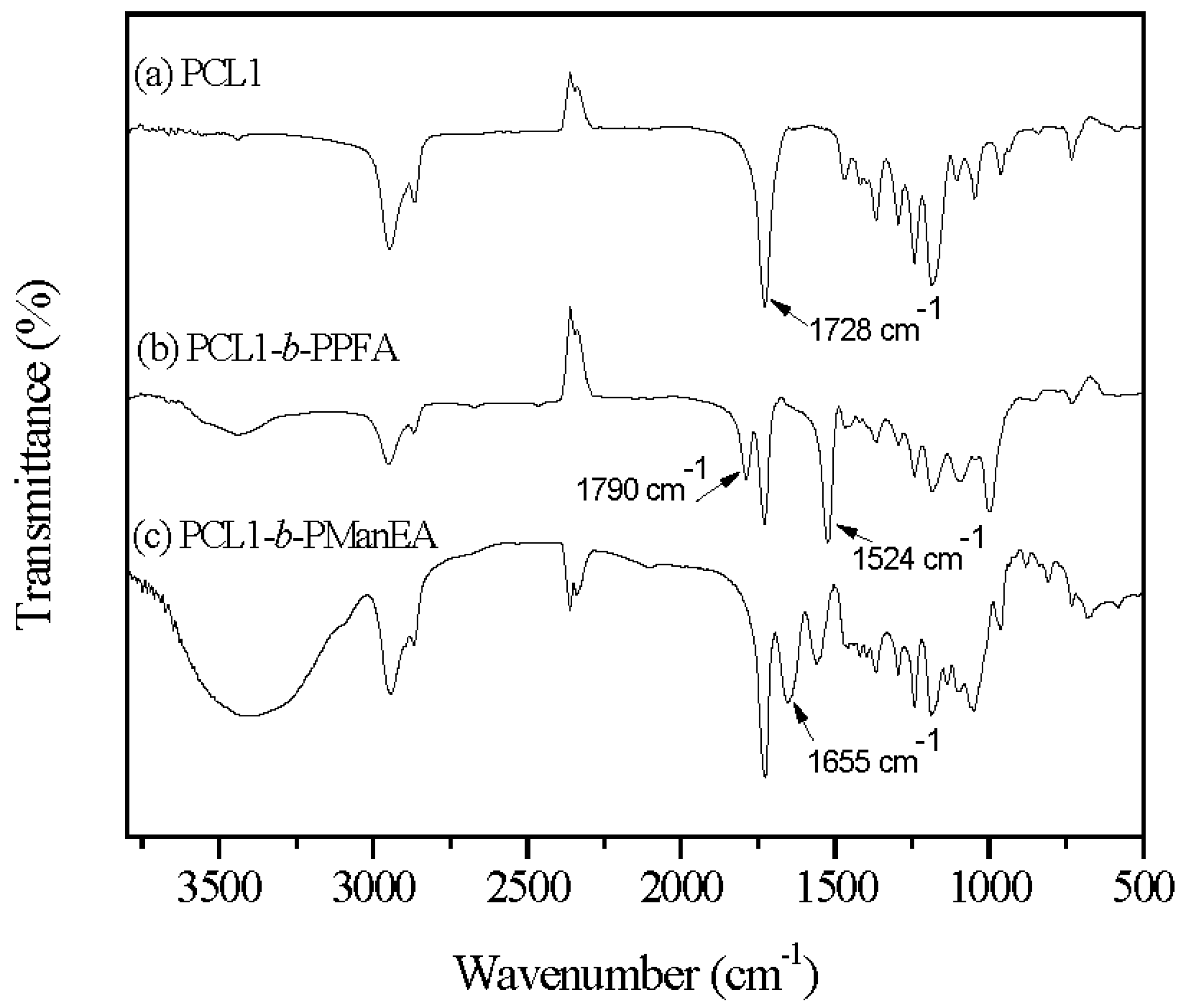
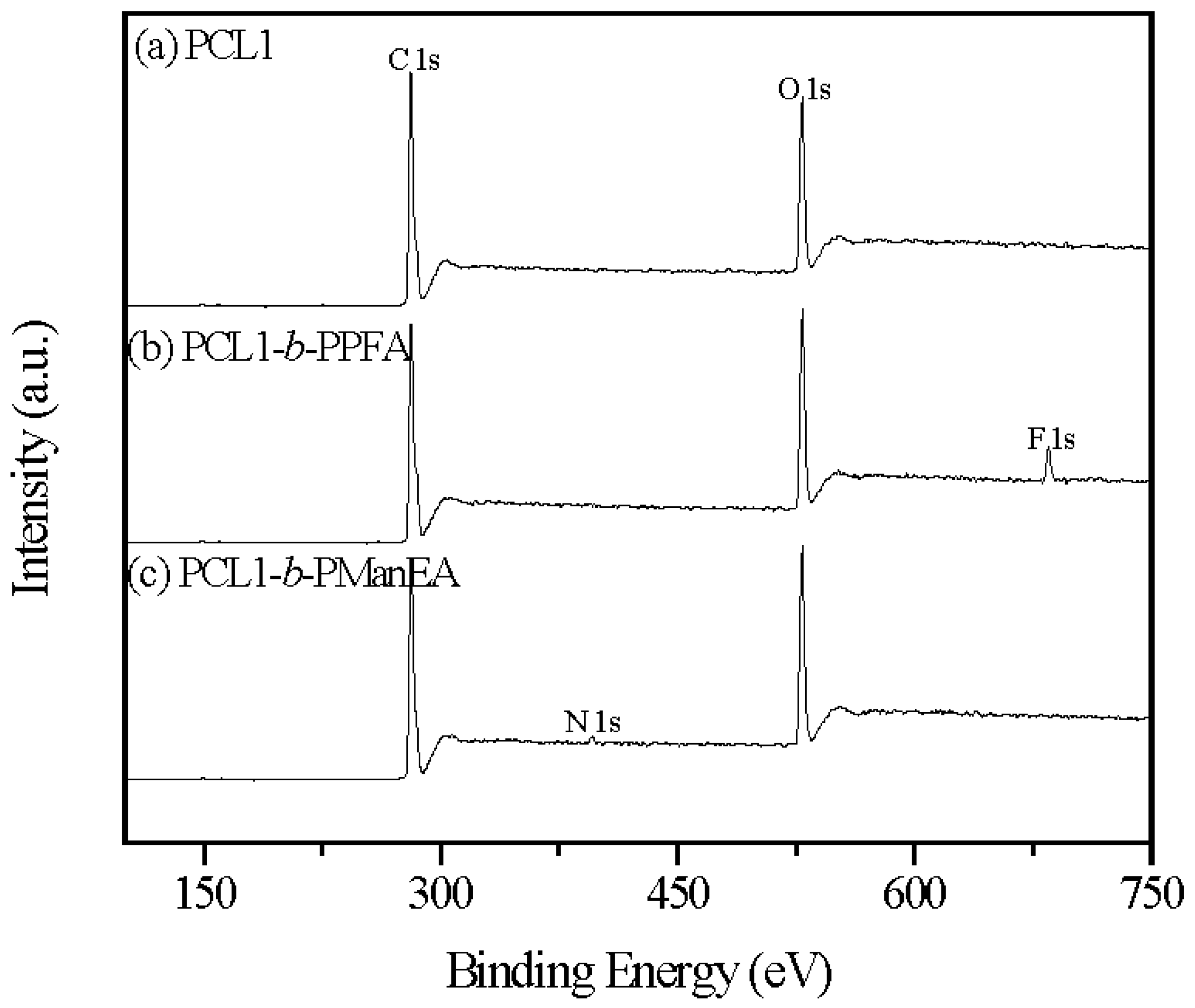
3.1. Synthesis of PCL-b-PManEA Block Copolymers
3.2. Preparation of DOX-Loaded PCL-b-PManEA Micelles and Their Lectin Conjugates
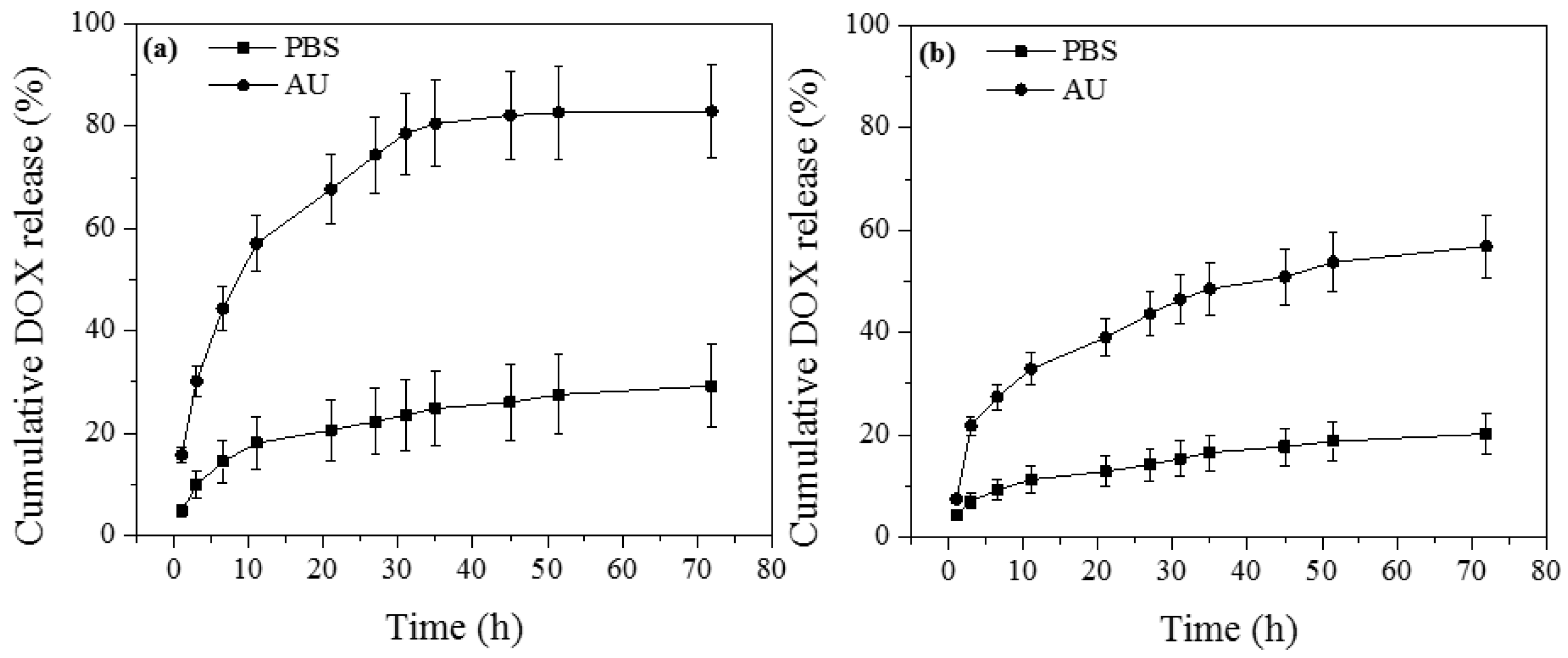
3.3. In Vitro Drug Release
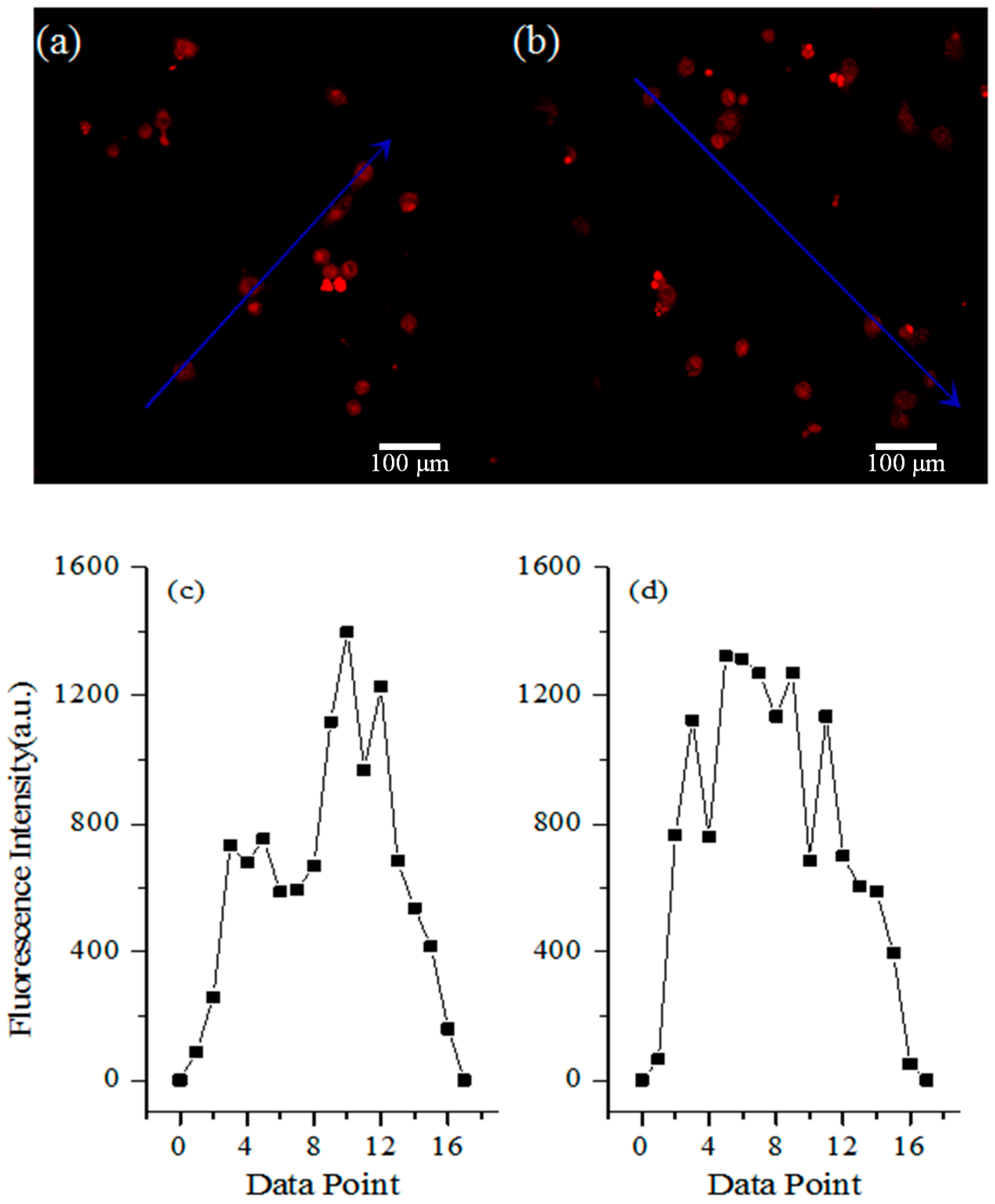
3.4. Mucoadhesive Study
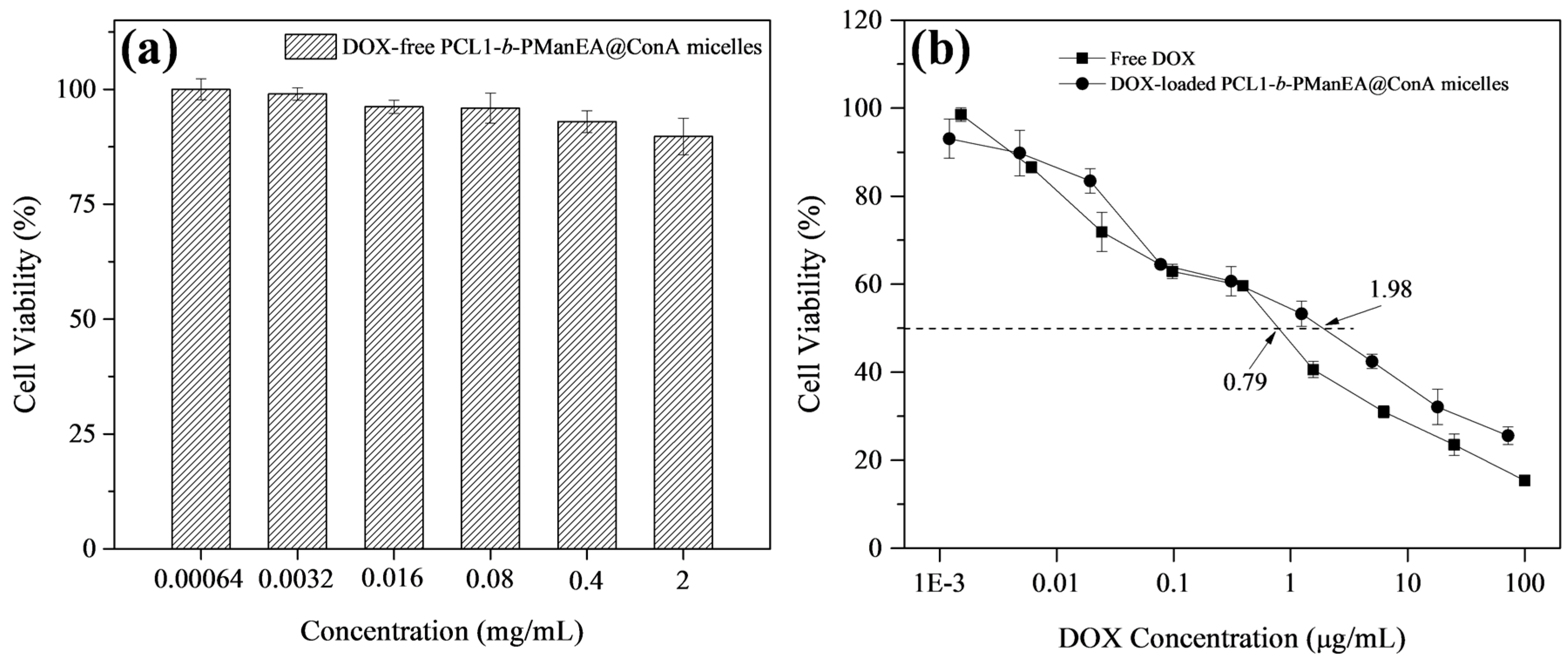
3.5. Cellular Uptake and In Vitro Cytotoxicity Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, Q.; Zhang, J.; Zhang, G.; Gan, Z. Synthesis and functions of well-defined polymer-drug conjugates as efficient nanocarriers for intravesical chemotherapy of bladder cancera. Macromol. Biosci. 2015, 15, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Allione, A.; Guarrera, S.; Turinetto, V.; Fiorito, G.; Viberti, C.; Russo, A.; Vineis, P.; Sacerdote, C.; Giachino, C.; et al. Abstract 4615: H2ax phosphorylation assays, gene expression and epigenomic profiles as markers in bladder cancer: An integrated approach. Cancer Res. 2015, 75, 4615. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, S.; Kaufman, J.; Huang, L.; de Miguel, F. Local drug delivery to bladder using technology innovations. Urol. Clin. N. Am. 2006, 33, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Matsui, Y.; So, A.I.; Gleave, M.E.; Baker, J.H.E.; Minchinton, A.I.; Manisali, I.; Liggins, R.; Brooks, D.E.; Burt, H.M. In vivo evaluation of mucoadhesive nanoparticulate docetaxel for intravesical treatment of non-muscle-invasive bladder cancer. Clin. Cancer Res. 2011, 17, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Harari, O.; Bianco-Peled, H. Evaluating the mucoadhesive properties of drug delivery systems based on hydrated thiolated alginate. J. Control. Release 2009, 136, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [PubMed]
- Dumitriu, S. Polymeric Biomaterials; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Leitner, V.M.; Walker, G.F.; Bernkop-Schnürch, A. Thiolated polymers: Evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56, 207–214. [Google Scholar] [CrossRef]
- Anande, N.M.; Jain, S.K.; Jain, N.K. Con-A conjugated mucoadhesive microspheres for the colonic delivery of diloxanide furoate. Int. J. Pharm. 2008, 359, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Liener, I.E.; Sharon, N.; Goldstein, I.J. The Lectins: Properties, Functions, and Applications in Biology and Medicine; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Yin, Y.S.; Chen, D.W.; Qiao, M.X.; Lu, Z.; Hu, H.Y. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. J. Control. Release 2006, 116, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.M.; Stone, G.M.; Peppas, N.A. Wheat germ agglutinin functionalized complexation hydrogels for oral insulin delivery. Biomacromolecules 2008, 9, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Gabor, F.; Bogner, E.; Weissenboeck, A.; Wirth, M. The lectin–cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, F.H.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z.Y. Biodegradable glycopolymer-b-poly(ε-caprolactone) block copolymer micelles: Versatile construction, tailored lactose functionality, and hepatoma-targeted drug delivery. J. Mater. Chem. B 2015, 3, 2308–2317. [Google Scholar] [CrossRef]
- Zhou, W.; Dai, X.H.; Dong, C.M. Biodegradable and biomimetic poly(ε-caprolactone)/poly(lactobionarnidoethyl methacrylate) biohybrids: Synthesis, lactose-installed nanoparticles and recognition properties. Macromol. Biosci. 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, R.; Pippig, F.; Nilles, K.; Theato, P.; Kikkeri, R.; Maglinao, M.; Lepenies, B.; Seeberger, P.H.; Borner, H.G. Modular approach toward bioactive fiber meshes carrying oligosaccharides. Macromolecules 2010, 43, 9239–9247. [Google Scholar] [CrossRef]
- Eberhardt, M.; Mruk, R.; Zentel, R.; Théato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: New precursor polymers for the synthesis of multifunctional materials. Eur. Polym. J. 2005, 41, 1569–1575. [Google Scholar] [CrossRef]
- Stickler, D.J.; Morgan, S.D. Modulation of crystalline proteus mirabilis biofilm development on urinary catheters. J. Med. Microbiol. 2006, 55, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Von der Ehe, C.; Weber, C.; Gottschaldt, M.; Schubert, U.S. Immobilized glycopolymers: Synthesis, methods and applications. Prog. Polym. Sci. 2016, 57, 64–102. [Google Scholar] [CrossRef]
- Xu, L.Q. Ruthenium(II)-terpyridine complexes-containing glyconanoparticles for one- and two-photon excited fluorescence imaging. Eur. Polym. J. 2015, 71, 279–288. [Google Scholar] [CrossRef]
- Xu, L.Q.; Huang, C.; Wang, R.; Neoh, K.G.; Kang, E.T.; Fu, G.D. Synthesis and characterization of fluorescent perylene bisimide-containing glycopolymers for escherichia coli conjugation and cell imaging. Polymer 2011, 52, 5764–5771. [Google Scholar] [CrossRef]
- Lee, Y.; Hanif, S.; Theato, P.; Zentel, R.; Lim, J.; Char, K. Facile synthesis of fluorescent polymer nanoparticles by covalent modification-nanoprecipitation of amine-reactive ester polymers. Macromol. Rapid Common. 2015, 36, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Q.; Chen, J.C.; Qian, S.S.; Zhang, A.K.; Fu, G.D.; Li, C.M.; Kang, E.T. Pegylated metalloporphyrin nanoparticles as a promising catalyst for the heterogeneous oxidation of cyclohexene in water. Macromol. Chem. Phys. 2015, 216, 417–426. [Google Scholar] [CrossRef]
- Xu, L.Q.; Jiang, H.; Neoh, K.G.; Kang, E.T.; Fu, G.D. Poly(dopamine acrylamide)-co-poly(propargyl acrylamide)-modified titanium surfaces for ‘click’ functionalization. Polym. Chem. 2012, 3, 920–927. [Google Scholar] [CrossRef]
- Sun, P.J.; Zhang, Y.; Shi, L.Q.; Gan, Z.H. Thermosensitive nanoparticles self-assembled from pcl-b-peo-b-pnipaam triblock copolymers and their potential for controlled drug release. Macromol. Biosci. 2010, 10, 621–631. [Google Scholar] [CrossRef] [PubMed]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconj. Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.; Rapoport, N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Su, Y.; Jin, C.Y.; Zhu, B.S.; Pang, Y.; Zhu, L.J.; Liu, J.Y.; Tu, C.L.; Yan, D.Y.; Zhu, X.Y. Supramolecular copolymer micelles based on the complementary multiple hydrogen bonds of nucleobases for drug delivery. Biomacromolecules 2011, 12, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.R.S.; Chen, G.J.; Stenzel, M.H. Synthesis of glycopolymers and their multivalent recognitions with lectins. Polym. Chem. 2010, 1, 1392–1412. [Google Scholar] [CrossRef]
- Krimmer, S.G.; Pan, H.Z.; Liu, J.H.; Yang, J.Y.; Kopecek, J. Synthesis and characterization of poly(ε-caprolactone)-block-poly n-(2-hydroxypropyl)methacrylamide micelles for drug delivery. Macromol. Biosci. 2011, 11, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Valimaa, T.; Laaksovirta, S. Degradation behaviour of self-reinforced 80L/20G PLGA devices in vitro. Biomaterials 2004, 25, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.; Francois, C.; Kedzierewicz, F.; Preat, W.; Hoffman, M.; Maincent, P. Stability study of nanoparticles of poly(ε-caprolactone), poly(d,l-lactide) and poly(d,l-lactide-co-glycolide). Biomaterials 1996, 17, 2191–2197. [Google Scholar] [CrossRef]
- Liu, Y.; Steele, T.; Kissel, T. Degradation of hyper-branched poly(ethylenimine)-graft-poly(caprolactone)-block-monomethoxyl-poly (ethylene glycol) as a potential gene delivery vector. Macromol. Rapid Commun. 2010, 31, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Bhaw-Luximon, A.; Meeram, L.M.; Jugdawa, Y.; Helbert, W.; Jhurry, D. Oligoagarose-g-polycaprolactone loaded nanoparticles for drug delivery applications. Polym. Chem. 2011, 2, 77–79. [Google Scholar] [CrossRef]
- Wang, X.-L.; Huang, Y.; Zhu, J.; Pan, Y.-B.; He, R.; Wang, Y.-Z. Chitosan-graft-poly(p-dioxanone) copolymers: Preparation, characterization, and properties. Carbohydr. Res. 2009, 344, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Takvorian, P.M.; Cali, A.; Orr, G.; Weiss, L.M. Glycosylation of the major polar tube protein of encephalitozoon hellem, a microsporidian parasite that infects humans. Infect. Immun. 2004, 72, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, X.; Hu, N.; Tam, U.C.; Blixt, O.; Zettl, A.; Bertozzi, C.R. Biocompatible carbon nanotubes generated by functionalization with glycodendrimers. Angew. Chem. Int. Ed. 2008, 47, 5022–5025. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.N.; Cai, X.Y.; Chen, J.C.; Hu, X.F.; Xu, L.Q. Conjugation of Lectin to Poly(ε-caprolactone)-block-glycopolymer Micelles for In Vitro Intravesical Drug Delivery. Polymers 2016, 8, 379. https://doi.org/10.3390/polym8110379
Li NN, Cai XY, Chen JC, Hu XF, Xu LQ. Conjugation of Lectin to Poly(ε-caprolactone)-block-glycopolymer Micelles for In Vitro Intravesical Drug Delivery. Polymers. 2016; 8(11):379. https://doi.org/10.3390/polym8110379
Chicago/Turabian StyleLi, Ning Ning, Xiao Yan Cai, Jiu Cun Chen, Xue Feng Hu, and Li Qun Xu. 2016. "Conjugation of Lectin to Poly(ε-caprolactone)-block-glycopolymer Micelles for In Vitro Intravesical Drug Delivery" Polymers 8, no. 11: 379. https://doi.org/10.3390/polym8110379




