Emerging Multifunctional NIR Photothermal Therapy Systems Based on Polypyrrole Nanoparticles
Abstract
:1. Introduction
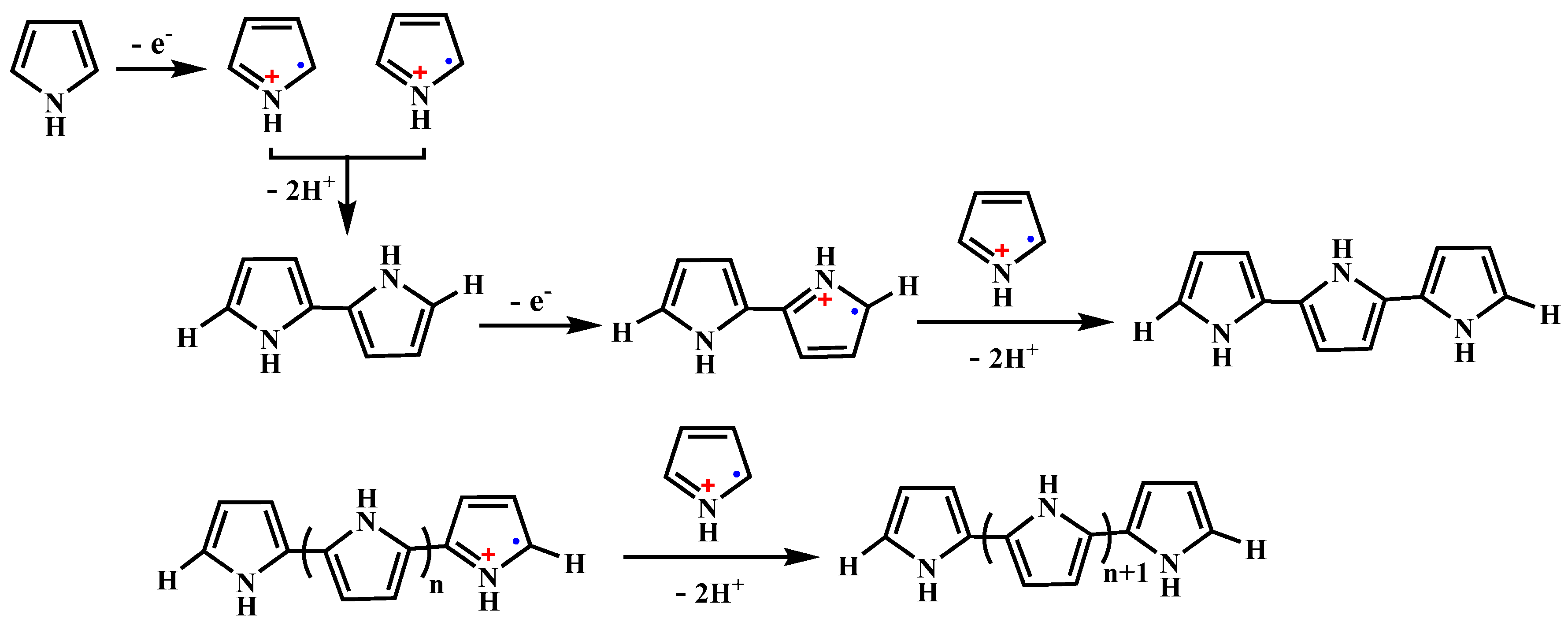
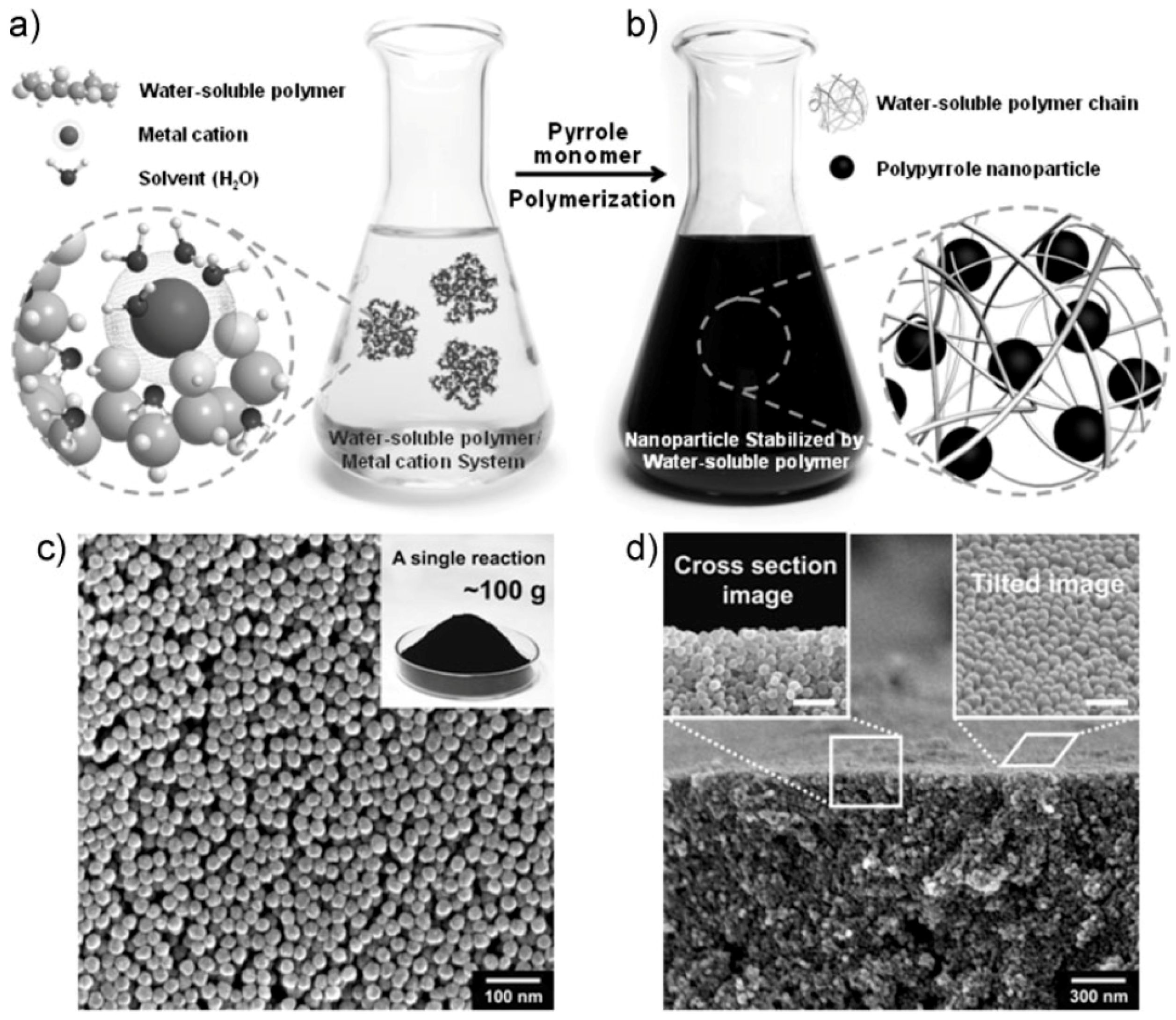
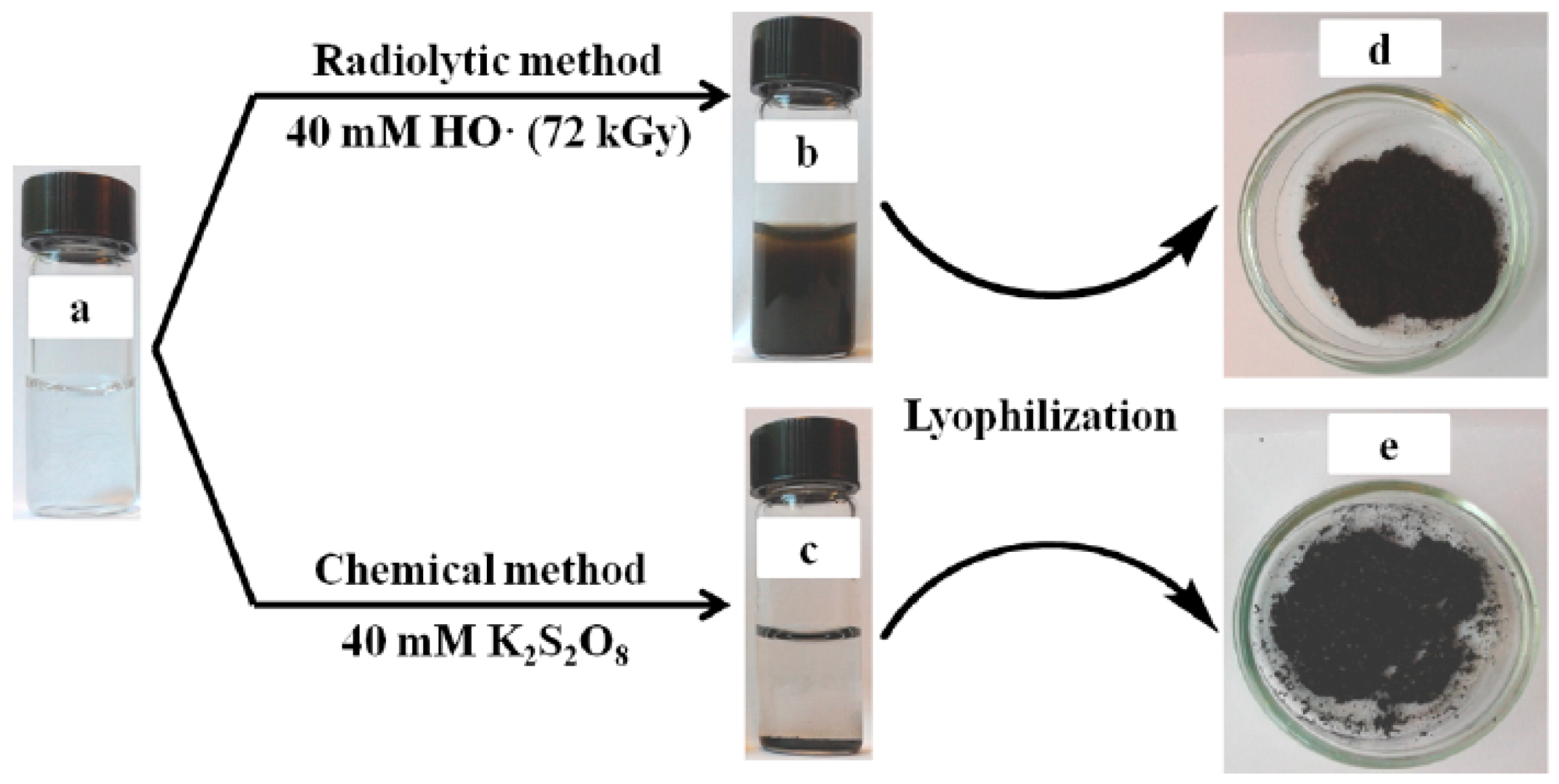
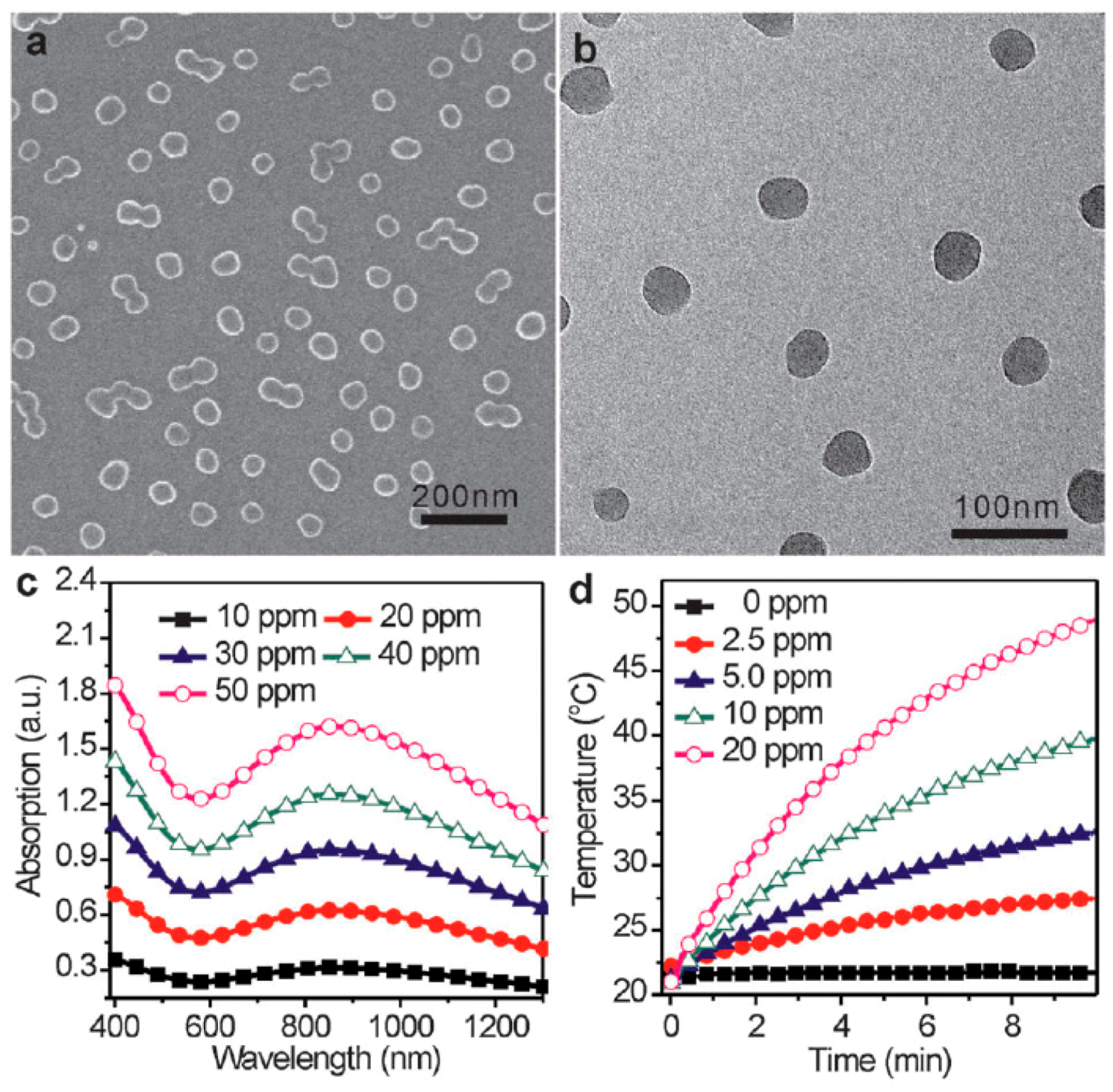
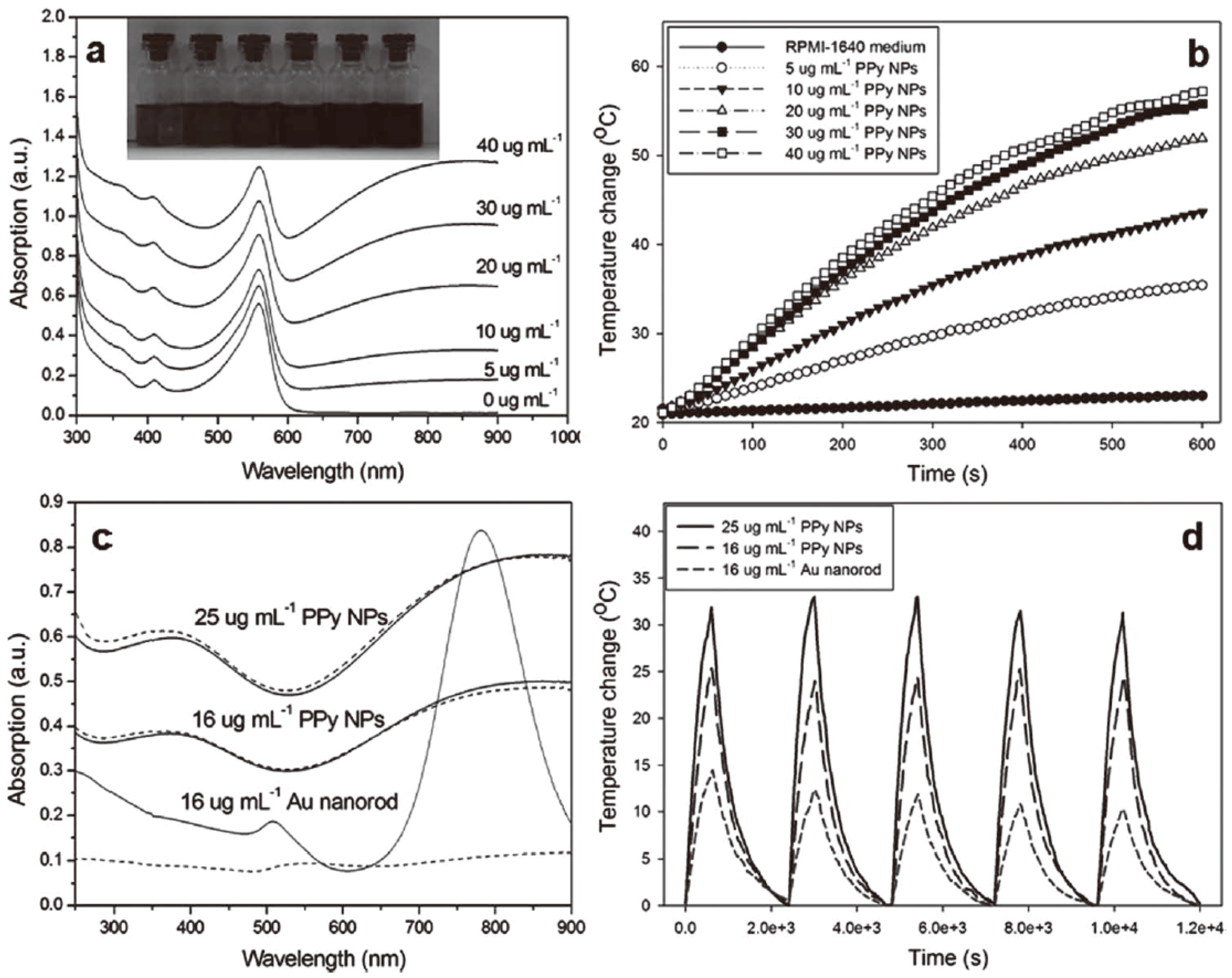
2. Synthesis and NIR-Triggered Photothermal Conversion Performance of PPy Nanoparticles (PPy NPs)
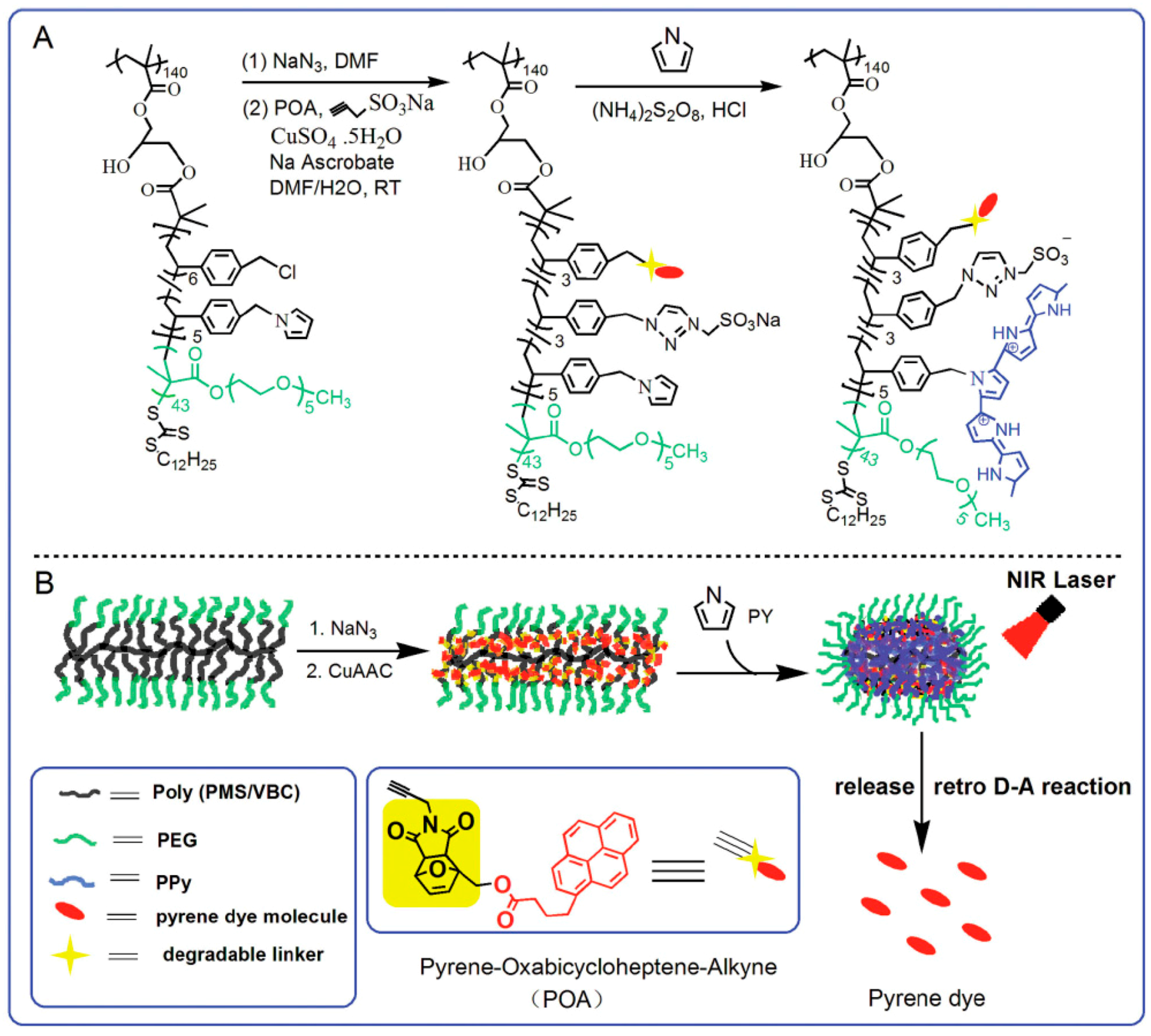
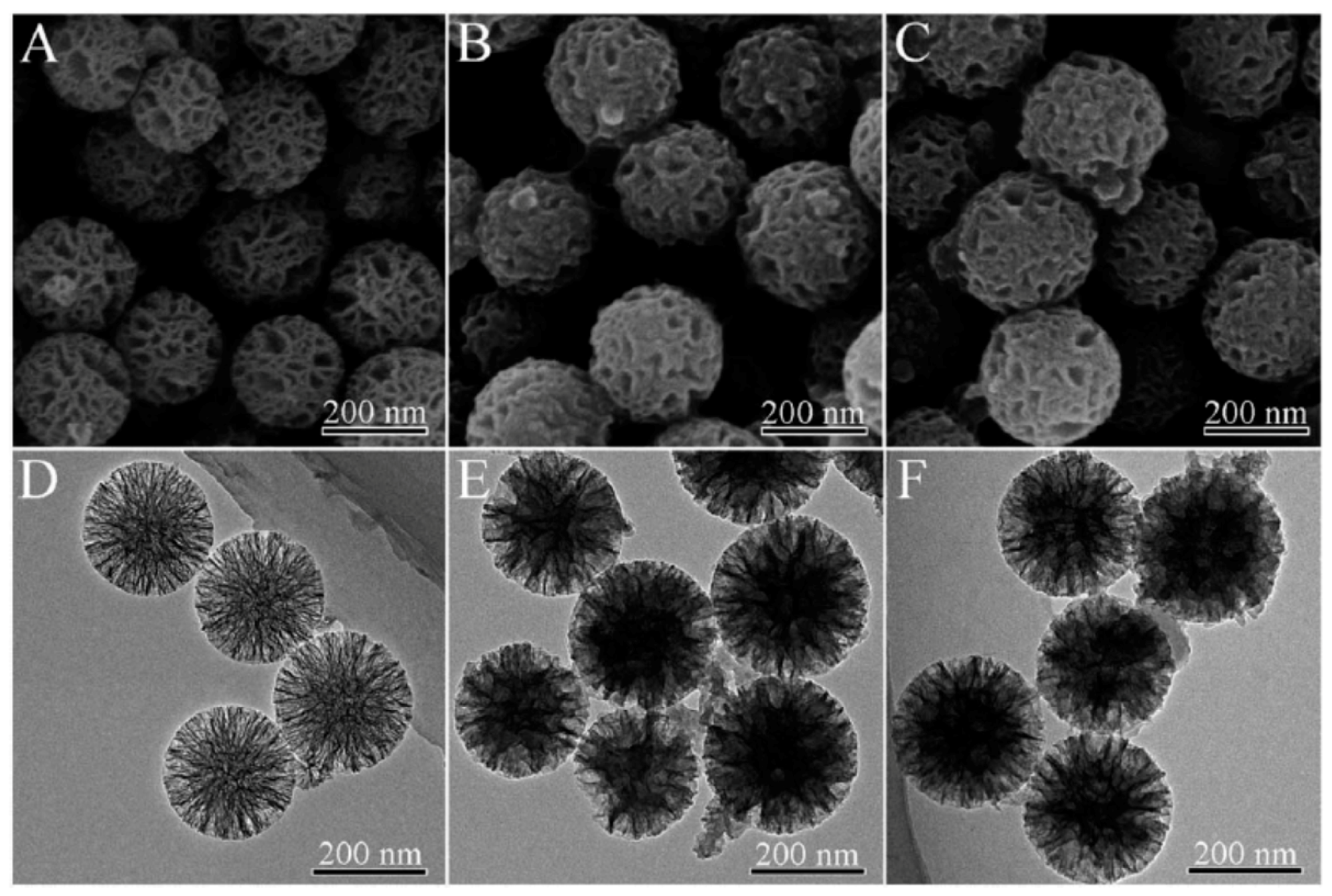
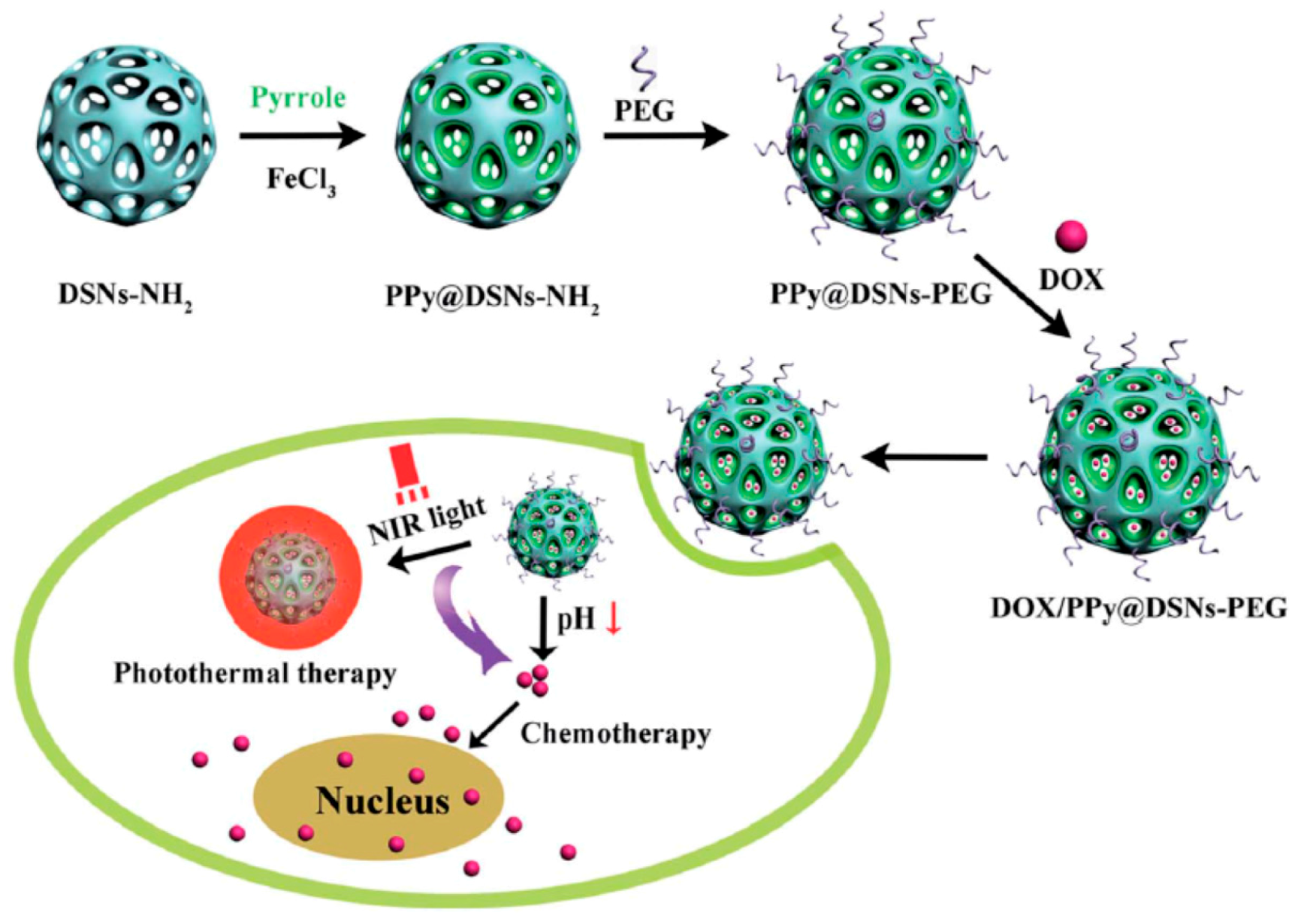
3. Multifunctional PPy-Based Therapy Platforms with NIR-Triggered PTT Effect
4. Conclusions and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Lee, S.M.; Ahn, R.W.; Chen, F.; Fought, A.J.; O’Halloran, T.V.; Cryns, V.L.; Nguyen, S.T. Biological evaluation of pH-responsive polymer-caged nanobins for breast cancer therapy. ACS Nano 2010, 4, 4971–4978. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, X.Z.; Ouyang, J.; Kong, J.M.; Zhong, W.; Xing, M.M.Q. pH and reduction dual-sensitive copolymeric micelles for intracellular doxorubicin delivery. Biomacromolecules 2011, 12, 3601–3611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Qian, Y.F.; Liu, T.; Hu, X.L.; Zhang, G.Y.; You, Y.Z.; Liu, S.Y. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 2011, 32, 6595–6605. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, J.Y.; Su, Y.; Wu, J.L.; Zhu, L.J.; Zhu, X.Y.; Yan, D.Y.; Zhu, B.S. Design and synthesis of thermo-responsive hyperbranched poly(amine-ester)s as acid-sensitive drug carriers. Polym. Chem. 2011, 2, 1661–1670. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T. Multi-targeting cancer chemotherapy using temperature-responsive drug carrier systems. React. Funct. Polym. 2012, 71, 235–244. [Google Scholar] [CrossRef]
- De la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I. Multiple stimuli-responsive hydrogels for metal-based drug therapy. Polymers 2012, 4, 964–985. [Google Scholar] [CrossRef]
- Lee, S.M.; Nguyen, S.T. Smart nanoscale drug delivery platforms from stimuli-responsive polymers and liposomes. Macromolecules 2013, 46, 9169–9180. [Google Scholar] [CrossRef]
- Sharma, M.; Waterhouse, G.I.N.; Loader, S.W.C.; Garg, S.; Svirskis, D. High surface area polypyrrole scaffolds for tunable drug delivery. Int. J. Pharm. 2013, 443, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Zhang, G.Y.; Ge, Z.S.; Liu, S.Y. Tumor-targeted redox-responsive nonviral gene delivery nanocarriers based on neutral-cationic brush block copolymers. Macromol. Rapid Commun. 2014, 35, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Cui, Z.R.; Yu, P.C.; Guo, C.Y.; Feng, B.; Jiang, T.Y.; Wang, S.L.; Yin, Q.; Zhong, D.F.; Yang, X.L.; et al. pH- and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S.Q. Polymeric-based particulate systems for delivery of therapeutic proteins. Pharm. Dev. Technol. 2016, 21, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Qiao, Z.Y.; Wang, H. Polymers with tertiary amine groups for drug delivery and bioimaging. Sci. China Chem. 2016, 59, 991–1002. [Google Scholar] [CrossRef]
- Lin, C.T.; Lin, I.C.; Sung, S.Y.; Su, Y.L.; Huang, Y.F.; Chiang, C.S.; Hu, S.H. Dual-targeted photopenetrative delivery of multiple micelles/hydrophobic drugs by a nanopea for enhanced tumor therapy. Adv. Funct. Mater. 2016, 26, 4169–4179. [Google Scholar] [CrossRef]
- Wang, L.H.; Wu, D.C.; Xu, H.X.; You, Y.Z. High DNA-binding affinity and gene-transfection efficacy of bioreducible cationic nanomicelles with a fluorinated core. Angew. Chem. Int. Ed. 2016, 55, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Kwon, O.S.; Jang, J. Conducting polymer nanomaterials for biomedical applications: Cellular interfacing and biosensing. Polym. Rev. 2013, 53, 407–442. [Google Scholar] [CrossRef]
- Francis, R.; Joy, N.; Aparna, E.; Vijayan, R. Polymer grafted inorganic nanoparticles, preparation, properties, and applications: A review. Polym. Rev. 2014, 54, 268–347. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, B.; Ma, X.J.; Liu, S.R.; Shang, X.D.; Yu, X.F. Tailor-made pH-responsive poly(choline phosphate) prodrug as a drug delivery system for rapid cellular internalization. Biomacromolecules 2016, 17, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.Z.; Cevenini, A.; Yazdi, I.K.; Parodi, A.; Evangelopoulos, M.; Corbo, C.; Scaria, S.; Hu, Y.; Haddix, S.G.; Corradetti, B.; et al. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials 2016, 87, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Ma, L.Y.; An, Y.X.; Li, Y.; Liu, Y.X.; Wang, L.; Guo, J.; Wang, J.H.; Zhou, J. Thermoresponsive nanogel-encapsulated PEDOT and HSP70 inhibitor for improving the depth of the photothermal therapeutic effect. Adv. Funct. Mater. 2016, 26, 4749–4759. [Google Scholar] [CrossRef]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T.K. Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.; Luo, Q.; Sun, L.; Li, X.; Zhu, H.Y.; Guan, P.J.; Wu, M.; Luo, K.; Gong, Q.Y. Enzyme- and pH-sensitive branched polymer-doxorubicin conjugate-based nanoscale drug delivery system for cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 11765–11778. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.Y.; Deng, H.P.; Su, Y.; He, L.; Wang, R.B.; Tong, G.S.; He, D.N.; Zhu, X.Y. Aptamer-functionalized and backbone redox-responsive hyperbranched polymer for targeted drug delivery in cancer therapy. Biomacromolecules 2016, 17, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Bu, Y.Z.; Zhang, L.N.; Wang, X.; Yang, Y.Y.; Zhuang, Y.P.; Yang, F.; Shen, H.; Wu, D.C. Facile construction of pH- and redox-responsive micelles from a biodegradable poly(β-hydroxyl amine) for drug delivery. Biomacromolecules 2016, 17, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; He, C.Y.; Tan, L.J.; Liu, B.Y.; Zhu, Z.G.; Gong, B.; Shen, Y.M.; Shao, Z.F. Synthesis and micellization of redox-responsive dynamic covalent multi-block copolymers. Polym. Chem. 2016, 7, 3145–3155. [Google Scholar] [CrossRef]
- Staruch, R.; Chopra, R.; Hynynen, K. Hyperthermia in bone generated with MR imaging–controlled focused ultrasound: Control strategies and drug delivery. Radiology 2012, 263, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Tovar-Carrillo, K.; Kobayashi, T. Ultrasound stimulated release of mimosa medicine from cellulose hydrogel matrix. Ultrason. Sonochem. 2016, 32, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, P.; He, Y.P.; Bo, X.W.; Li, X.L.; Li, D.D.; Chen, H.R.; Xu, H.X. Synergistic retention strategy of RGD active targeting and radiofrequency-enhanced permeability for intensified RF & chemotherapy synergistic tumor treatment. Biomaterials 2016, 99, 34–46. [Google Scholar] [PubMed]
- Marturano, V.; Cerruti, P.; Carfagna, C.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Photo-responsive polymer nanocapsules. Polymer 2015, 70, 222–230. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Xu, S.D.; Zhang, C.J.; Liu, B. Light-responsive AIE nanoparticles with cytosolic drug release to overcome drug resistance in cancer cells. Polym. Chem. 2016, 7, 3530–3539. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, Y.; Xie, M.; Wang, J.; Yan, X.W.; Li, Z.; Dong, W.G.; Huang, W.H. Near-infrared light-responsive hydrogel for specific recognition and photothermal site-release of circulating tumor cells. ACS Nano 2016, 10, 6201–6210. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.G.; Yu, J.C.; Chen, Y.L.; Hu, Q.Y.; Xiao, X.Z.; Sun, W.J.; Wang, C.; Feng, P.J.; Shen, Q.D.; Gu, Z. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gao, W.X.; Peng, X.Y.; Deng, X.; Sun, C.Z.; Wu, H.Y.; He, B. Near infrared light responsive hybrid nanoparticles for synergistic therapy. Biomaterials 2016, 100, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Nappini, S.; Fogli, S.; Castroflorio, B.; Bonini, M.; Bombelli, F.B.; Baglioni, P. Magnetic field responsive drug release from magnetoliposomes in biological fluids. J. Mater. Chem. B 2016, 4, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.F.; Odelius, K.; Edlund, U.; Zhao, C.S.; Albertsson, A.C. In situ synthesis of magnetic field-responsive hemicellulose hydrogels for drug delivery. Biomacromolecules 2016, 16, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.S.; Qu, X.G. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Khaletskaya, K.; Reboul, J.; Meilikhov, M.; Nakahama, M.; Diring, S.; Tsujimoto, M.; Isoda, S.; Kim, F.; Kamei, K.I.; Fischer, R.A.; et al. Integration of porous coordination polymers and gold nanorods into core-shell mesoscopic composites toward light-induced molecular release. J. Am. Chem. Soc. 2013, 135, 10998–11005. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.C.; Ninh, C.; Bettinger, C.J. Photoreconfigurable polymers for biomedical applications: Chemistry and macromolecular engineering. Biomacromolecules 2014, 15, 3474–3494. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, G.E.; Sosnofsky, C.T.; Ostrowski, A.D. Light-responsive iron(III)-polysaccharide coordination hydrogels for controlled delivery. ACS Appl. Mater. Interfaces 2015, 7, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.J.; Liao, W.C.; Sohn, Y.S.; Nechushtai, R.; Lu, C.H.; Willner, I. Light-responsive and pH-responsive DNA microcapsules for controlled release of loads. J. Am. Chem. Soc. 2016, 138, 8936–8945. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Shin, E.; Kim, B.S. Light-responsive micelles of spiropyran initiated hyperbranched polyglycerol for smart drug delivery. Biomacromolecules 2014, 15, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Lee, J.; Kim, J.; Kim, W.J. Visible light-induced singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy. Chem. Commun. 2015, 51, 9995–9998. [Google Scholar] [CrossRef] [PubMed]
- Szacilowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef] [PubMed]
- Carling, C.J.; Boyer, J.C.; Branda, N.R. Remote-control photoswitching using NIR light. J. Am. Chem. Soc. 2009, 131, 10838–10839. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Technol. 2014, 9, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.B.; Liu, J.J.; Wu, Y.F.; Feng, L.Z.; Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016. [Google Scholar] [CrossRef]
- Yavuz, M.S.; Cheng, Y.Y.; Chen, J.Y.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.W.; Kim, C.; Song, K.H.; Schwartz, A.G.; et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Welsher, K.; Tabakman, S.M.; Sherlock, S.P.; Wang, H.L.; Luong, R.; Dai, H.J. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010, 3, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Z.; Li, K.Y.; Shi, X.Z.; Gao, M.; Liu, J.; Liu, Z. Smart pH-responsive nanocarriers based on nano-graphene oxide for combined chemo- and photothermal therapy overcoming drug resistance. Adv. Healthc. Mater. 2014, 3, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.W.; Tang, M.H.; Sun, Y.G.; Zou, R.J.; Chen, Z.G.; Zhu, M.F.; Yang, S.P.; Wang, J.L.; Wang, J.H.; Hu, J.Q. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 2011, 23, 3542–3547. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.J.; Gu, X.; Gong, H.; Shi, X.Z.; Liu, T.; Wang, C.; Wang, X.Y.; Liu, G.; Xing, H.Y.; et al. PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.Z.; Feng, L.Z.; Sun, B.Q.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, K.; Lee, S.; Kim, D.H.; Lee, H. Near-infrared light-responsive nanomaterials for cancer theranostics. Adv. Rev. 2016, 8, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.Y.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 27, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Zhang, L.; Zhu, Y.; Shen, X.C.; Ji, S.C.; Tan, X.Y.; Cheng, L.; Liang, H. Water-soluble hyaluronic acid-hybridized polyaniline nanoparticles for effectively targeted photothermal therapy. J. Mater. Chem. B 2015, 3, 3767–3776. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, S.; Tian, Y.; Jiang, X.G.; Fu, D.L.; Shen, S.; Yang, W.L. Polydopamine-coated magnetic composite particles with an enhanced photothermal effect. ACS Appl. Mater. Interfaces 2015, 7, 15876–15884. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Lei, S.; Xie, Y.Y.; Wang, M.Z.; Yang, J.; Ge, X.W. Fabrication of high-performance magnetic lysozyme-imprinted microsphere and its NIR-responsive controlled release property. ACS Appl. Mater. Interfaces 2015, 7, 28606–28615. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, C.X.; Cheng, Z.Y.; Hou, Z.Y.; Huang, S.S.; Lin, J. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater. Sci. 2016, 4, 890–909. [Google Scholar] [CrossRef] [PubMed]
- McNeil, R.; Siudak, R.; Wardlawa, J.H.; Weiss, D.E. Electronic conduction in polymers I. The chemical structure of polypyrrole. Aust. J. Chem. 1963, 16, 1053–1075. [Google Scholar] [CrossRef]
- Dall’Olio, A.; Dascola, G.; Vacara, V.; Bocchi, V. Resonance paramagnetique electronique et conductivité d’un noir d’oxypyrrole electrolytique. C. R. Acad. Sci. C Paris 1968, 267, 433–435. [Google Scholar]
- Nord´en, B.; Krutmeijer, E. The Nobel Prize in Chemistry, 2000: Conductive Polymers (Advanced Information); The Royal Swedish Academy of Science: Stockholm, Sweden, 2000. [Google Scholar]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, A.; Bratengeier, C.; Gelmi, A.; Semeins, C.M.; Klein-Nulend, J.; Jager, E.W.H.; Bakker, A.D. Biocompatibility of polypyrrole with human primary osteoblasts and the effect of dopants. PLoS ONE 2015, 10, e0134023. [Google Scholar] [CrossRef] [PubMed]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, S.S.; Li, C.Y.; Qian, M.; Yan, X.Y.; Yao, S.C.; Peng, X.Y.; Wang, Y.; Huang, R.Q. Photothermal combined gene therapy achieved by polyethyleneimine-grafted oxidized mesoporous carbon nanospheres. Biomaterials 2016, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- An, X.N.; Zhu, A.J.; Luo, H.H.; Ke, H.T.; Chan, H.B.; Zhao, Y.L. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 10, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.B.; Deng, Z.J.; Li, Y.Y.; Li, C.H.; Wang, J.R.; Wang, S.M.; Qu, E.; Dai, Z.F. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 4, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, G.; Gohlke, U.; Ulrich, H. Dispersion polymerisation. Die Makromol. Chem. 1979, 3, 177–193. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Rodríguez, I.; Scharifker, B.R.; Mostany, J. In situ FTIR study of redox and overoxidation processes in polypyrrole films. J. Electroanal. Chem. 2000, 491, 117–125. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M. Electrosynthesized polymers for biosensing. Chem. Soc. Rev. 2011, 40, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, M.; Lin, X.Y.; Zhang, C.T.; Wei, H.T.; Zhang, H.; Yang, B. Polypyrrole-enveloped Pd and Fe3O4 nanoparticle binary hollow and bowl-like superstructures as recyclable catalysts for industrial wastewater treatment. ACS Appl. Mater. Interfaces 2014, 5, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.Y.; Ho, P.; Zhang, Z.G.; Ng, G.M.; Yao, K.; Guo, Z.B. Polypyrrole:FeOx·ZnO nanoparticle solar cells with breakthrough open-circuit voltage prepared from relatively stable liquid dispersions. RSC Adv. 2014, 4, 58608–58614. [Google Scholar] [CrossRef]
- Armes, S.P. Optimum reaction conditions for the polymerization of pyrrole by iron(III) chloride in aqueous solution. Synth. Met. 1987, 20, 365–371. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Armes, S.P.; Vincent, B. Dispersions of electrically conducting polypyrrole particles in aqueous media. Eur. J. Chem. Soc. Chem. Commun. 1987, 288–290. [Google Scholar] [CrossRef]
- Woo, H.Y.; Jung, W.G.; Ihmb, D.W.; Kim, J.Y. Synthesis and dispersion of polypyrrole nanoparticles in polyvinylpyrrolidone emulsion. Synth. Met. 2010, 160, 588–591. [Google Scholar] [CrossRef]
- Hong, J.Y.; Yoon, H.; Jang, J. Kinetic study of the formation of polypyrrole nanoparticles in water-soluble polymer/metal cation systems: A light-scattering analysis. Small 2010, 5, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Endo, Y.; Takahashi, T.; Otake, K.; Shono, A. New method for the synthesis of polypyrrole particle using water/oil emulsion. J. Chem. Eng. Jpn. 2013, 46, 550–555. [Google Scholar] [CrossRef]
- Dainton, F.S.; Rowbottom, J. Primary radiation yield in liquid water. Nature 1952, 169, 370–371. [Google Scholar] [CrossRef]
- Cui, Z.P.; Coletta, C.; Dazzi, A.; Lefrançois, P.; Gervais, M.; Néron, S.; Remita, S. Radiolytic method as a novel approach for the synthesis of nanostructured conducting polypyrrole. Langmuir 2014, 30, 14086–14094. [Google Scholar] [CrossRef] [PubMed]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Chen, M.; Fang, F.L.; Tang, S.; Zheng, N.F. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem. Commun. 2012, 48, 8934–8936. [Google Scholar] [CrossRef] [PubMed]
- Au, K.M.; Lu, Z.H.; Matcher, S.J.; Armes, S.P. Polypyrrole nanoparticles: A potential optical coherence tomography contrast agent for cancer imaging. Adv. Mater. 2011, 23, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.T.; Jiang, F.R.; Zou, R.J.; Liu, Q.; Chen, Z.G.; Zhu, M.F.; Yang, S.P.; Wang, J.L.; Wang, J.H.; Hu, J.Q. Hydrophilic Cu9S5 nanocrystals: A photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Cheng, S.H.; Wang, Y.J.; Chen, Y.C.; Chen, N.T.; Souris, J.; Chen, C.T.; Mou, C.Y.; Yang, C.S.; Lo, L.W. Near-infrared mesoporous silica nanoparticles for optical imaging: Characterization and in vivo biodistribution. Adv. Funct. Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Zha, Z.B.; Yue, X.L.; Ren, Q.S.; Dai, Z.F. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Doanea, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2991. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.G.; Cheng, L.; Wang, C.; Peng, R.; Liu, Z. Conjugated polymers for photothermal therapy of cancer. Polym. Chem. 2014, 5, 1573–1580. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.Y.; Zhao, J.F.; Liu, X.G.; Bu, J.; Yang, X.Y.; Huang, R.Q. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.L.; Wang, L.; Cao, Y.; Ma, Y.F.; Shen, H.; Zhang, M.X.; Zhang, Z.J. Efficient cancer ablation by combined photothermal and enhanced chemo-therapy based on carbon nanoparticles/doxorubicin@SiO2 nanocomposites. Carbon 2016, 97, 35–44. [Google Scholar] [CrossRef]
- Chiang, W.L.; Lin, T.T.; Sureshbabu, R.; Chia, W.T.; Hsiao, H.C.; Liu, H.Y.; Yang, C.M.; Sung, H.W. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. J. Control. Release 2015, 199, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.W.; Zhang, B.X.; Zheng, C.X.; Ji, R.; Ren, X.Y.; Guo, F.F.; Sun, S.L.; Shi, J.J.; Zhang, H.L.; Zhang, Z.Z.; et al. The tumor-targeting core-shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J. Control. Release 2015, 220, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Liu, Z.M.; Li, S.Y.; Su, C.K.; Qiu, X.J.; Zhong, H.Q.; Guo, Z.Y. Fabrication of graphene and AuNP core polyaniline shell nanocomposites as multifunctional theranostic platforms for SERS real-time monitoring and chemo-photothermal therapy. Theranostics 2016, 6, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, L.F.; Liao, X.J.; Huang, K. Controlled-release system of small molecules triggered by the photothermal effect of polypyrrole. Macromol. Rapid Commun. 2016, 37, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.K.; Yang, F.D.; Xue, Y.K.; Wei, X.T.; Song, L.J.; Liu, X.Z. Polypyrrole confined in dendrimer-like silica nanoparticles for combined photothermal and chemotherapy of cancer. RSC Adv. 2016, 6, 38931–38942. [Google Scholar] [CrossRef]
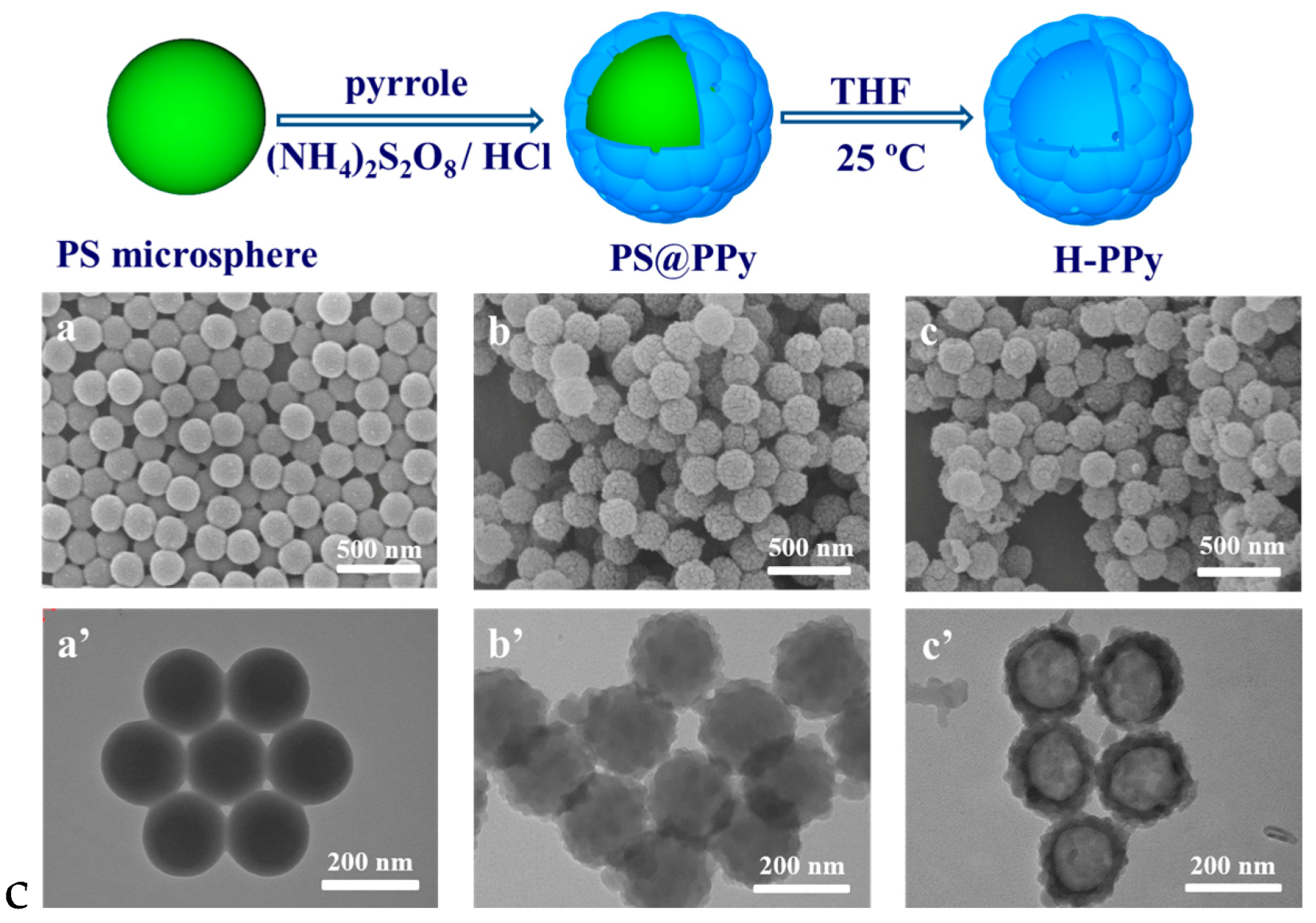
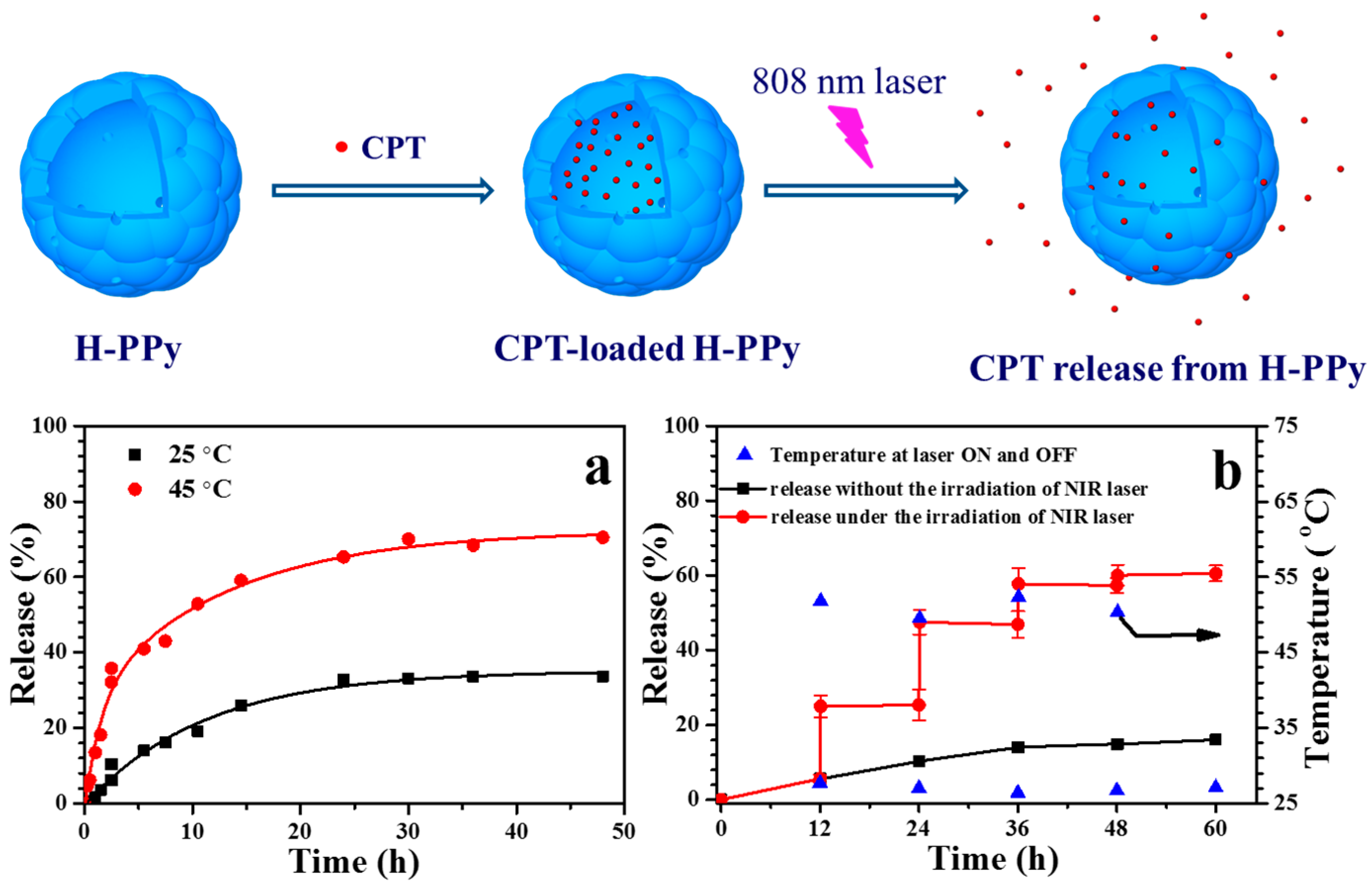
- Wang, J.; Lin, F.X.; Chen, J.X.; Wang, M.Z.; Ge, X.W. The preparation, drug loading and in vitro NIR photothermal-controlled release behavior of raspberry-like hollow polypyrrole microspheres. J. Mater. Chem. B 2015, 3, 9186–9193. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Tang, R.K. Spindle-like polypyrrole hollow nanocapsules as multifunctional platforms for highly effective chemo-photothermal combination therapy of cancer cells in vivo. Chem. Eur. J. 2014, 20, 11826–11834. [Google Scholar] [CrossRef] [PubMed]
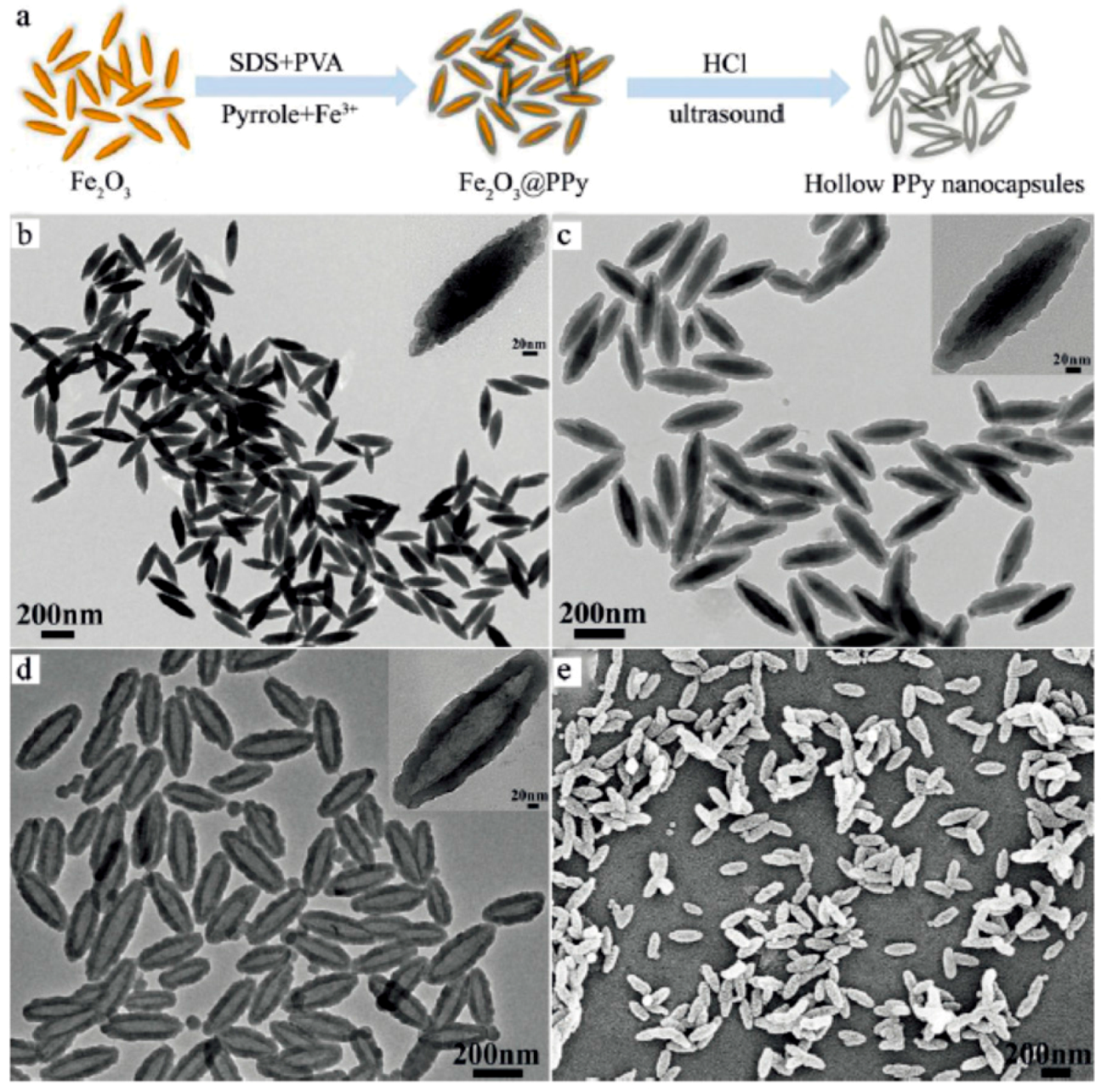
- Wang, C.; Xu, H.; Liang, C.; Liu, Y.M.; Li, Z.W.; Yang, G.B.; Cheng, H.; Li, Y.G.; Liu, Z. Iron oxide@polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 2013, 7, 6782–6795. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Khan, I.U.; Serra, C.A.; Messaddeq, N.; Jakhmola, A.; Vecchione, R.; Vandamme, T. One-step synthesis of iron oxide polypyrrole nanoparticles encapsulating ketoprofen as model of hydrophobic drug. Int. J. Pharm. 2016, 508, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Cheng, L.; Xiang, J.; Xu, H.; Feng, L.Z.; Shi, X.Z.; Liu, Z. Near-infrared absorbing polymeric nanoparticles as a versatile drug carrier for cancer combination therapy. Adv. Funct. Mater. 2013, 23, 6059–6067. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, Q.; Li, C.; Zou, R.J.; Li, B.; Song, G.S.; Xu, K.B.; Zheng, Y.; Hu, J.Q. Cu2-xSe@mSiO2-PEG core-shell nanoparticles: A low-toxic and efficient difunctional nanoplatform for chemo-photothermal therapy under near infrared light radiation with a safe power density. Nanoscale 2014, 6, 4361–4370. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Hu, Y.; Jiang, T.T.; Howard, K.A.; Li, Y.G.; Fan, X.L.; Sun, Y.; Besenbacher, F.; Yu, M. Human-serum-albumin-coated prussian blue nanoparticles as pH-/thermotriggered drug-delivery vehicles for cancer thermochemotherapy. Part. Part. Syst. Charact. 2016, 33, 53–62. [Google Scholar] [CrossRef]
- Forte, G.; Chiarotto, I.; Giannicchi, I.; Loreto, M.A.; Martinelli, A.; Micci, R.; Pepi, F.; Rossi, S.; Salvitti, C.; Stringaro, A.; et al. Characterization of naproxen-polymer conjugates for drug-delivery. J. Biomater. Sci. Polym. Ed. 2016, 27, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, B.Y.; Liang, J.; Bi, J.X.; Dai, S.; Qiao, S.Z. Developing functionalized dendrimer-like silica nanoparticles with hierarchical pores as advanced delivery nanocarriers. Adv. Mater. 2013, 25, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, J.; Li, W.W.; Rong, P.F.; Wang, Z.; Wang, S.J.; Wang, X.P.; Sun, X.L.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. Int. Ed. 2013, 52, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Chen, K.J.; Wu, T.H.; Wang, H.; Lin, W.Y.; Ohashi, M.; Chiou, P.Y.; Tseng, H.R. Photothermal effects of supramolecularly assembled gold colloids for targeted treatment of cancer cells. Angew. Chem. Int. Ed. 2010, 49, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Guo, C.R.; Li, J.; Zhou, D.; Liu, K.; Zhang, X.; Xu, T.S.; Zhang, H.; Wang, L.P.; Yang, B. Polypyrrole-coated chainlike gold nanoparticle architectures with the 808 nm photothermal transduction efficiency up to 70%. ACS Appl. Mater. Interfaces 2014, 6, 5860–5868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.W.; Li, T.T.; Lin, M.; Lin, X.Y.; Zhang, H.; Sun, H.C.; Yang, B. Composite photothermal platform of polypyrrole-enveloped Fe3O4 nanoparticle self-assembled superstructures. ACS Appl. Mater. Interfaces 2014, 6, 14552–14561. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyas, B. Nanoparticles in practice for molecular-imaging applications: An overview. Acta Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, H.J.; Chang, J. Progress in the field of constructing near-infrared light-responsive drug delivery platforms. J. Nanosci. Nanotechnol. 2016, 16, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
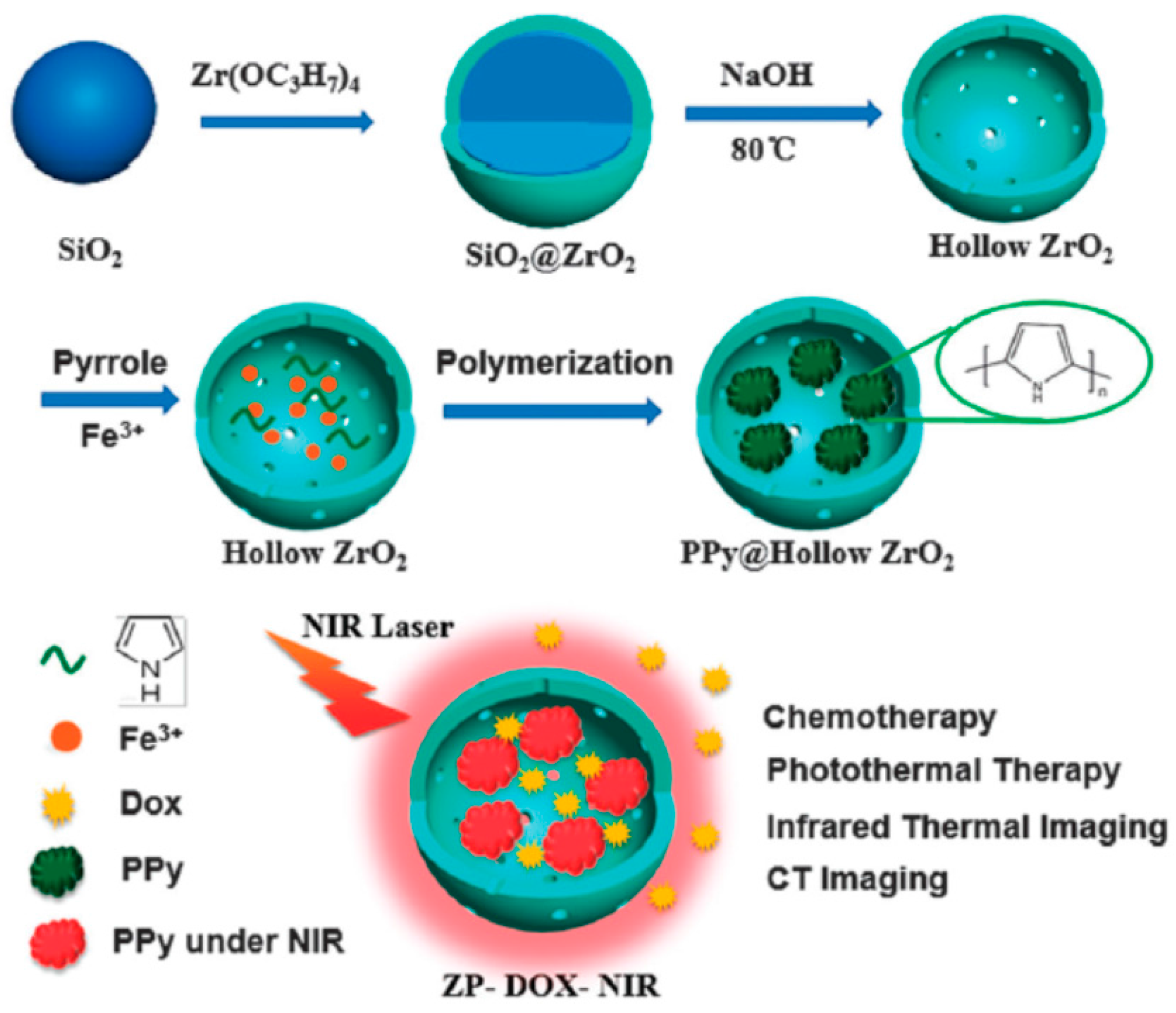
- Tan, L.F.; Liu, T.L.; Fu, C.H.; Wang, S.P.; Fu, S.Y.; Ren, J.; Meng, X.W. Hollow ZrO2/PPy nanoplatform for improved drug delivery and real-time CT monitoring in synergistic photothermal-chemo cancer therapy. J. Mater. Chem. B 2016, 4, 859–866. [Google Scholar] [CrossRef]
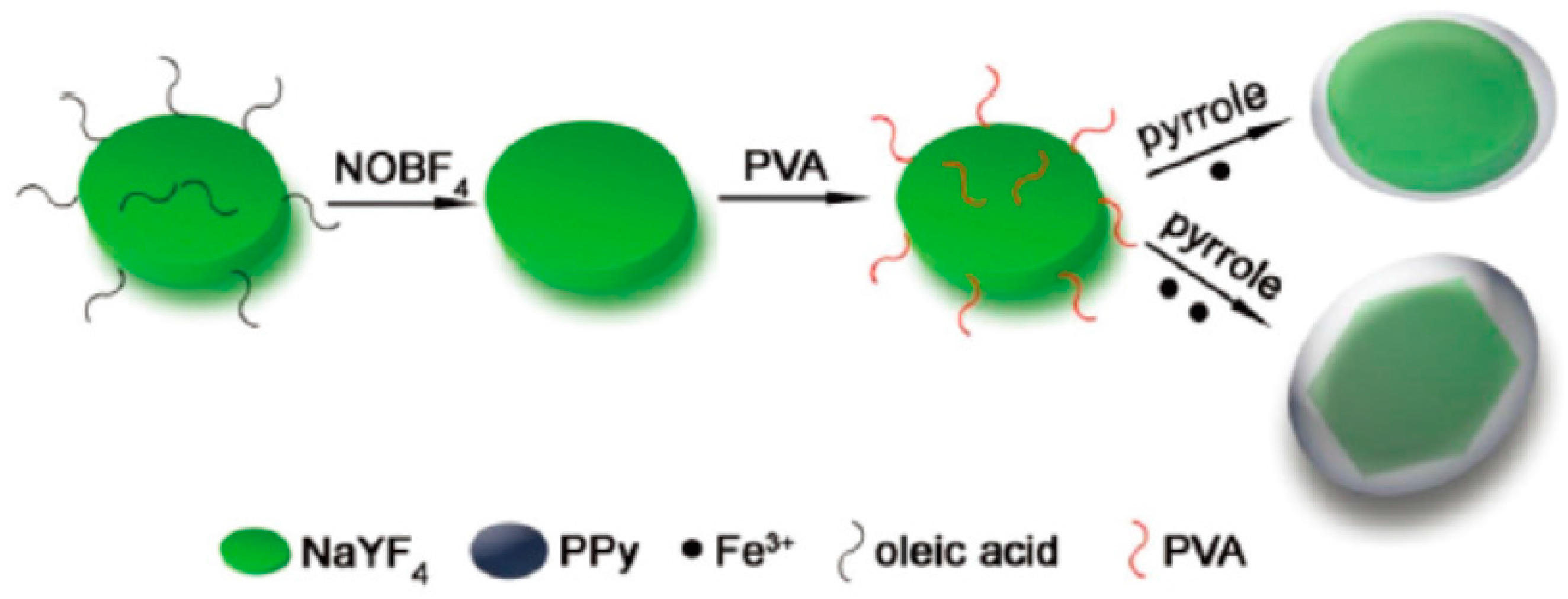
- Huang, X.J.; Li, B.; Peng, C.; Song, G.S.; Peng, Y.X.; Xiao, Z.Y.; Liu, X.J.; Yang, J.M.; Yu, L.; Hu, J.Q. NaYF4:Yb/Er@PPy core–shell nanoplates: An imaging-guided multimodal platform for photothermal therapy of cancers. Nanoscale 2016, 8, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Y.; Peng, C.; Jiang, X.H.; Peng, Y.X.; Huang, X.J.; Guan, G.Q.; Zhang, W.L.; Liu, X.M.; Qin, Z.Y.; Hu, J.Q. Polypyrrole-encapsulated iron tungstate nanocomposites: A versatile platform for multimodal tumor imaging and photothermal therapy. Nanoscale 2016, 8, 12917–12928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Wang, T.T.; Zhang, L.Y.; Li, L.; Wang, C.G. Near-infrared light and pH-responsive polypyrrole@polyacrylic acid/fluorescent mesoporous silica nanoparticles for imaging and chemo-photothermal cancer therapy. Chem. Eur. J. 2015, 21, 16162–16171. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ahn, K.O.; Jeong, K.C.; Choi, Y. Polypyrrole-based nanotheranostics for activatable fluorescence imaging and chemo/photothermal dual therapy of triplenegative breast cancer. Nanotechnology 2016, 27, 185102. [Google Scholar] [CrossRef] [PubMed]
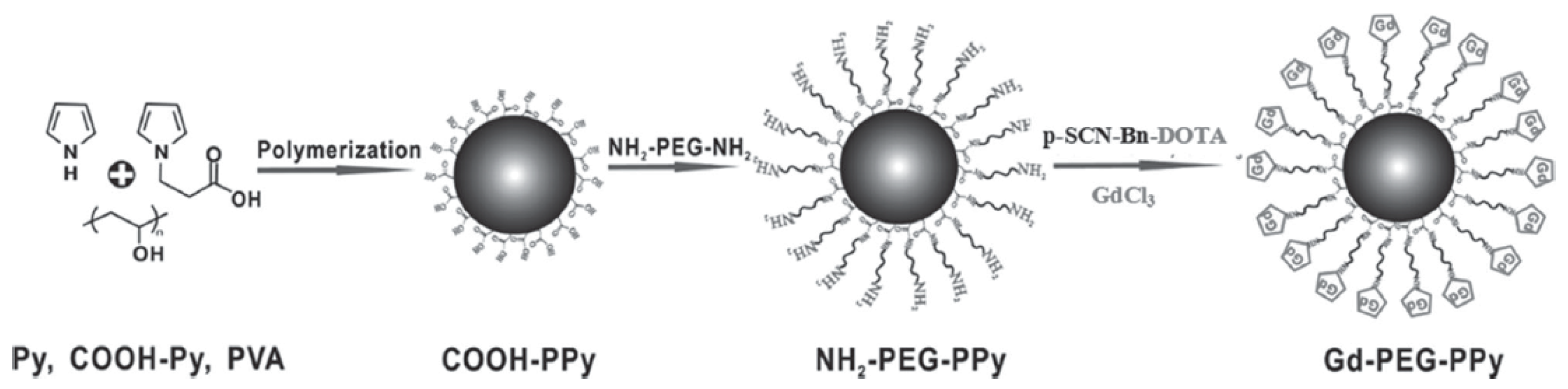
- Liang, X.L.; Li, Y.Y.; Li, X.D.; Jing, L.J.; Deng, Z.J.; Yue, X.L.; Li, C.H.; Dai, Z.F. PEGylated polypyrrole nanoparticles conjugating gadolinium chelates for dual-modal MRI/photoacoustic imaging guided photothermal therapy of cancer. Adv. Funct. Mater. 2015, 25, 1451–1462. [Google Scholar] [CrossRef]







© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M. Emerging Multifunctional NIR Photothermal Therapy Systems Based on Polypyrrole Nanoparticles. Polymers 2016, 8, 373. https://doi.org/10.3390/polym8100373
Wang M. Emerging Multifunctional NIR Photothermal Therapy Systems Based on Polypyrrole Nanoparticles. Polymers. 2016; 8(10):373. https://doi.org/10.3390/polym8100373
Chicago/Turabian StyleWang, Mozhen. 2016. "Emerging Multifunctional NIR Photothermal Therapy Systems Based on Polypyrrole Nanoparticles" Polymers 8, no. 10: 373. https://doi.org/10.3390/polym8100373




