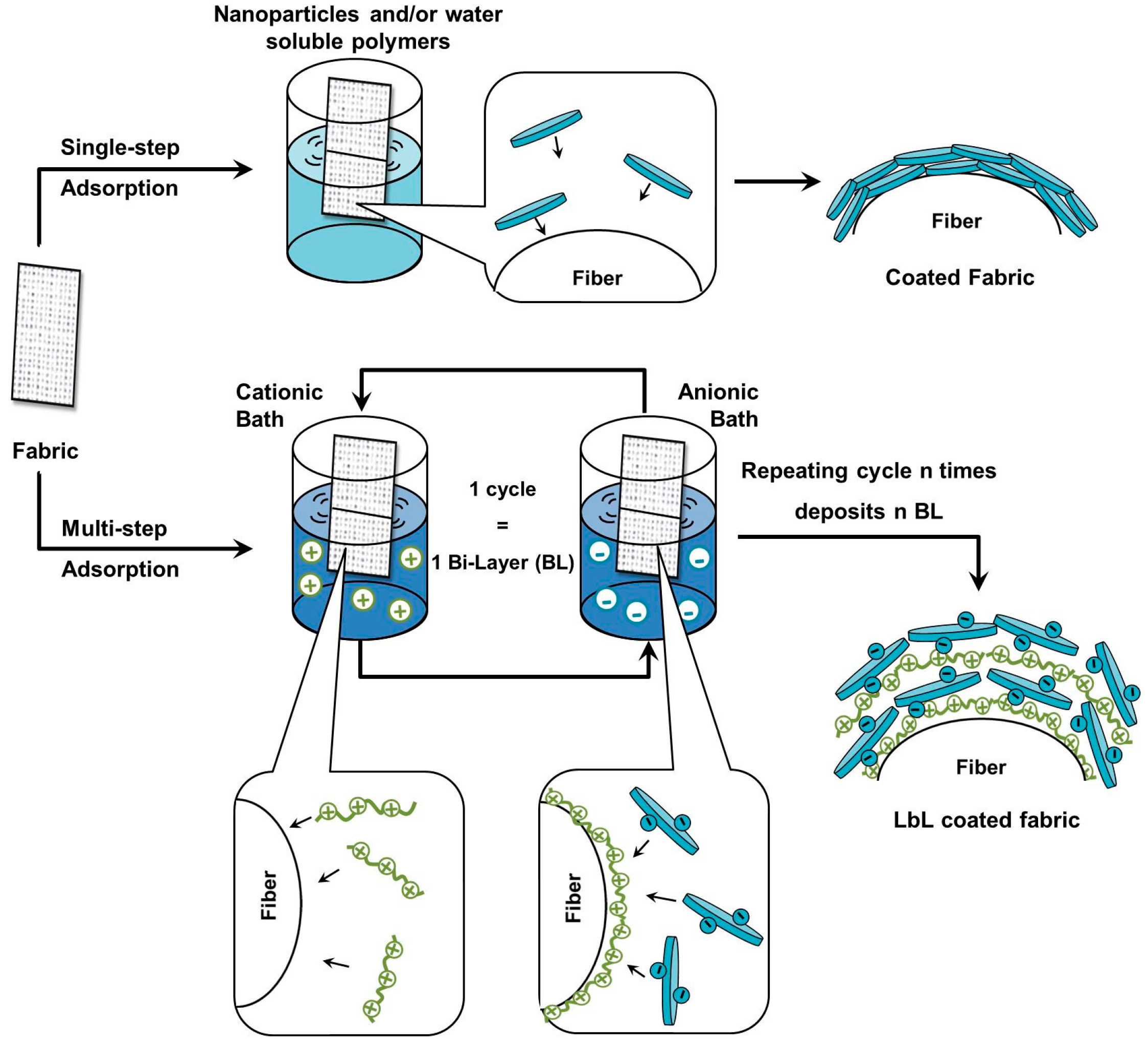
2.1.1. Polyester
As far as polyester fabrics are concerned, different NPs having either lamellar (e.g., montmorillonites (CNa), hydrotalcite (HT), and bohemite (OS)) or globular (silica, SiO
2), titania (TiO
2) and octapropylammonium polyhedral oligomeric silsesquioxane—POSS
®) shape were studied as potential FRs. The resulting properties were assessed by cone calorimetry under a 35 kW/m
2 heat flux. The collected data are summarised in
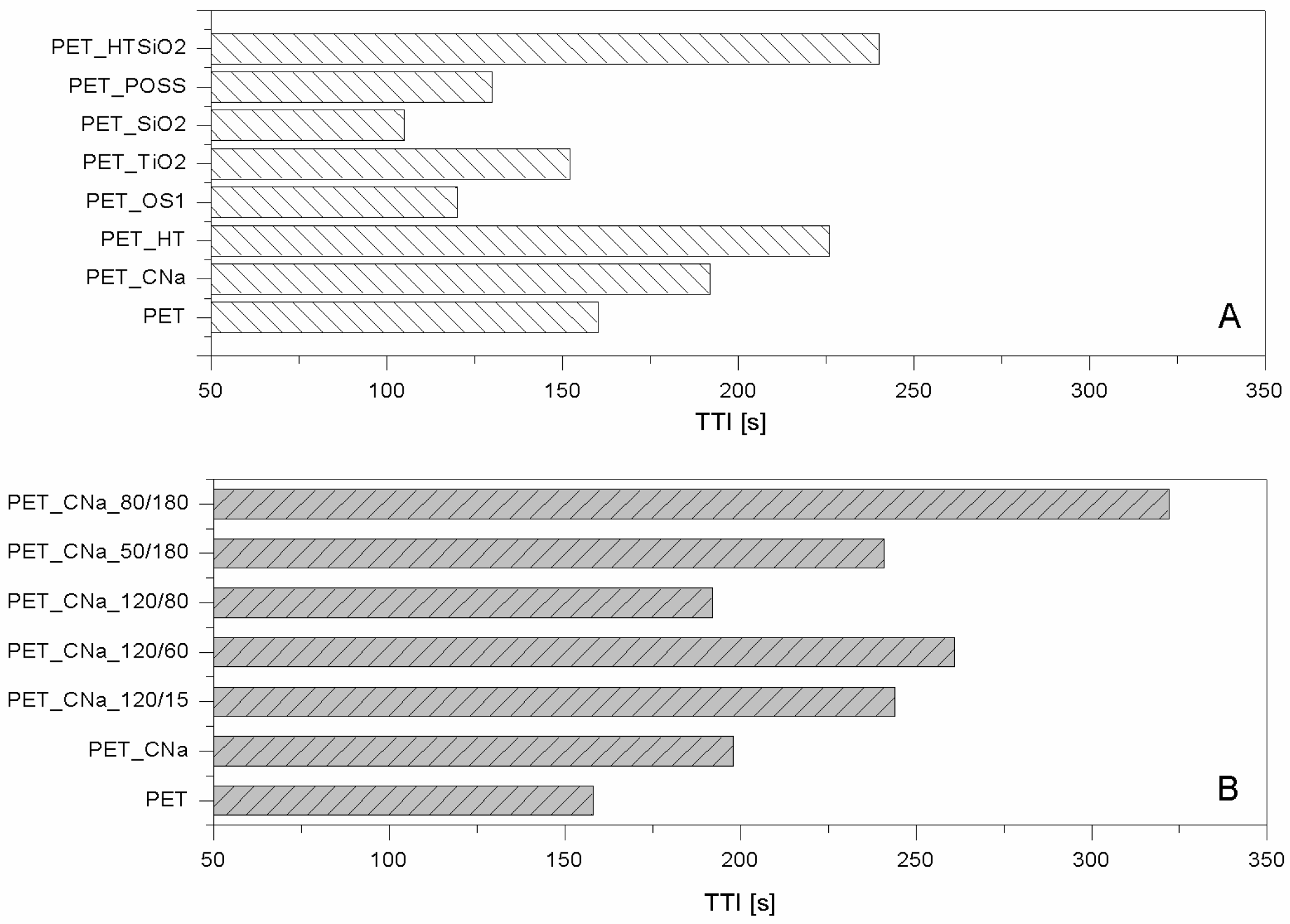
Table 1. CNa significantly increased PET Time to Ignition, TTI (192 vs. 160 s, respectively for PET_CNa vs. PET, see
Figure 2A) and reduced both Effective of Heat Combustion (EHC, 17.5 vs. 21.8 MJ/kg) and Heat Release Rate peak (PHRR, 80 vs. 90 kW/m
2) [
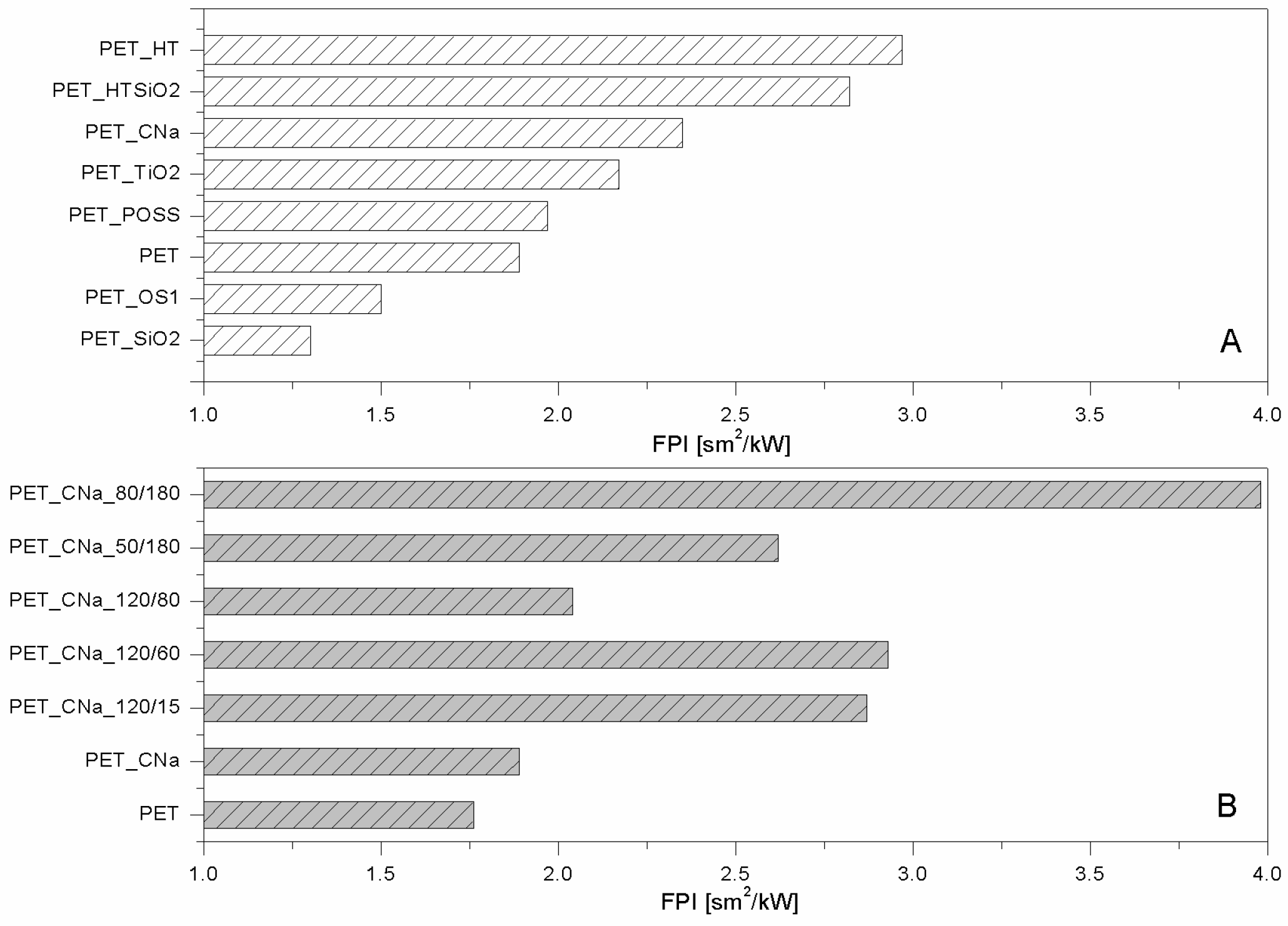
22]. As a consequence, Fire Performance Index (FPI) value of PET_CNa is higher than that of untreated PET (see
Figure 3A). This finding suggests that the PET combustion mechanism has been partially modified by the presence of a CNa monolayer deposited on the fabrics; indeed, such clay nanocoating acting as an insulating ceramic barrier somehow protected the polyester from heat, oxygen and mass transfer during combustion. Consequently, the ignition was postponed and the effective heat reduced as a lower amount of polymer burns. In addition, its presence caused a slight decrease of the CO and CO
2 yields. The Total Heat Release (THR) is almost constant within the experimental error.
The results already discussed have encouraged the pursuance of the approach of NP adsorption (and in particular the use of CNa due to its favourable aspect ratio and swelling properties in water), but have also highlighted the limitations derived from a deposition consisting of a single immersion step, in particular for non-hydrophilic substrates like polyester. Indeed, observing the morphology of CNa-treated fabrics (scanning electron microscopy—SEM—micrographs reported in ref. [
35]), it has been found that NPs do not completely and homogeneously cover PET fibres; as a consequence, the resulting flame retardant performances turned out to be strongly limited. Thus, in order to further increase the number of PET/CNa interactions, a pre-treatment etching of the fabric by cold oxygen plasma was carried out under different experimental conditions of power (50, 80, and 120 W) and time (15, 60, 80 and 180 s).
The results collected by cone calorimetry reported in
Table 2 show how much the plasma pre-treatment can improve CNa performances, by increasing both clay distribution densities on the surface and PET/CNa interactions. More specifically, the higher number of deposited NPs resulted in a drastic effect on PET TTI delay (see
Figure 2B), regardless of treatment power or time; despite this, the combustion kinetic parameters were not significantly affected (e.g., PHRR in
Table 2). An important aspect to highlight is the trend of TTI increase as a function of plasma conditions. At the highest power (namely, 120 w), 60 s of treatment gave the highest TTI increase (almost comparable with that of 15 s): longer treatment does not further increase PET TTI.
The sample exhibiting the highest performances was prepared by pre-treating PET fabrics at 80 w for 180 s; this sample displayed an increase of TTI up to 104% (from 158 to 322 s for PET and PET_CNa_80/180, respectively,
Figure 2B) and a small reduction of PHRR (10%); as a consequence, such a sample yielded the highest FPI value (
Table 2 and
Figure 3B). It is also interesting to observe the FPI trend as a function of plasma power and time, reported in
Figure 3B.
CNa performances have been compared with those of other two lamellar NPs, namely, a carbonate hydrotalcite and a p-toluenesulphonate bohemite.
Hydrotalcites are well known anionic nanoclays, having a chemical formula of Mg
6Al
2(CO
3)(OH
16)·4(H
2O) and organized as layered double hydroxides [
36]. The high water content present in their structure makes them potential flame retardant systems able to protect a polymer, as they form a ceramic layer during combustion, releasing a great amount of water upon heating. In this way, the degradation products released by the polymer are diluted, favouring a remarkable delay of its ignition and/or a reduction of combustion kinetics. For these reasons, PET fabrics were treated with a carbonate HT solution and subjected to cone calorimetry tests (see
Table 1) [
22]. In detail, HT is responsible for PET TTI increase from 166 up to 226 s (
Figure 2A) and PHRR decrease from 90 to 56 kW/m
2. Thus, FPI of PET_HT is drastically higher than that of untreated PET and also of PET_CNa (
Table 1 and
Figure 3B). This difference between these two values easily finds an explanation, comparing the TTI values of the two samples (192 vs. 226 s for PET_CNa and PET_HT, respectively). The presence of a higher water amount in the HT structure with respect to that internal in CNa galleries has a stronger effect in diluting the volatiles released by PET degradation before its ignition.
The third lamellar NP employed for treating PET fabrics is a p-toluenesulphonate bohemite. Bohemites theoretically have intrinsic flame retardant features as they are aluminium oxide hydroxides, γ-AlO(OH), that dehydrate in the range of 100–300 °C, releasing water and subsequently transforming into crystalline γ-Al
2O
3 phase at circa 420 °C [
36]. Similarly to HT, also bohemites should promote a strong diluting effect on the volatile products generated by polymers upon heating hence polymer ignition should be delayed. In addition, the ceramic barrier resulting from the presence of the just-formed alumina inhibits further combustion. Unfortunately, in spite of these considerations, when polyester fabrics are treated with such NP, PET TTI does not change in a significant way (
Table 1 and
Figure 2A); however, a reduction in EHC (15.0 vs. 15.8 MJ/kg for PET_OS1 and PET, respectively,
Table 1), PHRR (from 90 to 70 kW/m
2) and CO and CO
2 yields was registered.
Comparing the lamellar nanoparticles in terms of TTI (
Figure 1A) and FPI (
Figure 3A) improvements, the highest performances were achieved by treating PET with HT. On the other hand, including in this discussion a possible etching pre-treatment of fabrics, CNa exhibited the highest performances (namely, PET_CNa_80/180 sample).
As mentioned above, globular nanoparticles such as titania, silica, and octapropylammonium POSS have been taken into consideration as flame retardant systems for polyester, as well [
32]. In particular, these nanoparticles acting as smoke suppressants reduced the CO and CO
2 yields in a remarkable way (
Table 1). Unfortunately, silica particles did not impart good flame retardancy to polyester, as well visible comparing the corresponding FPI values (
Figure 3A). On the contrary, among the globular NPs, POSS exhibited the best performances by increasing PET TTI (
Figure 2A) and significantly reducing its PHRR as well as CO and CO
2 release.
These results together with the morphologies reported in ref. [
32] pointed out the presence of a low amount of titania and silica deposited on the fibre surfaces similarly to that observed for CNa. For this reason, an efficient physical barrier capable of protecting the substrate from fire was not achieved.
In order to overcome such a problem, the combination of lamellar and globular nanoparticles was proposed for reaching the optimal flame retardant performances of polyester [
32]. Thus, the features of HT and silica were combined in treating PET. To this aim, PET was initially treated with an HT suspension and then with a silica one; in doing so, two consequent layers were deposited on the PET fabrics. It is important to highlight that this approach somehow mimics what occurs in a LbL assembly and thus can be considered a sort of precursor of it. The results are the best collected for PET by using the approach of NP adsorption, as evidenced observing the TTI increase (
Table 1 and
Figure 2A) and FPI value (
Figure 3A). In addition, further analyses have shown that this system is able to reduce the production of CO and CO
2 in a remarkable way (
Table 3).
Beside the use of nanoparticles, water-soluble polymers or monomers to be polymerized in situ can be employed for the production of a fire protective treatment. In this approach an FR additive or FR monomer is employed in order to impart the final FR properties to the deposited coating. The main action of these coatings is similar to that of NPs: upon flame exposure the coating can swell into an expanded protective layer that limits heat transmission and volatiles release. The degree of expansion and the mechanism for barrier formation depend on the chemistry of the system.
Starch has been used as continuous phase in combination with sodium polyborate (SPB) evaluating different weight ratios between the polysaccharide and the FR [
37]. PET non-woven treated with the best formulation, achieved at a 0.134 ratio with a total add on of 48.4 wt %, and was found capable of enduring intensive heating by a premixed gas burner for 12 min.
Table 3 reports the performances obtained at different coating compositions.
The good performances achieved by the starch/SPB mixture were justified as follows: upon exposure to heat, SPB forms a foamed structure that insulates starch and improves its charring reactions, the release of water from starch enhances the foaming of SPB and dilutes the decomposition products, the SPB foam and starch carbonaceous residue insulate the substrate from heat and oxygen thus resulting in an FR action.
In another application PET fabrics were coated by polyaniline synthesized via in situ chemical polymerization of aniline in the presence of ammonium persulfate (as the oxidant), HCl, and H
3PO
4 (as the dopant) [
38]. The coating composition suggests intumescent formulation, the phosphoric acid works as acid source while the polyaniline represents both nitrogen and carbon sources. The effects of different oxidant to monomer ratios on the achieved FR properties were evaluated by LOI. Fabrics treated with a 1:2 molar ratio of ammonium persulfate to aniline resulting in the highest LOI (42%).
Recently, Luo et al. combined the two approaches described above by preparing a FR polyurethane (PU) waterborne resin, that was further, added with a commercial FR in order to exploit the synergistic effect of P–N systems to reduce the formation of flammable volatiles as well as catalyse char formation [
39]. In details, PET fabrics were coated with a waterborne polyurethane latex copolymerized with either Fyrol-6 or octahydro-2,7-di(
N,
N-dimethylamino)-1,6,3,8,2,7-dioxadiazadiphosphecine (ODDP) or the combination of both including Exolit
® OP550 in the final suspension. Regardless of the adopted formulation, treated PET fabrics (coating add-on was kept constant at 19 wt %) exhibited self-extinguishing behaviour in both horizontal and vertical flammability tests (UL 94 rating) as well as LOI values between ca. 25% and 27%.
Table 4 shows the detailed results.
With respect to nanoparticle adsorption, the use of a water-soluble polymer in combination with FR may offer different advantages such as better surface coverage, the possibility to include different FR additives and multiple crosslinking strategies for improving durability. On the other hand, the coating add-on is normally higher than NP adsorption with possible detrimental effects on the physical and comfort properties of the treated fabric.
2.1.2. Polyester-Cotton Blends
As mentioned in the Introduction, one of the biggest challenges for industrial applications is replacing PET with some blends containing cotton fibres. In this scenario, we investigated the NP adsorption approach in order to find the best nanoparticles able to protect two polyester-cotton (PET-COT) blends having 15 and 35 wt % of cotton, respectively. To this aim, we initially studied how such materials react to a 35 kW/m
2 heat flux to understand whether their behaviour was more similar to that of PET or cotton. This influenced the choice of NPs to employ.
Figure 4 reports the HRR curves for untreated PET, cotton, and two blends under investigation. It is clear that, regardless of the cotton amount, the TTIs of the two blends are more similar to those of cotton as compared with PET, indicating that cotton behaviour is dominating in the pre-ignition phase. Thus, on the basis of the results already collected for cotton [
22], CNa, HT and silica were selected.
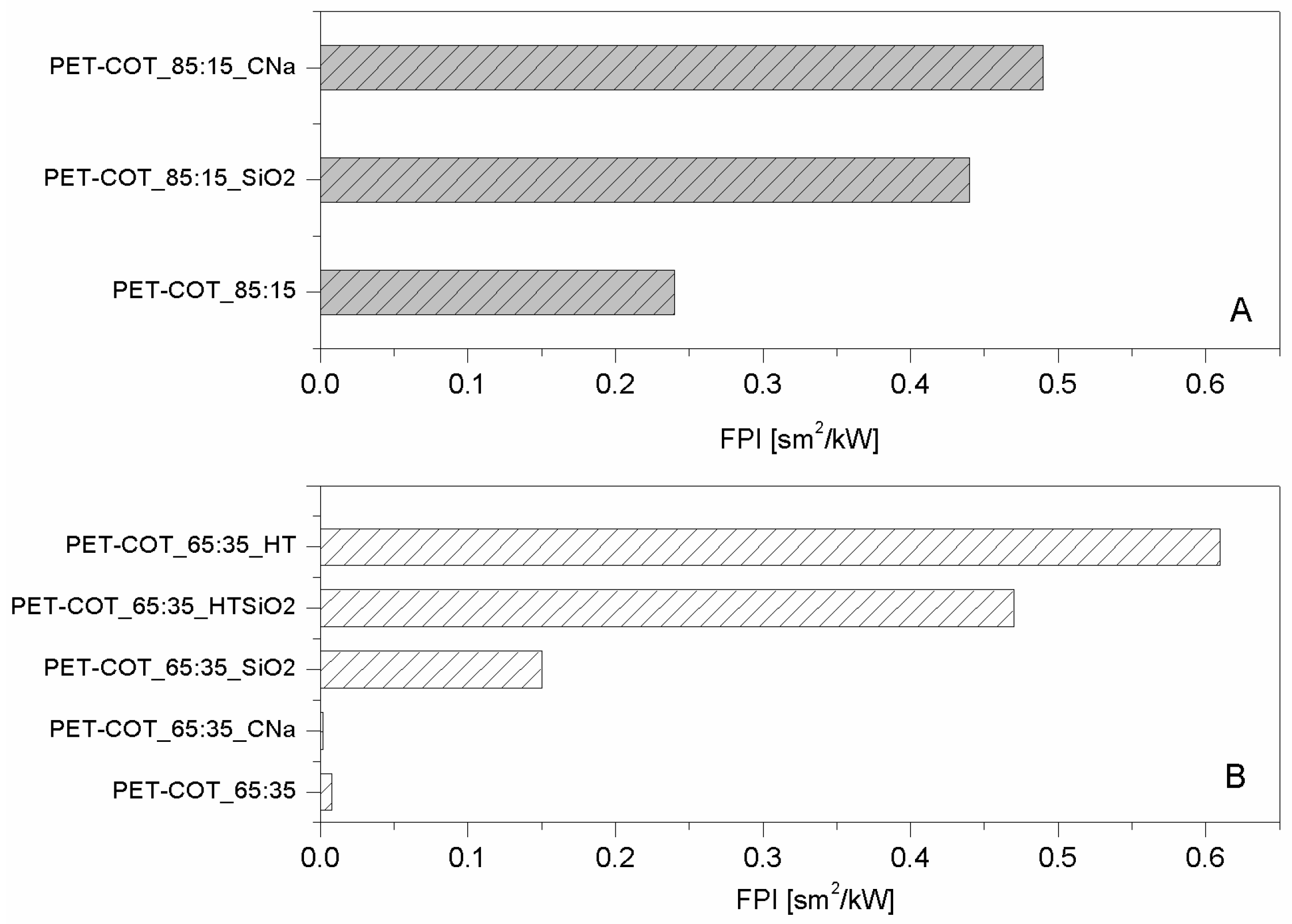
As far as CNa is concerned (
Table 5 and
Table 6), both blends exhibited a higher TTI (
Figure 5A,B) and a lower PHRR (
Figure 5C,D) due to the presence of NP. Referring to silica, the NP effect is comparable for the two substrates; no significant differences in performances were observed, also in terms of FPI value improvements (see
Figure 5). For the blend having a higher cotton amount, HT was tested as well, since as described before, alone or in combination with silica it exhibited good performances for both PET and cotton fabrics [
22]. In such cases, the best performances were achieved for the blend treated with one layer of HT, even though significant improvements were achieved also by coupling HT with silica. The latter results highlight how the subsequent adsorption of oppositely charged NPs in a layer by layer fashion can be the right route for the assembly of a performing flame retardant nanocoating.
For a possible industrial application, POSS has proven to be the most promising NP to improve PET flame retardancy. Indeed, following the ISO15025 standard, these samples did not burn when exposed to a 2.5 cm methane flame. Unfortunately, the same trend was not observed for a PET-COT blend. Thus, combination of a phosphorus-based flame retardant (namely, Pyrovatex
®-like [
6]) with POSS was experimented in order to enhance the overall flame retardancy level of such blends.
Figure 6 shows the collected results by cone calorimetry for PET-COT_65:35 treated with Pyrovatex
® at 10 wt % add-on (the add-on normally employed is 19 wt %) and different POSS amounts (namely, 5, 15, and 30 wt %). By increasing the POSS amount, TTI increases (up to 500% improvement) and PHRR decreases (up to 50% reduction) in a remarkable way (
Figure 7A,B, respectively).