Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Enzyme/Alginate/Chitosan Hydrosols and Sponges
| Enzyme Addition (E) | Polymers Ratio A:C | Variants (E/A/C) |
|---|---|---|
| (U) | (v/v) | (code) |
| N * | 0:1 | N/C |
| 1:3 | N/A/3C | |
| 1:1 | N/A/C | |
| 3:1 | N/3A/C | |
| 1:0 | N/A | |
| 1000 L | 0:1 | L/C |
| 1:3 | L/A/3C | |
| 1:1 | L/A/C | |
| 3:1 | L/3A/C | |
| 1:0 | L/A |
2.3. Reducing Sugar Assay of Enzyme/Alginate/Chitosan Hydrosols
2.4. Antioxidant Activity of Enzyme/Alginate/Chitosan Hydrosols
2.5. Rheological Characterization of Enzyme/Alginate/Chitosan Hydrosols
- 3 min to a maximum shear rate;
- 1 min at maximum shear rate; and
- 3 min to reach a shear rate of 0.
- 𝜏—shear stress (Pa);
- k—consistency index (Pa·s);
- γ—shear rate (s−1);
- n—flow behavior index (–);
- ηα—apparent viscosity (mPa·s).
2.6. Compressive Properties of Enzyme/Alginate/Chitosan Sponges
2.7. Solubility of Enzyme/Alginate/Chitosan Sponges
2.8. Scanning Electron Microscopy of Enzyme/Alginate/Chitosan Sponges
2.9. Statistical Analysis
3. Results and Discussion
3.1. Reducing Sugar
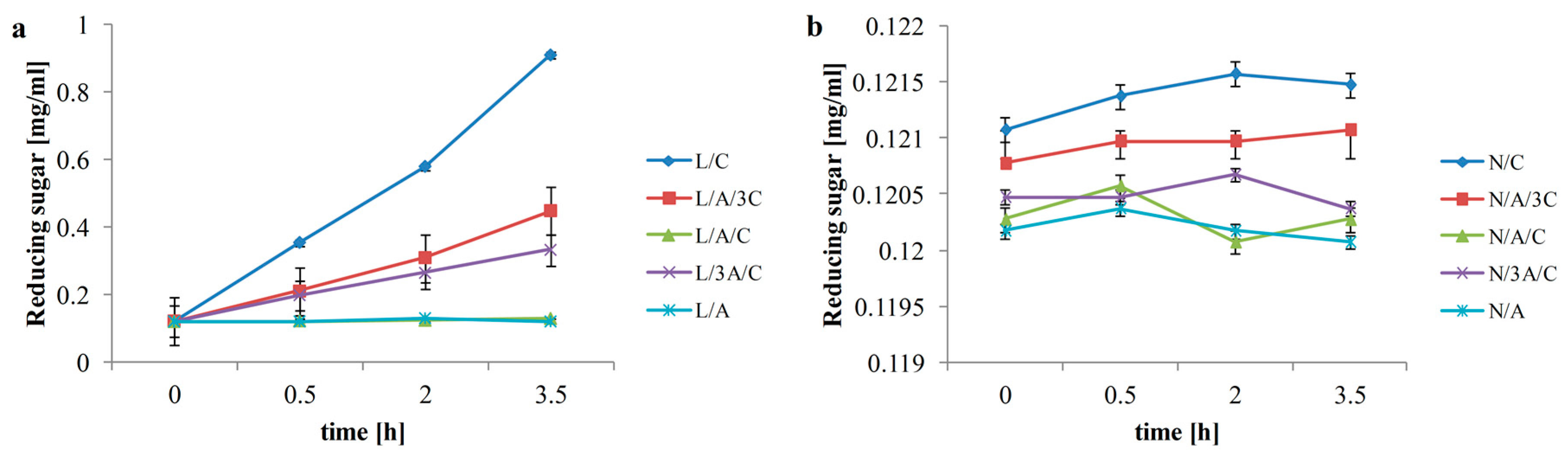
3.2. Antioxidant Activity
| Main effects | Interactions A/C | ||||
|---|---|---|---|---|---|
| A/C | E | ||||
| Polymers ratio (v/v) | Free radical scavenging of DPPH (μM·trolox/mL) | Polymers ratio (v/v) | Free radical scavenging of DPPH (μM·trolox/mL) | Polymers ratio (v/v) | Free radical scavenging of DPPH (μM·trolox/mL) |
| 0/1 | 202.89 ± 21.15 b | N | 198.41 ± 10.48 a | N/C | 231.33 ± 10.67 g |
| N/A/3C | 208.43 ± 2.02 def | ||||
| 1/3 | 181.71 ± 15.19 a | N/A/C | 220.27 ± 5.60 fg | ||
| N/3A/C | 206.01 ± 4.19 def | ||||
| 1/1 | 205.11 ± 4.39 b | N/A | 126 ± 13.15 b | ||
| L | 132.43 ± 10.08 b | L/C | 120.66 ± 3.13 b | ||
| 3/1 | 186.71 ± 10.13 a | L/A/3C | 121.33 ± 1.53 b | ||
| L/A/C | 193.33 ± 2.98 d | ||||
| 1/0 | 119.11 ± 11.32 c | L/3A/C | 146.8 ± 4.05 c | ||
| L/A | 80 ± 7.55 a | ||||
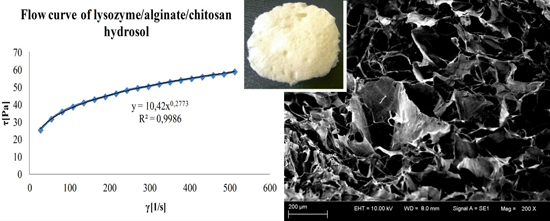
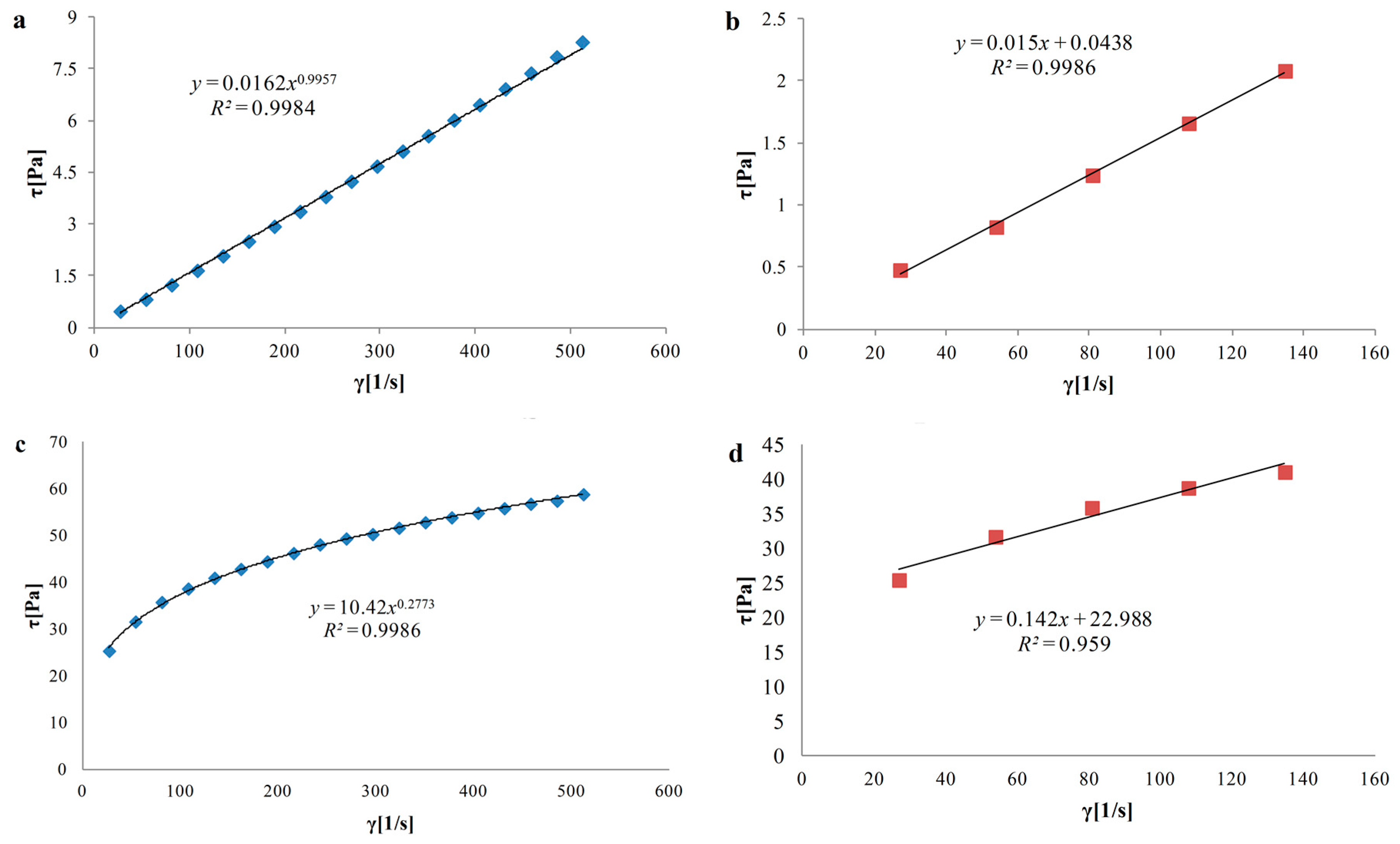
3.3. Rheological Properties of Hydrosols
| Variants (E/A/C) | Ostwald de Waele model | Herschel–Bulkley model | ||
|---|---|---|---|---|
| Consistency index k (Pa·s) | Flow behavior index n (–) | Apparent viscosity η (mPa·s) | Yield stress τ0 (Pa) | |
| N | ||||
| N/C | 0.02 ± 0.01 × 10−3 a | 0.996 ± 0.05 × 10−2 c | 15.6 ± 0.1 a | 0.04 ± 0.02 × 10−3 a |
| N/A/3C | 0.17 ± 0.01 × 10−2 b | 0.682 ± 0.03 × 10−2 b | 30.5 ± 0.5 b | 0.94 ± 0.05 × 10−2 b |
| N/A/C | 0.04 ± 0.50 × 10−2 d | 0.901 ± 0.04 × 10−2 g | 317.4 ± 0.4 g | 2.22 ± 0.1 × 10−2 d |
| N/3A/C | 2.46 ± 0.10 × 10−2 e | 0.492 ± 0.2 × 10−2 a | 151 ± 1.0 e | 9.62 ± 0.50 × 10−2 h |
| N/A | 0.68 ± 0.03 × 10−2 c | 0.713 ± 0.03 × 10−2 f | 140 ± 0.0 c | 2.86 ± 0.10 × 10−2 f |
| L | ||||
| L/C | 0.01 ± 0.49 × 10−5 a | 0.997 ± 0.05 × 10−2 c | 14.4 ± 0.1 a | 0.01 ± 0.49 × 10−5 a |
| L/A/3C | 0.18 ± 0.09 × 10−3 b | 0.677 ± 0.03 × 10−2 b | 28.6 ± 0.4 b | 1.04 ± 0.05 × 10−2 c |
| L/A/C | 10.42 ± 0.51 × 10−2 g | 0.277 ± 0.01 × 10−2 d | 189.8 ± 0.2 f | 22.99 ± 0.01 i |
| L/3A/C | 2.50 ± 0.12 × 10−2 f | 0.495 ± 0.02 × 10−2 a | 139.5 ± 0.5 c | 9.16 ± 0.45 × 10−2 g |
| L/A | 0.69 ± 0.03 × 10−2 c | 0.710 ± 0.04 × 10−2 e | 129.8 ± 0.2 cd | 2.38 ± 0.12 × 10−2 e |
3.4. Compressive Properties of Sponges
| Main effects | Interactions A/C | |||||||
|---|---|---|---|---|---|---|---|---|
| A/C | E | |||||||
| Polymers ratio (v/v) | σ10 (kPa) | E (kPa) | Enzyme addition | σ10 (kPa) | E (kPa) | Variants (E/A/C) | σ10 (kPa) | E (kPa) |
| 0/1 | 0.96 ± 0.27 a | 8.81 ± 1.97 a | N | 3.02 ± 0.69 a | 27.38 ± 5.28 a | N/C | 1.48 ± 0.31 ab | 11.89 ± 2.96 a |
| N/A/3C | 3.47 ± 0.35 b | 31.17 ± 3.32 c | ||||||
| 1/3 | 2.86 ± 0.33 b | 22.52 ± 4.32 b | N/A/C | 2.23 ± 0.92 ab | 26.14 ± 7.33 bc | |||
| N/3A/C | 7.14 ± 1.76 c | 59.01 ± 9.81 d | ||||||
| 1/1 | 2.08 ± 0.44 ab | 26.54 ± 3.43 b | N/A | 0.77 ± 0.22 a | 8.71 ± 1.62 a | |||
| L | 1.31 ± 0.23 b | 21.27 ± 4.52 b | L/C | 0.45 ± 0.10 a | 5.74 ± 1.11 a | |||
| 3/1 | 4.31 ± 1.50 c | 55.17 ± 4.78 c | L/A/3C | 2.27 ± 0.28 ab | 13.87 ± 2.79 ab | |||
| L/A/C | 1.92 ± 0.34 ab | 26.93 ± 2.25 bc | ||||||
| 1/0 | 0.60 ± 0.12 a | 8.60 ± 0.76 a | L/3A/C | 1.49 ± 0.50 ab | 51.32 ± 1.84 d | |||
| L/A | 0.44 ± 0.03 a | 8.49 ± 0.47 a | ||||||
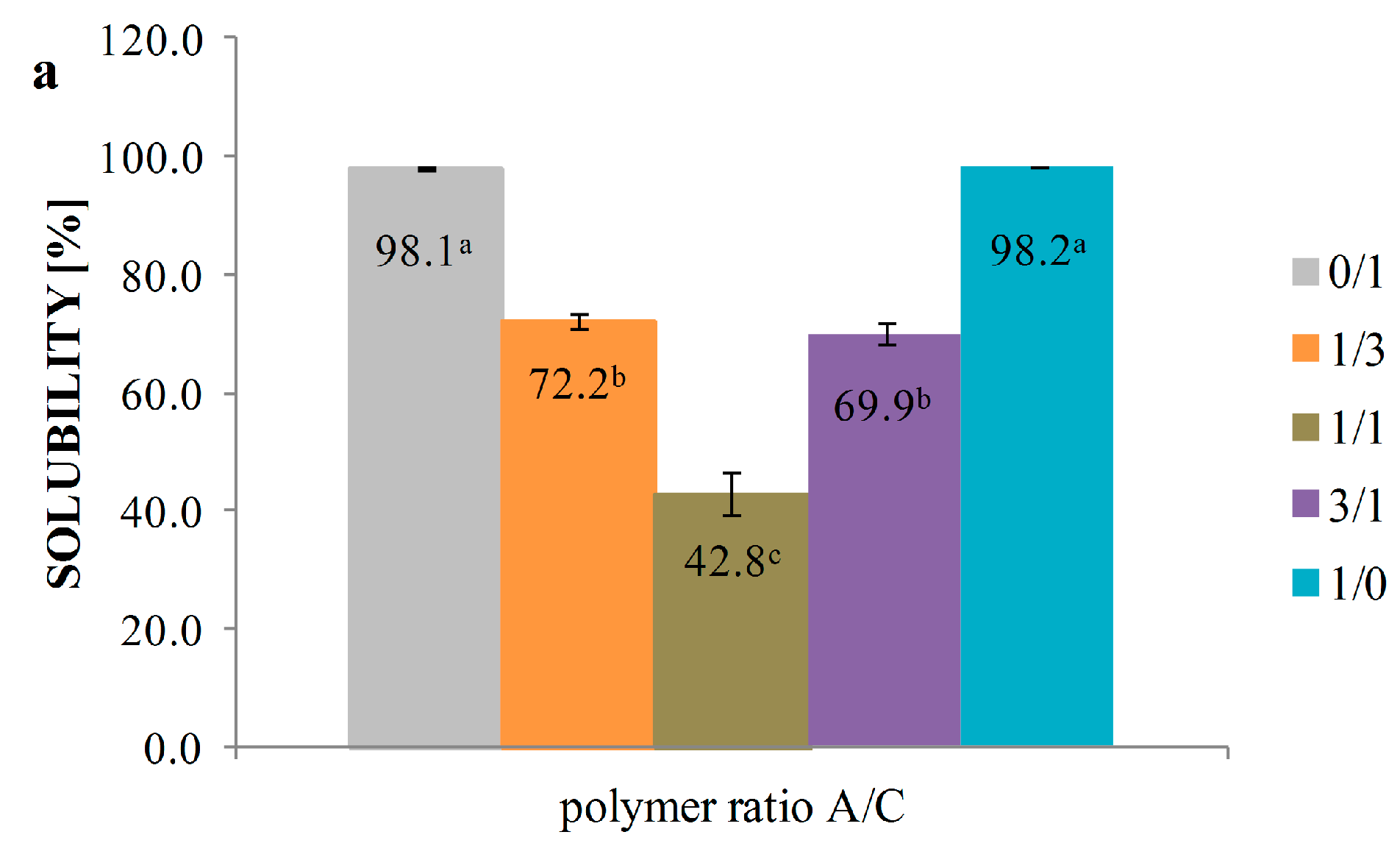
3.5. Solubility

3.6. Scanning Electron Microscopy
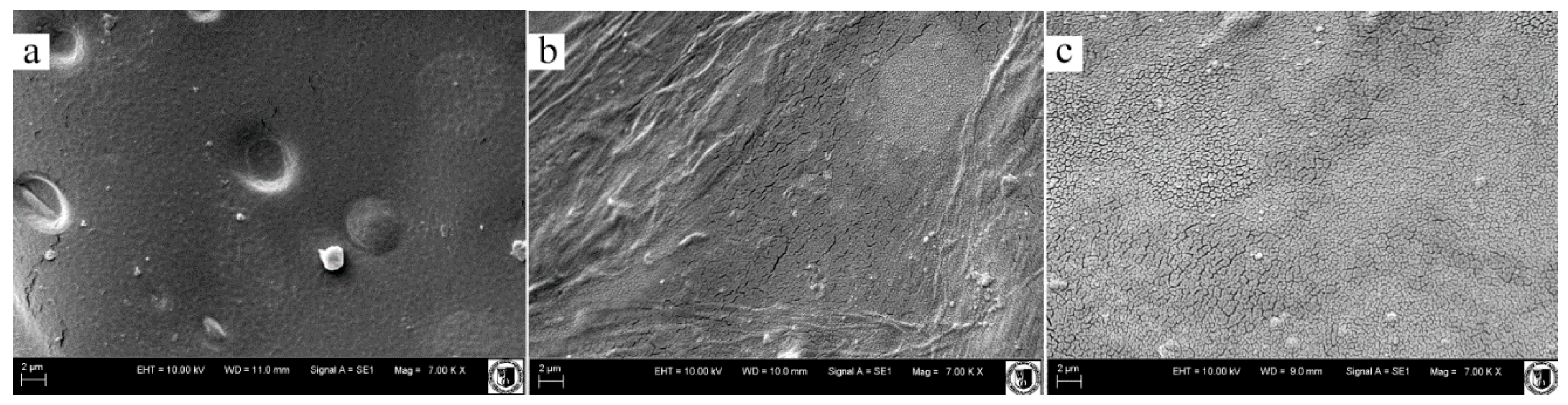
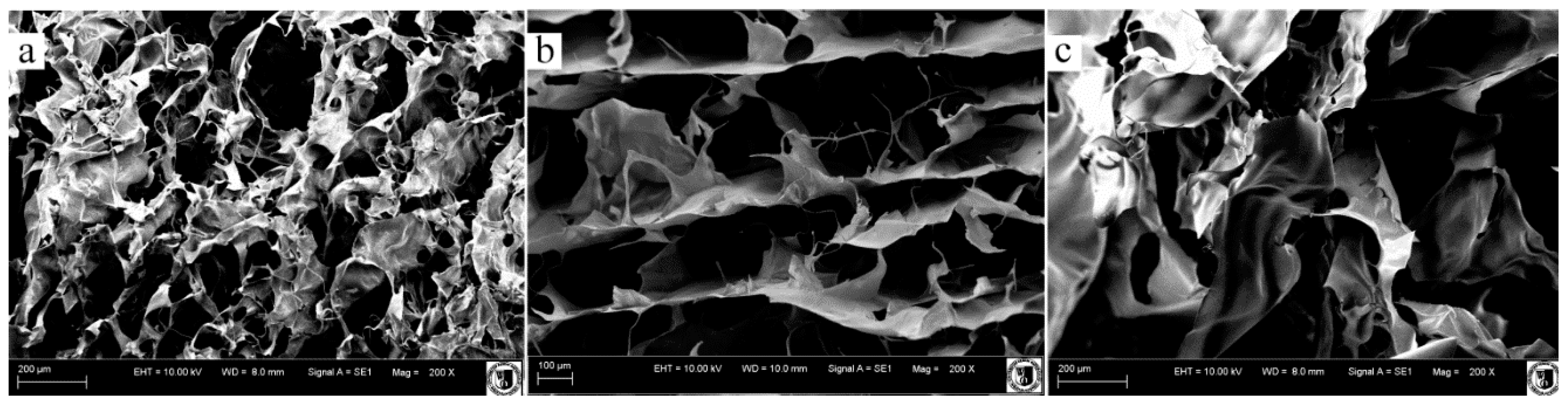
| Variants (E/A/C) | Pore size (μm) |
|---|---|
| N | |
| N/C | 109.2 ± 20.2 a |
| N/A/3C | 132.8 ± 22.9 ab |
| N/A/C | 124.9 ± 15.2 ab |
| N/3A/C | 140.6 ± 21.9 ab |
| N/A | 189.8 ± 26.8 bc |
| L | |
| L/C | 117.6 ± 17.8 ab |
| L/A/3C | 217.6 ± 19.8 c |
| L/A/C | 120.0 ± 12.4 ab |
| L/3A/C | 218.4 ± 35.4 c |
| L/A | 224.6 ± 36.1 c |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Zhu, Y.; Che, L.; Shen, O.; Zhang, J.; Li, X. Oligoamines conjugated chitosan derivatives: Synthesis, characterization, in vitro and in vivo biocompatibility evaluations. Carbohydr. Polym. 2011, 83, 1153–1161. [Google Scholar] [CrossRef]
- Sun, T.; Yao, Q.; Zhou, D.; Mao, F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorganic Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Upadhyay, L.; Tewari, R.P.; Dutta, J.; Huang, Y.B.; Dutta, P.K. Chitosan-pectin-alginate as a novel scaffold for tissue engineering applications. Indian J. Biotechnol. 2013, 12, 475–482. [Google Scholar]
- Hsieh, W.C.; Chang, C.P.; Lin, S.M. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2007, 57, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolyte complex formation between alginate and chitosan as a function of pH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Oliviero, M.; Maio, D.E.; Iannace, S.; Netti, P.A. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Sapir, Y.; Kryukov, O.; Cohen, S. Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials 2011, 32, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Kim, D.J.; Wood, D.L.; Zhang, M. Influence of processing parameters on pore structure of 3D porous chitosan-alginate polyelectrolyte complex scaffolds. J. Biomed. Mater. Res. A 2011, 98, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Feng, T.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Sreeram, K.J.; Nidhin, M.; Nair, B.U. Synthesis of aligned hematite nanoparticles on chitosan-alginate films. Colloids Surf. B Biointerfaces 2009, 71, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Nair, P.D. Novel porous, polysaccharide scaffolds for tissue engineering application. Trends Biomater. Artif. Organs 2005, 18, 219–224. [Google Scholar]
- Holme, H.K.; Davidsen, L.; Kristiansen, A.; Smidsrød, O. Kinetics and mechanisms of the depolymerization of alginate and chitosan in aqueous solution. Carbohydr. Polym. 2008, 73, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Gardrat, C.; Castellan, A.; Jarmoluk, A.; Coma, V. The use of lysozyme to prepare biologically active chito-oligomers. Polimeros 2015, 25, 35–41. [Google Scholar]
- Lin, H.; Wang, H.Y.; Xue, C.H.; Ye, M. Preparation of chitosan oligomers by immobilized papain. Enzym. Microb. Technol. 2002, 31, 588–592. [Google Scholar] [CrossRef]
- Wu, S.J. Preparation of water soluble chitosan by hydrolysis with commercial α-amylase containing chitosanase activity. Food Chem. 2011, 128, 769–772. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.Z.; Gianficoa, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Teroa, J. HPLC method for evaluation of the free radical scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Pinotti, A.; Martino, M.N.; Zaritzky, N.E. Characterization of composite hydrocolloid films. Carbohydr. Polym. 2004, 56, 339–345. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- ASTM D1621-10. Standard Test Method For Compressive Properties Of Rigid Cellular Plastics; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Amid, B.T.; Mirhosseini, H. Effect of different purification techniques on the characteristics of heteropolysaccharide-protein biopolymer from durian seed. Molecules 2012, 17, 10875–10892. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Haden, P. Statistical Analysis with the General Linear Model. 2006. Available online: http://r-dir.com/statistics/e-books.html (accessed on 13 August 2015).
- Leśnierowski, G.; Kijowski, J. Lysozyme. In Bioactive Egg Compounds; Huopalhti, R., Lopez-Fandino, R., Anton, M., Schade, R., Eds.; Springer: Berlin, Germany, 2007. [Google Scholar]
- An, Q.-D.; Zhang, G.-L.; Wu, H.-T.; Zhang, Z.-C.; Zheng, G.-S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry. Relevance to the biomedical science. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar]
- Zimoch-Korzycka, A.; Jarmoluk, A. The use of chitosan, lysozyme, and the nano-silver as antimicrobial ingredients of edible protective hydrosols applied into the surface of meat. J. Food Sci. Technol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lin, Y.; Xiangling, C.; Baotang, Z.; Zhan, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Moraes, I.C.F.; Fasolin, L.H.; Cunha, R.L.; Menegalli, F.C. Dynamic and steady-shear rheological properties of xanthan and guar gums dispersed in yellow passion fruit pulp (Passiflora edulis f. flavicarpa). Braz. J. Chem. Eng. 2011, 03, 483–494. [Google Scholar] [CrossRef]
- Kelesoglu, S.; Pettersen, B.; Sjoblom, J. Flow properties of water in North Sea heavy crude oil emulsions. J. Pet. Sci. Eng. 2012, 100, 14–23. [Google Scholar] [CrossRef]
- Björn, A.; Segura de La Monja, P.; Karlsson, A.; Ejlertsson, J.; Svensson, B.H. Rheological Characterization, Biogas, Dr. Sunil Kumar (Ed.), InTech. 2012. Available online: http://www.intechopen.com/books/biogas/rheological-characterization (accessed on 03 November 2015).
- Alphonse, P.; Varghese, A.; Tendero, C. Stable hydrosols for TiO2 coatings. J. Sol-Gel Sci. Technol. 2010, 56, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Haywood, A. The Effect of Polymer Solutions on the Settling Behaviour of Sand Particles. 2011. Available online: http://personalpages.manchester.ac.uk/staff/p.martin/publications/The_effect_of_polymer_solutions_on_the_settling_behaviour_of_sand_particles.pdf (accessed on 03 November 2015).
- Khunawattanakul, W.; Puttipipatkhachorn, S.; Rades, T.; Pongjanyakul, T. Chitosan–magnesium aluminum silicate composite dispersions: Characterization of rheology flocculate size and zeta potential. Int. J. Pharm. 2008, 351, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Lin, Y.C.; Chen, H.H. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterisation and antibacterial activity. Food Chem. 2009, 116, 47–53. [Google Scholar] [CrossRef]
- Mengíbar, M.; Ganan, M.; Miralles, B.; Carrascosa, A.V.; Martínez-Rodriguez, A.J.; Peter, M.G.; Heras, A. Antibacterial activity of products of depolymerization of chitosans with lysozyme and chitosanase against Campylobacter jejuni. Carbohydr. Polym. 2011, 84, 844–848. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and characteristics of chitosan sponge as a tissue engineering scaffold. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ilina, A.V.; Tkacheva, Y.V.; Varlamov, V.P. Depolymerization of high-molecular-weight chitosan by the enzyme preparation Celloviridine G20x. Appl. Biochem. Microbiol. 2002, 38, 112–115. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan Based polyelectrolyte complexes as potential carrier materials in drug delivery Systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Gershon, Z.; Nussinovitch, A. Characteristics of enzymatically produced agar-starch sponges. Food Hydrocoll. 1998, 12, 105–110. [Google Scholar]
- Dai, M.; Zheng, X.L.; Xu, X.; Kong, X.Y.; Li, X.Y.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z. Chitosan-alginate sponge: Preparation and application in curcumin delivery for dermal wound healing in rat. J. Biomed. Biotechnol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Wrzeszcz, K.; Marycz, K.; Zimoch, A.; Jarmoluk, A. Polymerization reaction of sodium alginate—Natural polymer used in tissue engineering. Chem. Rev. 2013, 92, 1018–1022. (In Polish) [Google Scholar]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimoch-Korzycka, A.; Kulig, D.; Jarmoluk, A.; Marycz, K.; Matuszczak, W. Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process. Polymers 2016, 8, 8. https://doi.org/10.3390/polym8010008
Zimoch-Korzycka A, Kulig D, Jarmoluk A, Marycz K, Matuszczak W. Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process. Polymers. 2016; 8(1):8. https://doi.org/10.3390/polym8010008
Chicago/Turabian StyleZimoch-Korzycka, Anna, Dominika Kulig, Andrzej Jarmoluk, Krzysztof Marycz, and Weronika Matuszczak. 2016. "Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process" Polymers 8, no. 1: 8. https://doi.org/10.3390/polym8010008