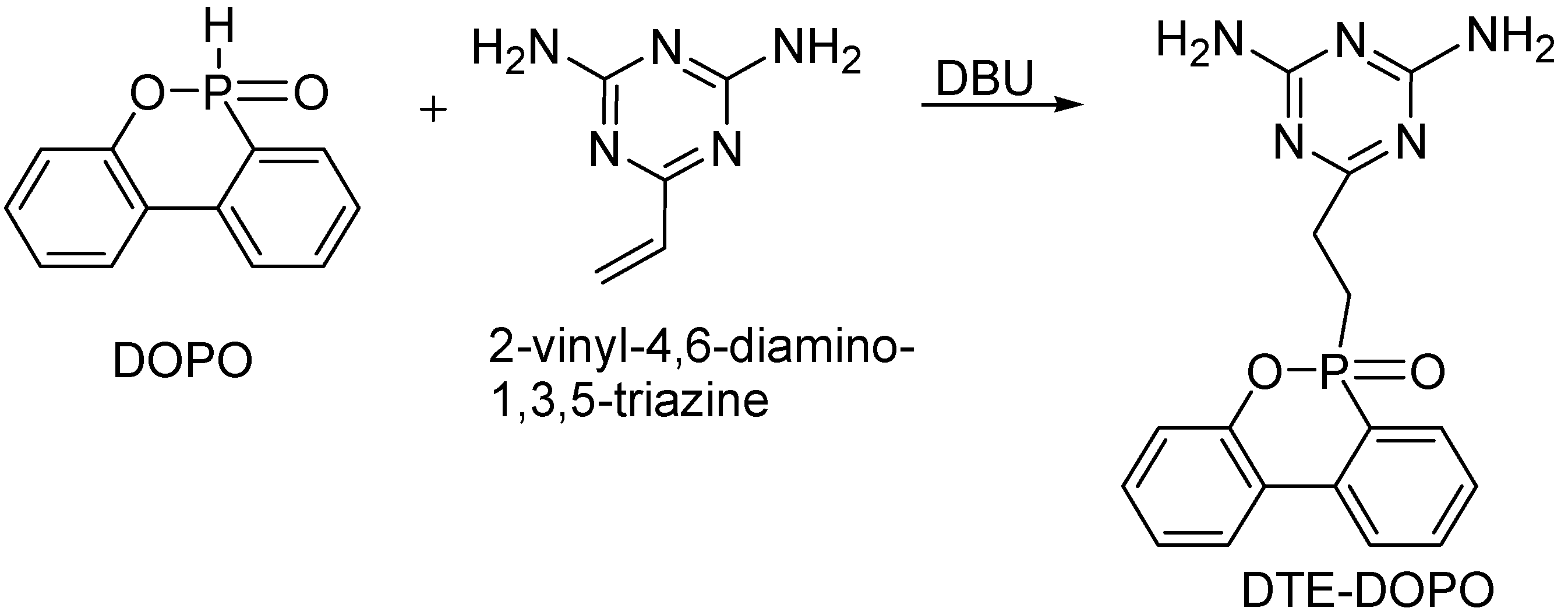
A successful strategy to maintain the desired thermal and mechanical properties of the PA6 is to develop a formulation with a minimum loading of the FR additive. The melting behavior of the newly developed DTE-DOPO FR additive is a possible advantage for the compounding process. A meltable additive may exhibit increased compatibility with PA6 in comparison with the non-meltable FR additive Exolit® OP 1230. To evaluate the influence of the new additive on the characteristics of PA6 and to compare its efficiency with the commercial Exolit® OP 1230, a formulation containing Exolit® OP 1230 was also prepared. The two formulations were obtained using compounding by melt extrusion with a loading of 17.1% additive for the PA6/DTE-DOPO formulation and a corresponding P content of 1.46%, while for the PA6/Exolit formulation the additive loading was of 16.8% with a P content of 3.99%. The efficiency of a P-based FR additive is, in general, dependent on the phosphorus content of the formulation. Thus, it should be noted that, for the same additive loading the P content in the PA6/Exolit formulation is almost three times higher than that of the PA6/DTE-DOPO formulation.
3.2.1. Rheological Properties
To evaluate the influence of the additives on the processability and eventual mechanical properties of the formulations, rheological measurements were carried out on the PA6 formulations and on the neat PA6. The loss modulus (
G”) reflects the viscous behavior (liquid-like properties) of the polymer and represents the ability of a material to dissipate energy, while the storage modulus (
G’) is a measure for its elasticity (solid-like properties) showing the ability of the polymer to store energy [
36].
To compare the flow behavior of thermoplastic formulations it is useful to limit the rheological experiments to the linear viscoelastic region (LVR). According to the strain sweep measurements carried out with neat PA6 and both formulations, the complex viscosity (µ*) registered a linear plateau in the 1%–10% strain region (data not shown). This result determined the selection of 1% strain for the following rheological measurements.
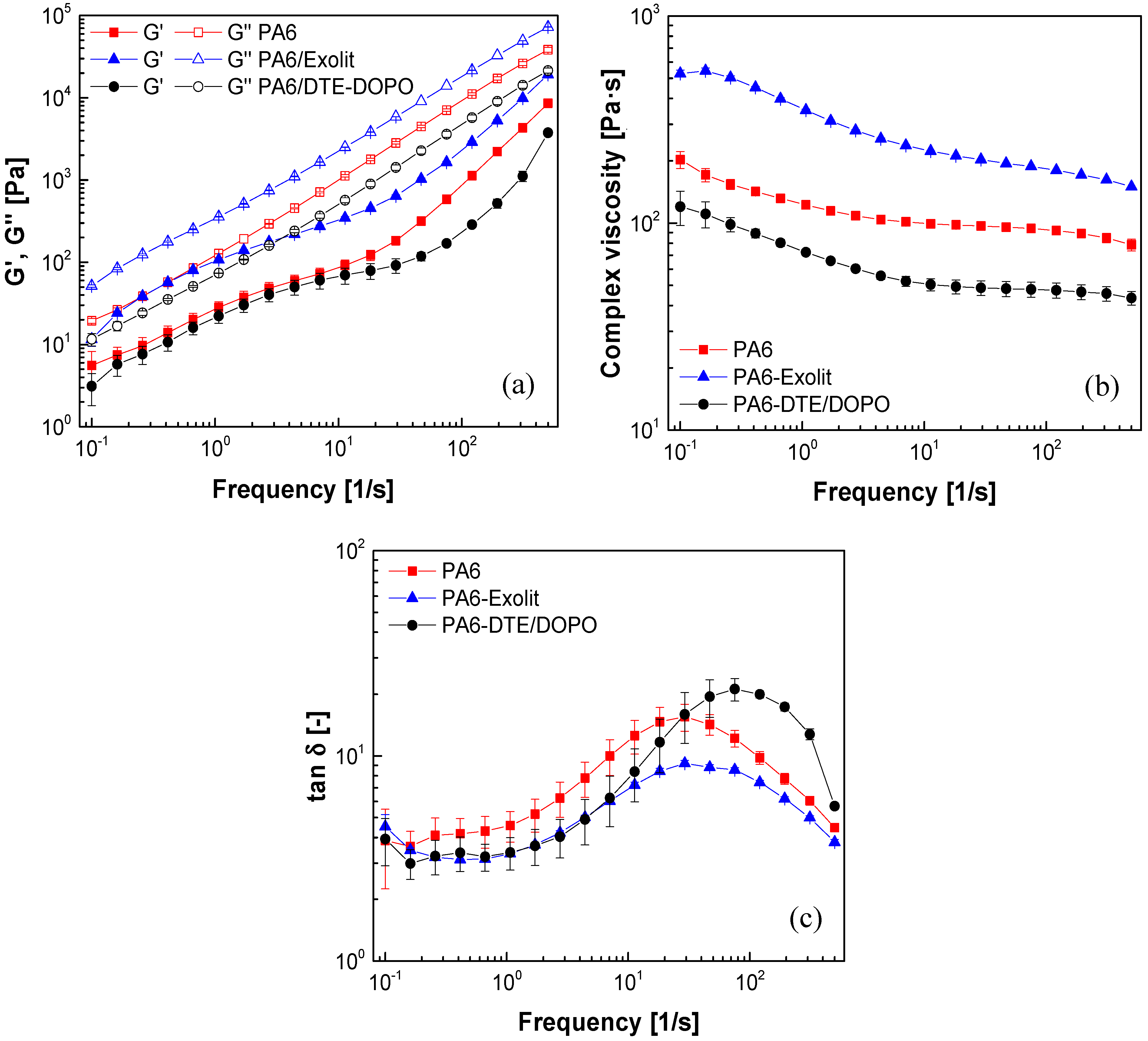
Analyzing the evolution of
G’ and
G” as a function of the applied frequency (
Figure 1a), the loss modulus is almost linear over the whole range of frequencies, while the storage modulus shows a viscoelastic relaxation at low frequencies for both formulations. This deviation from the expected behavior is representative of interfacial interactions between the dispersed FR domains and the polymeric matrix phase, therefore indicating at least a partial miscible blend. As expected, neat PA6 and both PA6 formulations exhibited higher values of loss modulus compared to those of storage modulus in all studied frequency domain, which is typical of a viscous material. The incorporation of Exolit
® OP 1230 into PA6 increases the complex viscosity of this formulation and led to higher values of
G’ and
G” respect to the neat PA6 (
Figure 1b). This could be explained by the limited motion of the polymer chains due to the incorporation of the FR. Contrary to this result, the incorporation of
DTE-DOPO additive to PA6 decreases both storage and loss modulus and reduces the complex viscosity when compared to neat PA6 for the whole range of measured frequencies. This might indicate the internal lubricating or plasticizing effect of the additive on the polymer structure, which leads to the improvement in the flexibility of the PA6/DTE-DOPO formulation.
Figure 1.
Evolution of (a) loss and storage modulus; (b) complex viscosity; and (c) loss factor (tanδ) as a function of frequency for PA6 formulations at T = 250 °C and 1% strain.
Figure 1.
Evolution of (a) loss and storage modulus; (b) complex viscosity; and (c) loss factor (tanδ) as a function of frequency for PA6 formulations at T = 250 °C and 1% strain.
From the variation of the loss factor (tanδ) with frequency (
Figure 1c) it can be noticed that the values of tanδ decrease with the introduction of Exolit
® OP 1230 into the PA6 formulation, which emphasize the increase in the storage modulus and, therefore, may indicate improved elastic properties by the introduction of this additive. The maximum value of tanδ for the PA6/DTE-DOPO formulations shifted to higher frequency compared to that of neat PA6, probably due to changes in the microstructure associated to the addition of the flame retardant.
3.2.2. Thermal Stability
The thermal stability of an additive is an important criterion for its application in high-temperature processing of polymers as well as in the final use of the obtained FR material. In the initial stage of combustion, the thermo-oxidative decomposition of flame-retardant polymer is a decisive process due to the presence of a high concentration of oxygen radicals [
22]. Moreover, the thermal behavior of a formulation in a free-burning phase of a fire,
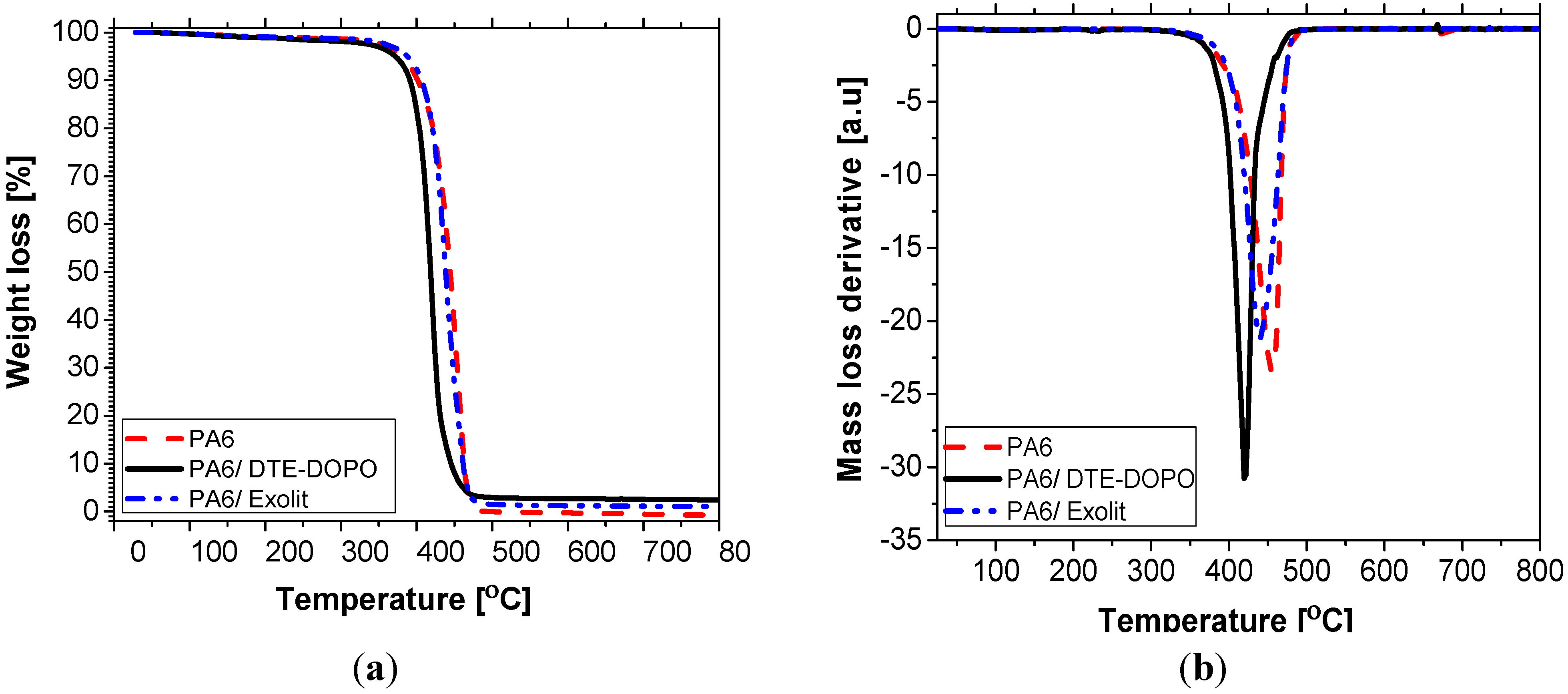
i.e., when the access to oxygen of the burning material is hindered, is best evaluated in a nitrogen environment. Considering these two different atmospheres, thermogravimetric analysis can simulate the burning conditions as in a UL94 vertical burning test, using a defined heating program and normal pressure. In this context, the thermal decomposition of FR formulations was measured in air (20% oxygen) and nitrogen environments. The representative TGA curves in N
2 of neat PA6 and PA6 formulations, showing the weight loss profile and the corresponding first order derivatives (DTG), are presented in
Figure 2. For air conditions, readers are referred to the
supplementary files.
Figure 2.
TGA (a) and DTG curves (b) of PA6 formulations in nitrogen.
Figure 2.
TGA (a) and DTG curves (b) of PA6 formulations in nitrogen.
The initial decomposition temperature (
T5), the temperature of the maximum mass loss rate (
Tmax), estimated on the basis of the DTG maximum peak, and the char yield at 700 °C (
WI) measured in both environments are listed in
Table 1.
Table 1.
Thermal properties of PA6 formulations in nitrogen and air environment.
Table 1.
Thermal properties of PA6 formulations in nitrogen and air environment.
| Sample | N2 Environment | Air Environment |
|---|
| T5 a [°C] | Tmax b [°C] | W700 c [wt%] | T5 a [°C] | Tmax b [°C] | W700 c [wt%] |
|---|
| PA6 | 388 ± 8.4 | 455 ± 1.0 | 0.28 ± 0.1 | 382 ± 2.0 | 449 ± 1.0/549 ± 1.0 | 0 |
| PA6/DTE-DOPO | 372 ± 0.4 | 413 ± 0.8 | 2.4 ± 0.3 | 356 ± 1.4 | 406 ± 0.8/547 ± 1.0/725 ± 1.0 | 6.05 ± 0.01 |
| PA6/Exolit | 386 ± 0.8 | 430 ± 2.0 | 0.9 ± 0.2 | 373 ± 1.0 | 424 ± 2.0/586 ± 1.4 | 8.9 ± 0.1 |
The thermal decomposition behavior of neat PA6 and PA6 formulations with different FR systems based on non-meltable melamine derivatives has been well studied [
7,
37,
38,
39,
40]. In the nitrogen environment (
Figure 2), PA6 has a
T5 value of approximately 388 °C, decomposed in one stage process with
Tmax of about 455 °C, leaving negligible char at 700 °C (0.28 wt%), which can be attributed to the partial decomposition of the cross-linked structure at the final stage of the decomposition process. The incorporation of
DTE-DOPO in the PA6 has led to a reduction of the initial decomposition temperature (
T5 = 372 °C), while the degradation process registered one step with a decrease of 40 °C for the temperature of the maximum mass loss rate (
Tmax = 413 °C). The TGA curve registered a steeper slope of the decomposition rate compared to PA6 suggesting a catalyzing effect of
DTE-DOPO FR additive on the decomposition of PA6. The highest amount of char was observed for this formulation (2.4 wt%) as compared to the neat PA6 and to the PA6/ Exolit
® OP 1230 formulation. When Exolit
® OP 1230 was introduced into PA6 the thermal degradation did not register a significant change as compared to the neat PA6 polymer. Thus, the value of
T5 was of about 386 °C, the decomposition process registered a single stage with a reduction of 20 °C in the value of
Tmax (430 °C), while the char yield had a value of 0.9 wt%. Similar data, regarding the influence of the additive on thermal stability, were obtained for different polymers and FR based on DOPO derivatives [
26,
27,
28]. The incorporation of various DOPO-based FR additives led to a decrease in thermal stability of the corresponding polymers. This behavior was attributed to the decomposition or vaporization of the FR additive in the initial stage of polymer degradation, and also to the interactions of products obtained from the decomposition reactions of the polymer and additive. In fact, the thermal decomposition of Exolit
® OP 1230 in the PA6 formulation might be the result of the degradation of the additive and its vaporization, which might constitute the dominant process [
41]. Former studies on PA6 formulations containing melamine or melamine derivatives also reveal an increased destabilization of the PA6 polymer caused by the incorporation of FR additives [
8,
38,
39]. Considering the small reduction of
T5 in the PA6/DTE-DOPO formulation as compared to neat PA6, it seems that the thermal decomposition process of this formulation may be initiated with the release of the FR additive. Additionally, an increased rate of the main degradation process,
i.e., sharp curves in the DTG, indicates that the decomposition of the additive may catalyze the scission of the PA6 chains due to the acidic character of the DOPO derivatives [
23]. The TGA data in nitrogen suggests that when
DTE-DOPO additive was combined with PA6, it resulted in a slight increase in the char formation as compared to the PA6/Exolit
® OP 1230 formulation. Additionally, the PA6/DTE-DOPO formulation maintains a higher thermal stability. Without a detailed analysis of the decomposition products the evaluation of the FR mechanism of the
DTE-DOPO additive in the PA6 formulation is not feasible.
The thermal oxidative degradation behavior of PA6 and PA6 formulations was investigated using a simulated air environment (
Figure S2 in
Supplementary files). In an air environment the degradation pathway follows a multistep mechanism and more
Tmax values are registered when compared to a nitrogen environment. These steps correspond to the maximum of different process and not to the global maximum degradation of the whole process.
As expected, the values of
T5 for all samples slightly decreased compared to the values obtained in N
2 atmosphere (
Table 1). The degradation process for PA6 and PA6/Exolit formulations exhibited two steps while the PA6/DTE-DOPO formulation presented three steps of decomposition. The earlier decomposition temperature (approximately 7 °C less than in N
2) and the increased number of decomposition stages for all PA6 formulations, indicate a thermo-oxidative behavior of all samples. The major decomposition step of PA6 could be observed between 449 and 549 °C and it is attributed to the volatilization of the small chain fragments and monomer units present in the polymer, which may be related to oxidative-induced cross-linking of the chains. In the case of the PA6/DTE-DOPO formulation, the first degradation step at 406 °C may be due to the decomposition of the FR additive, the second decomposition step registered at 547 °C could be attributed to the degradation of the triazine ring, while the third maximum mass loss rate at 725 °C might be ascribed to the degradation of the remaining products. Literature data also show that the decomposition of the –NH
2 unit and of the triazine ring at about 400 and 550 °C, respectively, may lead to the production of nonflammable gas, which can help to the formation of a nonflammable gas barrier region [
39]. For the PA6/Exolit formulation,
Tmax1 observed at 424 °C may be attributed to the destabilization of polymer chains caused by the decomposition or volatilization of the additive, while
Tmax2 may be due to the degradation of the system. Such a phenomenon is common for gas-phase active FR for PA6 due to the fire inhibition mechanism of the additives, being attributed by other authors to the formation of volatile diethyl phosphinic acid following the decomposition of its aluminium salt [
40,
42]. Both PA6 formulations promoted char formation at 700 °C while the absence of the residue in the case of neat PA6 polymer indicated the total decomposition of polymer into gaseous products. The higher amount of char registered for PA6/Exolit (8.9 wt%) when compared to the PA6/DTE-DOPO formulation (6.05 wt%) may be due to the generation of polyphosphoric acid and its salts in the case of Exolit
® OP 1230, which facilitates the formation of the carbonaceous layer [
43]. It is known that the introduction of melamine destabilizes PA6 and leaves no char residue above 600 °C, while DOPO-based FR can exhibit both condensed and gas phase activity [
8,
41]. The 6.05 wt% char in the case of PA6/DTE-DOPO could be explained by the chemical modification of the melamine derivative which enables, as expected from the TGA data of the additive, the promotion of a carbonaceous layer. Higher values for char residue in air were found by other authors for the analyzed PA6,6/OMMT nanoclay system. They concluded that the barrier effect of the silicate layer had a large contribution in the formation of carbonaceous char on the surface of the nanocomposite [
44]. The amount of char obtained in the case of the PA6/DTE-DOPO formulation may indicate, to a certain extent, a condensed-phase activity. Further decomposition studies using PCFC and DIP-MS measurements were carried out to understand the mode of action of the FR additive and will be discussed in detailed in the following sections.
Incorporation of FR additives in PA6 by means of compounding may change the physical and structural properties of the polymer due to the use of heat and shear stress during processing. DSC measurements were used to identify the changes in the molecular structure based on the melting behavior of PA6 formulations. It is known that DSC curves obtained from the first heating cycle reflect the influence of processing conditions, storage conditions and/or sample preparation. Some subtle thermal transitions like side group rotations, segmental motions of the polymer chain, and the specific details of the melting endotherm may be caused by the process history. Therefore, it is important to compare a second heating cycle DSC curve with the first one to distinguish properties characteristic of the polymer with those properties imparted by the process [
45]. The results obtained from the first heating cycle (
Figure 3a) provide information regarding the melting characteristics which influences the mechanical properties of the formulation [
46,
47,
48].
Figure 3.
DSC measurements for PA6 formulations on first (a) and second (b) heating scan.
Figure 3.
DSC measurements for PA6 formulations on first (a) and second (b) heating scan.
The endothermic peak at 224 °C registered for neat PA6 was attributed to the melting of α-phase crystals. Compared to the base polymer, the peak for the PA6/DTE-DOPO formulation shifted to 219 °C, while the peak for PA6/Exolit sample was found in the same region as in PA6, at 224.9 °C. As mentioned earlier, the nature of the peaks registered at 65, 94, and 239 °C for PA6/DTE-DOPO during the first heating may be explained by the influence of processing. The two small endothermic peaks registered below 100 °C may be caused by the evaporation of absorbed water from the PA6 polymer, while the small peak after the melting process may be due to a subtle thermal transition. These peaks do not appear in the second heating curve, i.e., when the thermal history of the polymer has been deleted, confirming the effect of processing. The decrease of melting point for the DTE-DOPO formulation may be attributed to the incorporation of the meltable additive which acts as a plasticizer reducing the density of the amide group interactions, which has direct influence on the ability of forming hydrogen bonding. Thus, as a result of the decreased value of melting point of the PA6/DTE-DOPO formulation, lower processing temperatures could be applied in the compounding step of PA6 and the DTE-DOPO additive. However, due to the non-melting nature of Exolit OP1230, those lower processing temperatures would produce rather inhomogeneous formulations of PA6/Exolit as a result of the limiting interaction of polymer chains with the additive.
The curves recorded in the second heating cycle (
Figure 3b) give information regarding the possible interaction between PA6 and FR additives. The values obtained for the melting temperature of the PA6 formulations were similar to those registered in the first heating cycle: 220.9 °C for neat PA6, 216.3 °C for PA6/DTE-DOPO, and 221 °C for PA6/Exolit. The similarity in shape and position of the melting peaks exhibited in both heating cycles indicate that no polymer degradation happened during thermal processing.
3.2.3. Mechanical Properties of PA6 Formulations
The incorporation of FR additives may influence the mechanical properties of the PA6 formulations as indicated by rheological and DSC measurements. Therefore, various mechanical tests were performed to investigate the effect of introduction of FR additives. The results for neat PA6 and PA6 formulations containing FR additives are listed in
Table 2.
Table 2.
Mechanical properties of PA6 formulations.
Table 2.
Mechanical properties of PA6 formulations.
| Sample | Yield strength [MPa] | Elongation at yield strength [%] | Young’s modulus [GPa] | Hardness [MPa] | Impact strength [kJ/m2] |
|---|
| PA6 | 62.5 ± 6.9 | 6.5 ± 1.0 | 1.19 ± 0.11 | 144.4 ± 4.9 | do not break |
| PA6/DTE-DOPO | 69.1 ± 6.6 | 6.3 ± 0.7 | 1.25 ± 0.06 | 162.1 ± 9.1 | 4.53 ± 0.11 |
| PA6/Exolit | 47.3 ± 6.2 | 6.2 ± 1.2 | 1.17 ± 0.03 | 136.7 ± 5.0 | 5.58 ± 0.06 |
It can be observed that the introduction of the DTE-DOPO FR additive in the PA6 has a slight reinforcing effect due to the increased value of yield strength (69.07 MPa) while the elongation at yield strength (6.3%) is similar in comparison to PA6. In contrast, the incorporation of Exolit® OP 1230 has led to a significant decrease in the yield strength (47.3 MPa), indicating that permanent deformation occurs at lower stress for this formulation.
The evaluation of the Young’s modulus showed higher mean values for PA6/DTE-DOPO (1.25 GPa) with respect to neat PA6 (1.19 GPa) and the PA6/Exolit formulation (1.17 GPa), demonstrating that the incorporation of DTE-DOPO into PA6 helps in obtaining a more rigid material. Moreover, the hardness, defined as a material’s resistance of its surface to the penetration of a harder body, was as well higher for PA6/DTE-DOPO (162 MPa). On the contrary, the PA6-Exolit formulation exhibited the lowest of the hardness values. This result sustains the higher resistance of the newly-developed DTE-DOPO FR additive which exhibits a strengthening effect on PA6.
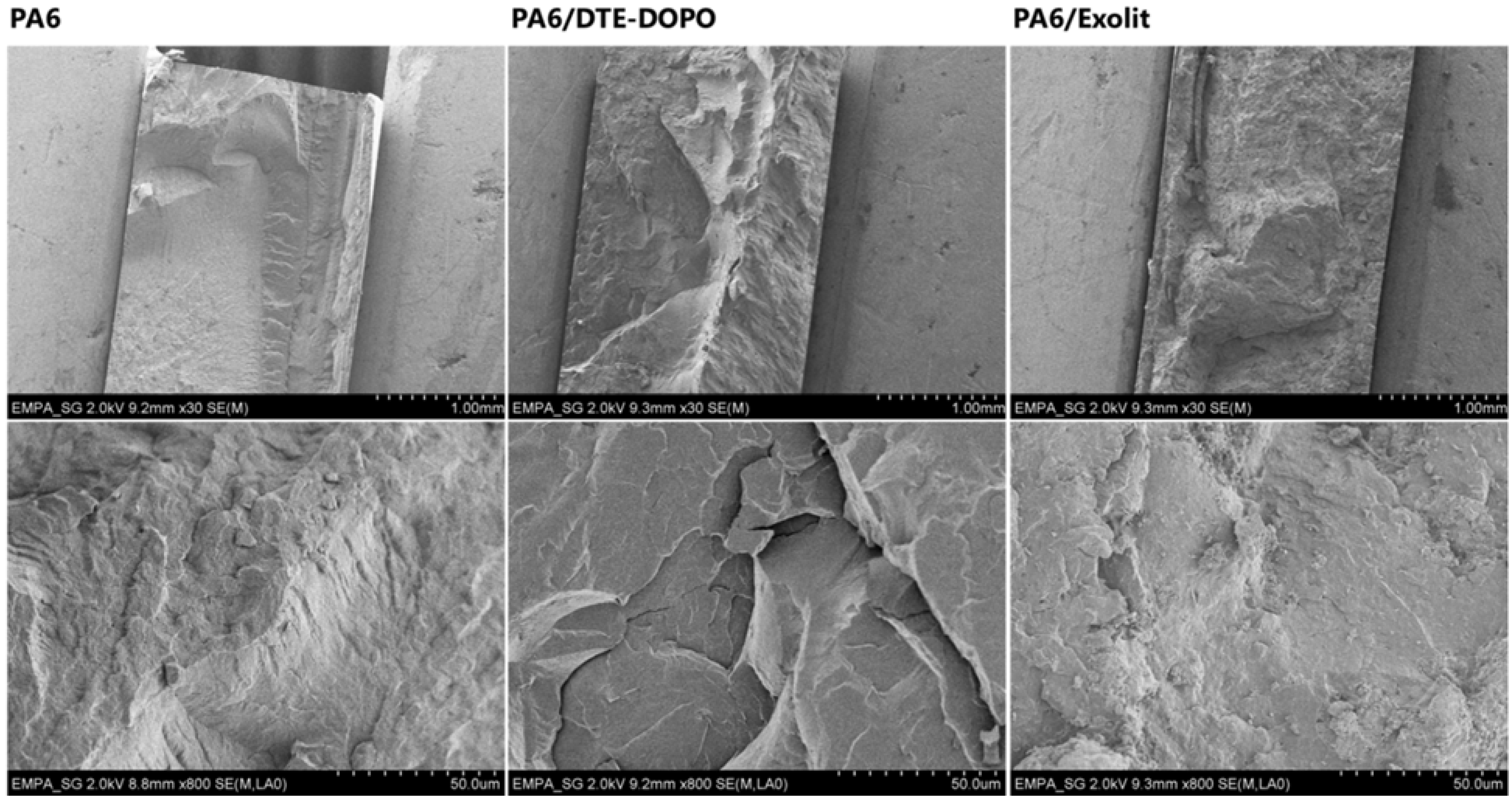
Finally, the results from the impact strength measurements showed no breaking for neat PA6, while PA6/DTE-DOPO and PA6/Exolit formulations registered values of 4.53 kJ/m
2 and 5.58 kJ/m
2, respectively. Observing SEM images the cryogenic fracture of the formulations, one can distinguish distinct lines of fracture propagation for the DTE-DOPO formulation which are associated to an inherent brittleness. Contrary, the other formulations do not present such distinct lines, confirming the lower toughness of the DTE-DOPO formulation. This corroborates the decrease of toughness for the formulation containing
DTE-DOPO what can also be observed in the SEM image from the fracture at liquid nitrogen temperature of PA6/DTE-DOPO formulation (
Figure 4), as well as from the lowest complex modulus (
G*) of this formulation obtained from the rheological measurements (
Figure S3 in
Supplementary files).
The results are similar with the data obtained by rheological measurements where lower values of the loss modulus were registered as compared to neat PA6, suggesting a good interfacial compatibility which restricts the movement of the chain segments, resulting in a lower toughness [
49]. The better compatibility of the PA6/DTE-DOPO formulation when compared to PA6/Exolit may be explained by the nature of the two FR additives:
DTE-DOPO melts during the compounding process enabling a better homogeneity with PA6, while Exolit
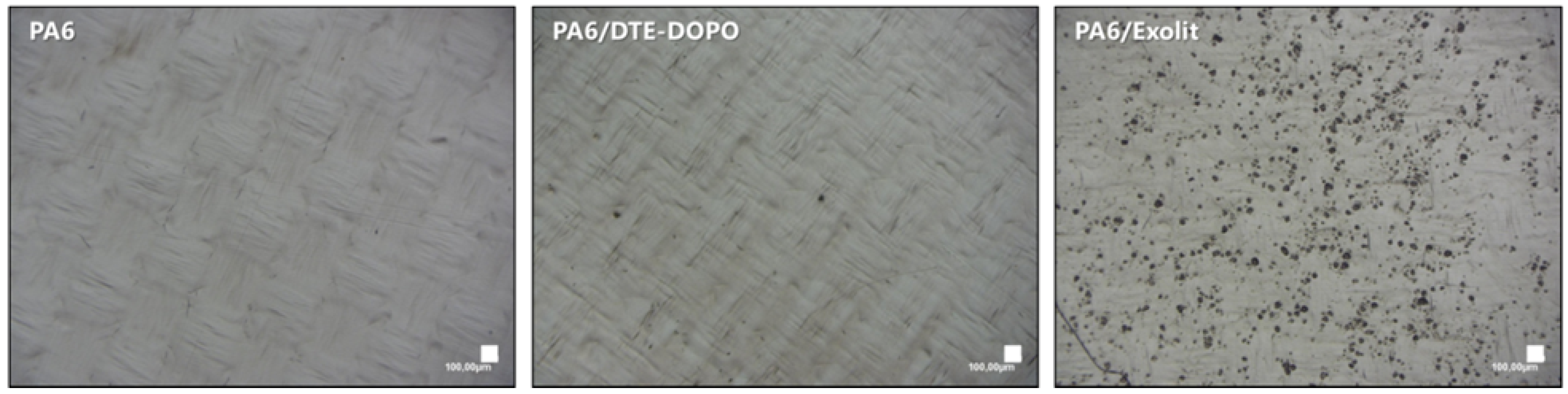
® OP 1230 is a non-meltable salt which may lead to a less uniform distribution in the polymer matrix. To corroborate this statement the distribution of FR additives within the PA6 matrix was analyzed. As can be seen in
Figure 5 the microstructure of the PA6/DTE-DOPO formulation is similar to the neat PA6 while the non-melted Exolit
® OP 1230 particles are clearly visible in PA6/Exolit formulation.
Figure 4.
SEM images of the fracture of neat PA6 and PA6/ FRs formulations after their treatment in liquid N2. Upper row shows the images of the materials located in between the walls of the metallic holder. Lower row presents higher magnification images.
Figure 4.
SEM images of the fracture of neat PA6 and PA6/ FRs formulations after their treatment in liquid N2. Upper row shows the images of the materials located in between the walls of the metallic holder. Lower row presents higher magnification images.
Figure 5.
Transmission optical microscope images of the films prepared from PA6, PA6/DTE-DOPO, and PA6/Exolit formulations by hot compression at 240 °C for 5 min. Bar scales represent 100 µm. For the PA6/Exolit formulation the black points of the image account for the distribution of the Exolit particles within the PA6 matrix.
Figure 5.
Transmission optical microscope images of the films prepared from PA6, PA6/DTE-DOPO, and PA6/Exolit formulations by hot compression at 240 °C for 5 min. Bar scales represent 100 µm. For the PA6/Exolit formulation the black points of the image account for the distribution of the Exolit particles within the PA6 matrix.
3.2.4. Flame Retardancy of PA6 Formulations-PCFC and UL94 Tests
PCFC is a complimentary technique of cone calorimetry which investigates the combustion behavior and the efficiency of flame retardants. The main results obtained from the PCFC measurements (
Table 3 and
Figure 6) are the peak of the heat release rate (
pHRR) defined as the heat release rate divided by the constant heating rate, the temperature at the maximum heat release rate (
TM) and the total heat released per unit initial mass (
THR) [
50].
Table 3.
PCFC data for PA6 and PA6 formulations.
Table 3.
PCFC data for PA6 and PA6 formulations.
| Sample | TM a [°C] | pHRR b [W/g] | THR c [kJ/g] | W800 d [%] | P content [wt%] |
|---|
| PA6 | 476.5 ± 0.2 | 590 ± 8.4 | 28.5 ± 0.2 | 1.01 ± 0.9 | 0 |
| PA6/DTE-DOPO | 445.5 ± 0.3 | 738 ± 14 | 27.4 ± 1.6 | 3.77 ± 0.9 | 1.46 ± 0.08 |
| PA6/Exolit | 450.9 ± 1.7 | 538 ± 14 | 27.4 ± 0.6 | 1.41 ± 0.9 | 3.99 ± 0.07 |
Figure 6.
HRR versus temperature for PA6 formulations (a) and comparative HRR and mass loss derivative for the PA6/DTE-DOPO formulation (b).
Figure 6.
HRR versus temperature for PA6 formulations (a) and comparative HRR and mass loss derivative for the PA6/DTE-DOPO formulation (b).
For neat PA6 the value for the peak heat release rate (
pHRR) was of about 590 W/g at a temperature of 476 °C, for the PA6/DTE-DOPO formulation the
pHRR registered a value of 738 W/g at a
TM of about 445 °C, while for the PA6/Exolit formulation the peak heat release rate was 538 W/g at a temperature of 451 °C. Thus, it can be noticed that the newly-developed triazine-DOPO-based PA6/FR formulation has a higher value of peak heat release rate as compared to the neat PA6, while the PA6 formulation based on the commercially available flame retardant Exolit
® OP 1230 registered a lower value of
pHRR as compared to the base polymer (
Figure 6a). For both FR additives, the temperature for peak heat release rate decreased compared to the virgin PA6,
i.e., 31 and 26 °C less for PA6/DTE-DOPO and PA6/Exolit, respectively. The increase in the value of
pHRR might indicate limited condensed phase effect and a primary gas-phase mechanism of flame inhibition in the case of PA6/DTE-DOPO formulation, while the reduction of
pHRR for PA6/Exolit formulation may be correlated with a comparatively higher condensed-phase activity. Increased
pHRR for PA6/DTE-DOPO formulation observed in PCFC correlates well with the TGA data (DTG curves) presented earlier. This may be due to catalytic decomposition of the PA6 resulting from the interaction of PA6 with the acidic DOPO derivatives. Charring in fire is an anaerobic process, so that the residues obtained after PCFC measurements might be an indication for an additional evidence of a condensed-phase activity in case of the PA6/DTE-DOPO as presented in
Table 3. Other studies based on the PA6 formulation containing organic phosphinate also concluded an additional gas-phase activity for the formulation containing Exolit
® OP 1230 additive as a result of the release of phosphinic acid derivatives. The authors suggested that Exolit
® OP 1230 decomposes, forming diethylphosphinic acid and ethane in vapor phase and aluminium phosphates acts in condensed phase [
51]. Thus, a combined gas and condensed-phase activity can be considered for our PA6/Exolit formulation.
Since constant heating rates are used in both the PCFC and TGA methods, the heat release rate in the PCFC is related to the mass loss rate observed in TGA measurements. In
Figure 6b is presented the heat release rate data together with the mass loss derivative (in N
2) for the PA6/DTE-DOPO formulation. A very good correlation was observed: the thermal degradation exhibited one-step in both curves, while the temperature at the maximum heat release rate was shifted to higher temperatures because of the higher heating rate used in the PCFC (60 °C/min for PCFC
versus 10 °C/min for TGA). Thus, PCFC delivered similar information as the TGA, but in the form of the heat release rate, which is the accepted measure for fire risks [
52].
From the parameters registered by PCFC, a good predictor of combustion efficiency is the total heat release (THR). A slight decrease in
THR for the PA6 formulations compared to the neat PA6 is a possible evidence for the improvement of flame spread and fire load [
53]. Both PA6 formulations registered a reduction in the mean value of
THR of approximately 1 kJ/g relative to PA6 (
Table 3). Therefore, based on the
THR values obtained from the PCFC measurements it can be assumed that the newly-developed
DTE-DOPO FR additive may exhibit a similar flame-retardant behavior compared to Exolit
® OP 1230. In addition, phosphorus content is a determining factor in the efficiency of P containing FRs [
12,
22]. From the data reported in
Table 3 it is obvious that the P content of the PA6/DTE-DOPO formulation (1.46%) is nearly three times lower compared to the PA6/Exolit formulation (3.99%), at a similar FR additive weight percent loading. Therefore, their comparable effectiveness of flame retardancy may suggest a good performance of the triazine-DOPO-based FR additive. The flame-retardant efficiency of the new
DTE-DOPO-based FR in PA6 might be also facilitated by the release of melamine derivatives through the decomposition of FR additive resulting in a dilution of fuel, as implied by the TGA analysis. It is known that an efficient gas-phase flame-retardant activity is determined by the release in the same temperature range of both FR additive and the decomposition products of the polymer [
26]. Therefore, the slight reduction of thermal stability of the triazine-DOPO-based FR compared to neat PA6 may support a gas-phase fire inhibition mechanism.
It should be mentioned that the well-defined conditions of the combustion in PCFC differ essentially from the small scale fire tests in which the fire behavior of the sample also controls the fire scenario. Important fire retardancy mechanisms, such as flame inhibition in the gas phase are active in real fires but not in the complete combustion from the PCFC measurements. While PCFC is a reasonable approach for measurements within the mg-scale with respect to characterizing the intrinsic fire hazard of a material, it cannot replace tests like LOI, UL 94 or the cone calorimeter. PCFC does not measure important physical effects occurring on larger scales, such as dripping, wicking, barrier formation, insulation and flame inhibition. Thus, combining the PCFC data with results from other small scale fire tests enables a quantitative insight into the effectiveness of charring and flame inhibition.
The UL 94 test was also used to determine the efficiency of FRs since it is the most important and widely used method for screening the flammability of polymeric materials. It measures the resistance to sustain ignition by a small flame in a specific, well-defined scenario rather than evaluating a material property. UL 94 flame resistance is determined not only by the thermal combustion properties measured in the PCFC but also by factors such as the viscosity of the melt, which determines dripping behavior, thermal conductivity, specific heat, and the specimen geometry [
35]. The relevant data obtained by UL 94 test are presented in
Table 4.
Table 4.
Results of UL94 flammability test for PA6 formulations.
Table 4.
Results of UL94 flammability test for PA6 formulations.
| Sample | Rating | Cotton ignition | t1 a/t2 b [s] |
|---|
| PA6 | V2 | yes | 2/0 |
| PA6/DTE-DOPO | V0 | no | 0/0 |
| PA6/Exolit | V0 | no | 2/3 |
As expected, PA6 exhibited a self-sustained burning behavior achieving a V2 rating (due to the ignition of the cotton placed under the sample). As a result the recorded afterflame time for the first flame application (t1) was of 2 s. No significant char formation was observed during the UL 94 test, similar to the results obtained by TGA analyses in air. The best test results in terms of rating and afterflame times were obtained in the case of the PA6/DTE-DOPO formulation. No burning drips were observed which led to an eventual V0 classification. Lower concentration of DTE-DOPO (i.e., 15%) resulted in V2 ratings and thus the experimental data related to this formulation is not presented in this work. The absence of afterflame times and the formation of non-burning residue are solid evidence for the gas-phase fire inhibition of the DTE-DOPO additive. Therefore, the observations drawn by UL 94 test are consistent with the ones obtained by PCFC and TGA measurements. In the case of the PA6/Exolit formulation a V0 rating was also achieved but the recorded afterflame times (t1/t2 = 2/3 s) indicated that the self-sustained burning was not fully suppressed. It is known that as the degradation of polymer chains occurs, low viscosity melt drips are formed, which may result in the reduction of the exothermic effect caused by the combustion process. Thus, when the melted polymer drips away from the burning material, the heat is removed from the sample leading to the extinction of fire. Along with the observations obtained from the PCFC and TGA measurements, namely a decrease value of total heat release and a comparable thermal stability, it can be inferred that the enhanced flame resistance of the DTE-DOPO FR additive may be mainly due to a gas-phase activity. The slight char residue observed in both TGA and PCFC experiments together with the non-burning melted drips observed in UL 94 test may support an additional condensed phase mechanism of the additive.
3.2.5. Direct Insertion Probe Mass Spectrometry (DIP-MS)
Combining the data obtained from TGA, PCFC, and UL 94 tests a predominant gas-phase action for the
DTE-DOPO FR additive seems to be dominant in the improvement of fire performance for PA6/DTE-DOPO formulation. In general, an effective flame retardant additive should decompose and release active species in the gas phase at a temperature close to the degradation temperature of the polymer matrix [
26]. Thus, to further analyze the flame retardant behavior of the additives, direct insertion probe mass spectrometry measurements were performed to identify the volatile products released during the thermal decomposition of PA6 formulations.
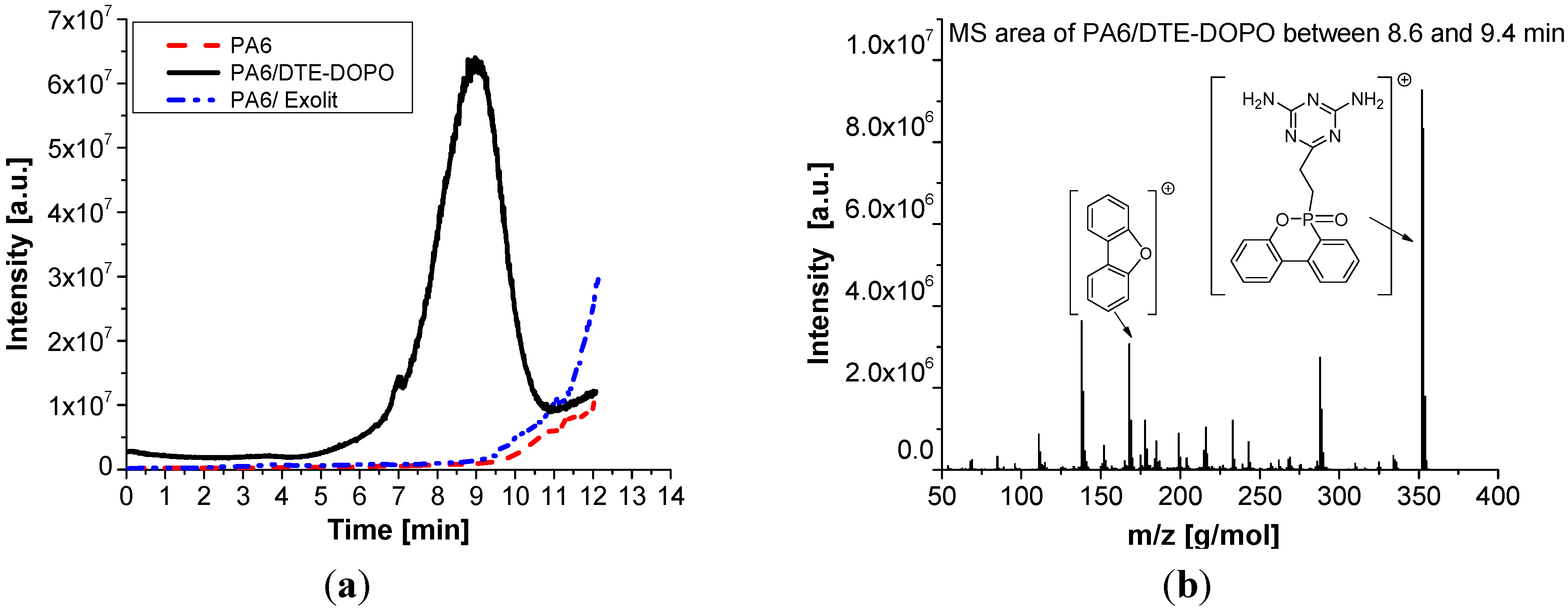
From
Figure 7a it can be noticed that the PA6/DTE-DOPO formulation decomposes or volatizes earlier compared to PA6 and PA6/Exolit formulations, as expected from the initial decomposition temperature measured by TGA. The PA6/DTE-DOPO formulation starts to volatilize or decompose at about 4.6 min and has a maximum decomposition rate at 8.9 min which corresponds to a temperature of approximately 440 °C. After 11 min most of the FR additive was released in the gas phase, but a certain amount of
DTE-DOPO may still remain in the polymer. This was also suggested by the condensed phase activity showed in TGA, PCFC, and UL 94 measurements. The catalyzing effect of the
DTE-DOPO additive on the PA6 formulation shown in TGA behavior can be also observed in the DIP-MS curve, while the efficiency can be confirmed by the long-term presence of the additive in the system (about 6 min). The PA6/Exolit formulation starts to decompose and volatilize at
ca. 9 min, in the same temperature range of PA6, which corresponds to a temperature of approximately 450 °C, while the maximum intensity cannot be detected due to the limitation of the instrument settings.
Figure 7.
DIP-MS data of PA6 formulations: total ion chromatogram (a) and the corresponding mass profile between 8.6 and 9.4 min (b).
Figure 7.
DIP-MS data of PA6 formulations: total ion chromatogram (a) and the corresponding mass profile between 8.6 and 9.4 min (b).
Extracted MS data from total ion chromatogram of the PA6/DTE-DOPO formulation between 8.6 and 9.4 min are presented in
Figure 7b. It can be observed that the
DTE-DOPO FR additive is present as the predominant product in the gas phase as well as some other fragments with a lower intensity, which enhances its flame inhibition action.