1. Introduction
Biologics are becoming a major class of therapeutics and are expected to grow annually at a rate of ~13% [
1]. The biologically derived molecules usually have a short half-life and are prone to physical and chemical instabilities [
2,
3,
4,
5,
6]. Due to short biological half-life, these molecules are delivered by frequent injections. It has been demonstrated that smart polymers can be used to develop delivery systems in order to overcome dosing frequency and increase patient compliance [
7,
8,
9,
10]. One of such polymers is thermosensitive polymer which is soluble in water at room temperature but turns into gel at body temperature and releases the incorporated biologics at a controlled rate for longer duration in biologically and conformationally stable form after a single subcutaneous injection [
11].
When delivering therapeutics using thermosensitive
in situ gel forming delivery systems it is important to take into consideration the size and hydrophobicity of the therapeutics. The amphiphilic nature of these copolymers allows for the sol-gel transition in which the hydrophobic portion of the copolymer is partitioned toward the inside of the gel depot. Therefore, it is thought that a more hydrophobic therapeutic will also be partitioned toward the inside of the depot and complement the hydrophobicity of the core which will provide resistance to interactions with water. In addition, once pores form as therapeutic is released, the size of the therapeutic will dictate the size of the pores needed for release. Large pores will be required for larger therapeutics which causes increased exposure of copolymer to the aqueous environment and thus, faster breakdown of the gel depot which in turn propagates faster drug release. The hydrolytic and enzymatic degradation of hydrogels have been studied [
12,
13,
14]. However, studies on the type of molecules affecting degradation of triblock copolymers are lacking.
By comparing the breakdown of polymer, pore size, and release of model molecules, we planned to establish a relationship between physico-chemical properties of incorporated molecules on controlling the release from thermosensitive copolymer delivery systems. In this study we compared the effect of size and hydrophobicity of three molecules (risperidone (Mw = 410.48 Da; Ko/w = 3.49), insulin (Mw = 5808 Da; Ko/w = 0.02), and bovine serum albumin (BSA) (Mw = ~66,400 Da; Ko/w = 0.007)) on the polymer degradation, pore size, and in vitro release of incorporated molecules from the thermosensitive delivery systems.
2. Materials and Methods
2.1. Materials
Polyelthylene glycol (1500 Da) was purchased from Sigma Aldrich Co. (St. Louis, MO, USA). d,l-lactide was procured from Alfa Aesar (Ward Hill, MA, USA). Stannous octoate was obtained from Pfaltz and Bauer Inc. (Waterbury, CT, USA). MicroBCA protein assay kit was purchased from Pierce Biotechnology Inc. (Rockford, IL, USA). Human recombinant insulin (Incelligent SG) was procured from Millipore Corporation (Norcross, GA, USA).
2.2. Synthesis of Thermosensitive Polymer
The copolymer 1500–1500–1500 (4500 Da) chain length was synthesized by the ring opening polymerization of
d,
l-lactide, catalyzed by stannous octoate, using polyethylene glycol (PEG 1500 Da) as an initiator. The synthesis and characterization of the above copolymer are presented in our earlier publication [
15].
2.3. Preparation of Delivery Systems
Aqueous solution of thermosensitive copolymer was prepared at a concentration of 30% w/w by stirring at 4 °C. BSA, risperidone, and insulin were dispersed into the aqueous copolymer solution at room temperature, and homogenized at 8000 rpm for 30 s. The delivery system prepared without adding drug was used as control. Injectability of the delivery system was examined by passing through a 25 gauge (25G) needle.
2.4. In Vitro Release Behavior of the Delivery Systems
One milliliter of copolymer based delivery system with the help of 25 G needle was injected into the polypropylene tubes and incubated at 37 °C, allowing formulation to change into gel. A pre-warmed phosphate buffer saline (PBS, pH 7.4, 10 mL) was added slowly over the gel depot as release medium, and the entire assembly was incubated at 37 °C in a water bath. The release medium was replaced intermittently for the entire study period, and the amount of protein released was quantified by Pierce Micro BCA™ protein assay kit [
16]. An Agilent 1120 compact LC system was used to determine the amount of risperidone released [
17]. Briefly, an Agilent Eclipse Plus C18 column (4.6 mm × 150 mm, 5 μm, Agilent, Santa Clara, CA, USA) was used, and risperidone analysis was performed using isocratic elution. The mobile phase consisted of methanol and ammonium acetate buffer (10 mM, pH 5.5) in the ratio of 85:15 at a flow rate of 1 mL/min. Run time was 5 min and absorbance was monitored continuously at 280 nm. EZChrom Elite™ 3.3.2 software (Agilent, Santa Clara, CA, USA) was used for data acquisition and analysis. The concentration correction was performed according to the method described by Hayton and Chen [
18].
2.5. Mass Loss of Polymer Hydrogels during In Vitro Release
The delivery systems prepared for in vitro release studies were also evaluated for percent mass loss. In order to study the effect of size and Ko/w of incorporated molecules on the polymer mass loss, the release medium was removed at fixed time intervals. The delivery systems were freeze-dried, weighed and the percent mass loss was calculated.
2.6. Hydrolytic Degradation and Drug Release Behaviors of the Delivery Systems
The delivery systems were evaluated to study the time dependent hydrolytic degradation of the PLA–PEG–PLA copolymer for mass loss. Proton Nuclear Magnetic Resonance (Proton NMR, Varian Inc., Palo Alto, CA, USA) and Gel Permeation Chromatography (GPC, Waters 515, Milford, MA, USA) were used to determine the reduction in molecular weight during hydrolytic degradation.
2.7. Hydrolytic Degradation of Copolymer Determined by Proton NMR
The freeze dried copolymer residues obtained at particular time points were dissolved in deuterated chloroform (CDCl3), and proton NMR spectra was recorded on a Varian Spectrometer at 400 MHz and 25 °C. Tetramethylsilane (TMS) signal was used for calibration and its signal was taken as the zero chemical shift. The proton NMR signals were integrated and the ratio of lactic acid (LA) to ethylene glycol (EG) moieties was used to determine the degradation behavior.
2.8. Hydrolytic Degradation of Copolymer Determined by GPC
GPC was used to study the change in molecular weight, and molecular weight distribution of copolymers during hydrolytic degradation. The freeze dried copolymer residues acquired at 0, 30, 60 and 90 days were dissolved in tetrahydrofuran (THF). The samples were analyzed using GPC (Waters 515, Milford, MA, USA) equipped with Waters 2410 refractive index detector. Styragel® HR4E and HR5E columns (Milford, MA, USA) were used. The analysis was based on the calibration using polystyrene standards and tetrahydrofuran (THF) as a carrier solvent at 30 °C with a flow rate of 1 mL/min and sample volume 100 μL.
2.9. Morphology of Polymeric Delivery Systems Determined by Scanning Electron Microscopy (Cryo-SEM)
The surface morphology and pore size of the delivery system were visualized using Cryo-SEM. The formulations were injected into the polypropylene tubes and incubated at 37 °C, to change into gel. The in vitro release study was carried out as per the procedure described previously. At 30 and 60 days the release medium was decanted, and the delivery system was flash frozen by immersing in liquid nitrogen to avoid the ice crystal formation during freezing, as well as to minimize the alteration in gel structure. The clean surface of the frozen sample was obtained by cutting down the excess frozen sample by a sharp, cold scalpel. The frozen copolymer mounted brass was visualized using JEOL JSM- 6490LV (JEOL USA, Inc., Peabody, MA, USA) high performance variable pressure SEM with a laser beam (15 kV acceleration voltage) under low vacuum, and at 1500× magnification. Sublimation under low vacuum (35 Pa) helped to remove the frozen layer of water from the surface. Two hundred pores were measured for their sizes. The formulation without incorporated molecule (control) was maintained at room temperature (25 °C), and 37 °C. A small amount of copolymer solution maintained at room temperature was mounted on a brass mount, and immediately the mount was immersed into liquid nitrogen to flash freeze the copolymer solution. The surface of the delivery system was cut with the cold scalpel, the frozen copolymer mounted brass was visualized immediately (0 min), and at 5 min under SEM with same conditions.
2.10. Statistical Analysis
For statistical analysis, a single factor ANOVA was performed using Minitab 16 statistical software (Minitab Inc., State College, PA, USA). A p-value of less than 0.05 was considered to be significant. Data are expressed as mean ± standard deviation (SD) and n is the sample size.
3. Results and Discussion
In vitro release characteristics of small protein insulin, large hydrophilic protein BSA, and a small hydrophobic molecule, risperidone from the polymeric delivery system containing 30%
w/
w PLA–PEG–PLA are shown in
Figure 1. The polymer degradation pattern of hydrogels in the presence of these different molecules was studied using NMR and GPC, while the gel morphology was visualized using Cryo-SEM.
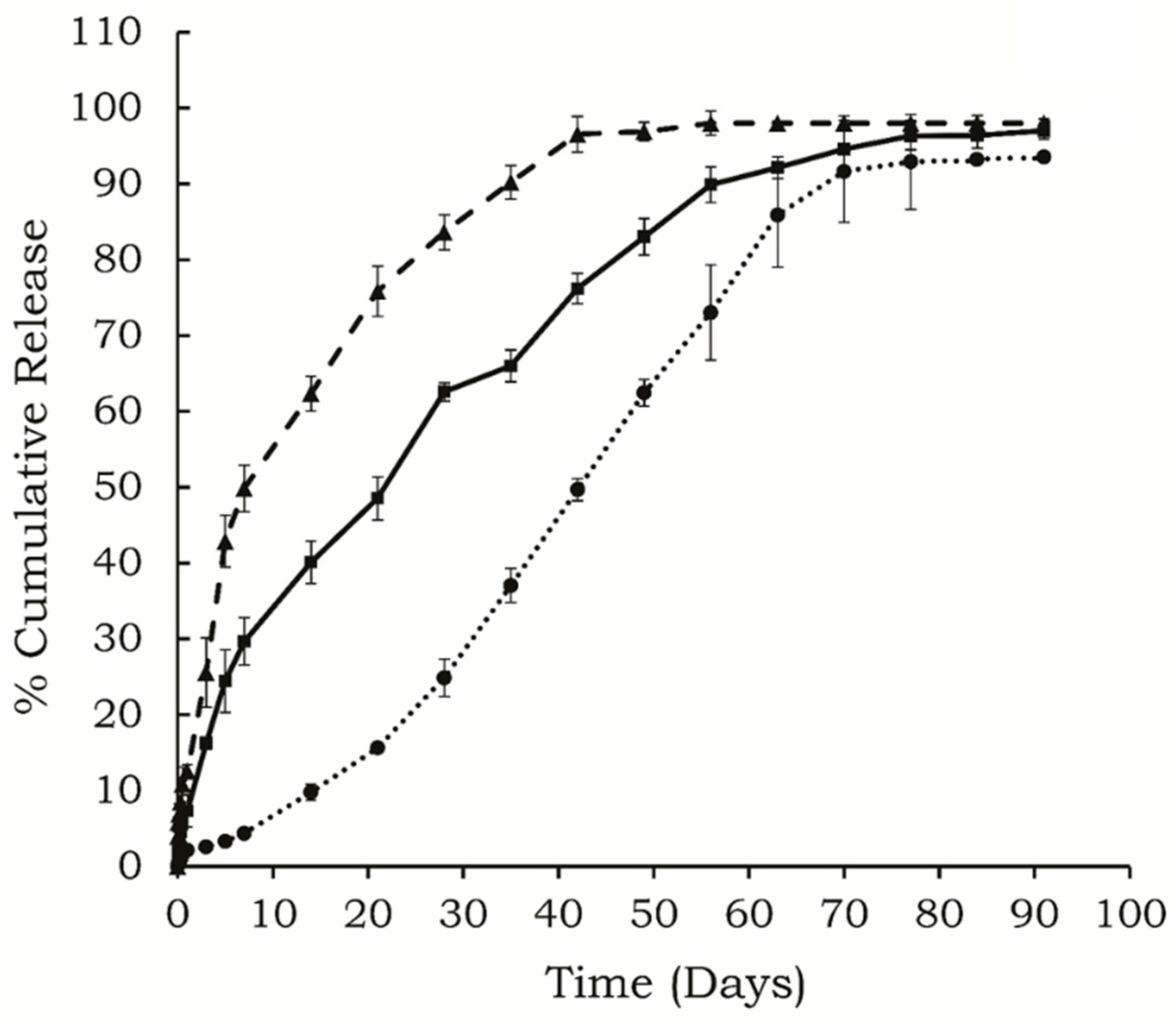
Figure 1.
In vitro release profiles of (●) risperidone, (▲) BSA, and (■) insulin released from 30% w/w copolymer containing delivery systems, (n = 4, mean ± SD, drug loading: 0.3% w/v).
Figure 1.
In vitro release profiles of (●) risperidone, (▲) BSA, and (■) insulin released from 30% w/w copolymer containing delivery systems, (n = 4, mean ± SD, drug loading: 0.3% w/v).
3.1. In Vitro Release
Figure 1 shows
in vitro release profile of risperidone, insulin, and BSA from the delivery systems. It was observed that only 10% of the total risperidone was released in the initial 15 days, and the overall release period lasted for approximately 77 days. BSA showed the highest initial burst release (13.5% ± 0.9%), followed by insulin (7.3% ± 3.1%). The BSA was released rapidly over the initial 7 days, followed by a slow release phase up to 42 days. Insulin was released over 70 days in a controlled manner after an initial burst release. The correlation coefficients for BSA, insulin, and risperidone were 0.76, 0.86 and 0.98, respectively, for zero order release kinetics. These three molecules differ in their size and
Ko/w partition coefficient, which leads to differences in their distribution in the copolymeric delivery system.
PLA–PEG–PLA forms micelles in aqueous environment with hydrophilic PEG facing the aqueous phase forming the ‘shell’ and hydrophobic PLA forming the “core” region. Due to the core-shell structure the partitioning of drug molecules depends on their
Ko/w partition coefficient and influences different release profiles. Hydrophobic drug usually partitions into the micellar core and results in prolonged release [
19]. Therefore, along with the polymer structure and concentration, the release profile also depends on the physical and chemical properties of the incorporated molecule.
The size of molecules is another key factor affecting its release from the porous hydrogel. A larger protein is supposed to be released slower than a smaller protein, because it takes more time for a larger molecule to diffuse out through the narrow interconnected channels of the hydrogel matrix [
20]. BSA is an ellipsoid protein with ~66,400 Da molecular weight, while insulin is a small protein (~6,000 Da), and hence, BSA should be released slower than insulin; however, it was observed that the release of BSA was much faster than insulin [
21,
22]. Water solubility is another feature which influences the release pattern and duration of various molecules and may be partly responsible for the observed release profiles. Molecules with high water solubility show immediate release from the hydrogel probably due to faster dissolution and diffusion from the delivery system. BSA has high water solubility (aqueous solubility: ~40 mg/mL) [
22], while risperidone is a small hydrophobic molecule (molecular formula: C
23H
27FN
4O
2) with very low water solubility about 2.8 μg/mL [
23]. Insulin also has limited solubility at neutral pH (~0.1 mg/mL) [
2]. Our studies indicated that BSA showed highest initial burst release and released over shorter duration, followed by insulin and risperidone.
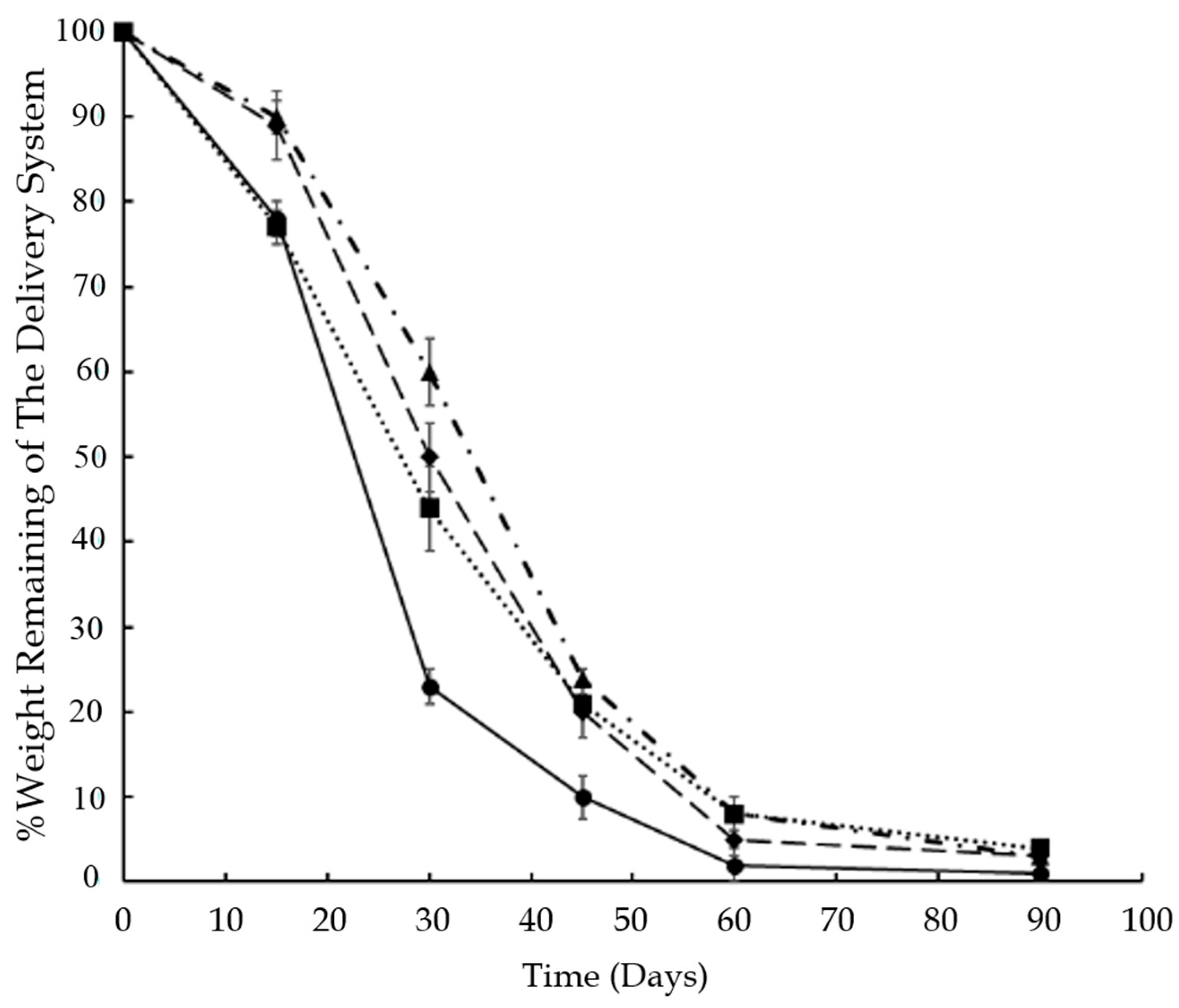
3.2. Mass Loss of Polymer Hydrogels during In Vitro Release
It was observed that the hydrophobicity of the incorporated molecule affected the mass loss in the hydrogels. The degradation behavior of the delivery system in the presence of different molecules can be visualized in
Figure 2. The delivery system containing BSA degraded faster as compared to risperidone and insulin containing delivery systems, and the mass loss was significantly higher (
p < 0.05) until 45 days of release.
Figure 2.
Weight loss of the delivery system during in vitro release of (●) BSA, (▲) risperidone; (♦) insulin and (■) control, from 30% w/w copolymer containing delivery systems, (n = 4; mean ± SD).
Figure 2.
Weight loss of the delivery system during in vitro release of (●) BSA, (▲) risperidone; (♦) insulin and (■) control, from 30% w/w copolymer containing delivery systems, (n = 4; mean ± SD).
3.3. Hydrolytic Degradation of Polymer Hydrogels Determined by Proton NMR
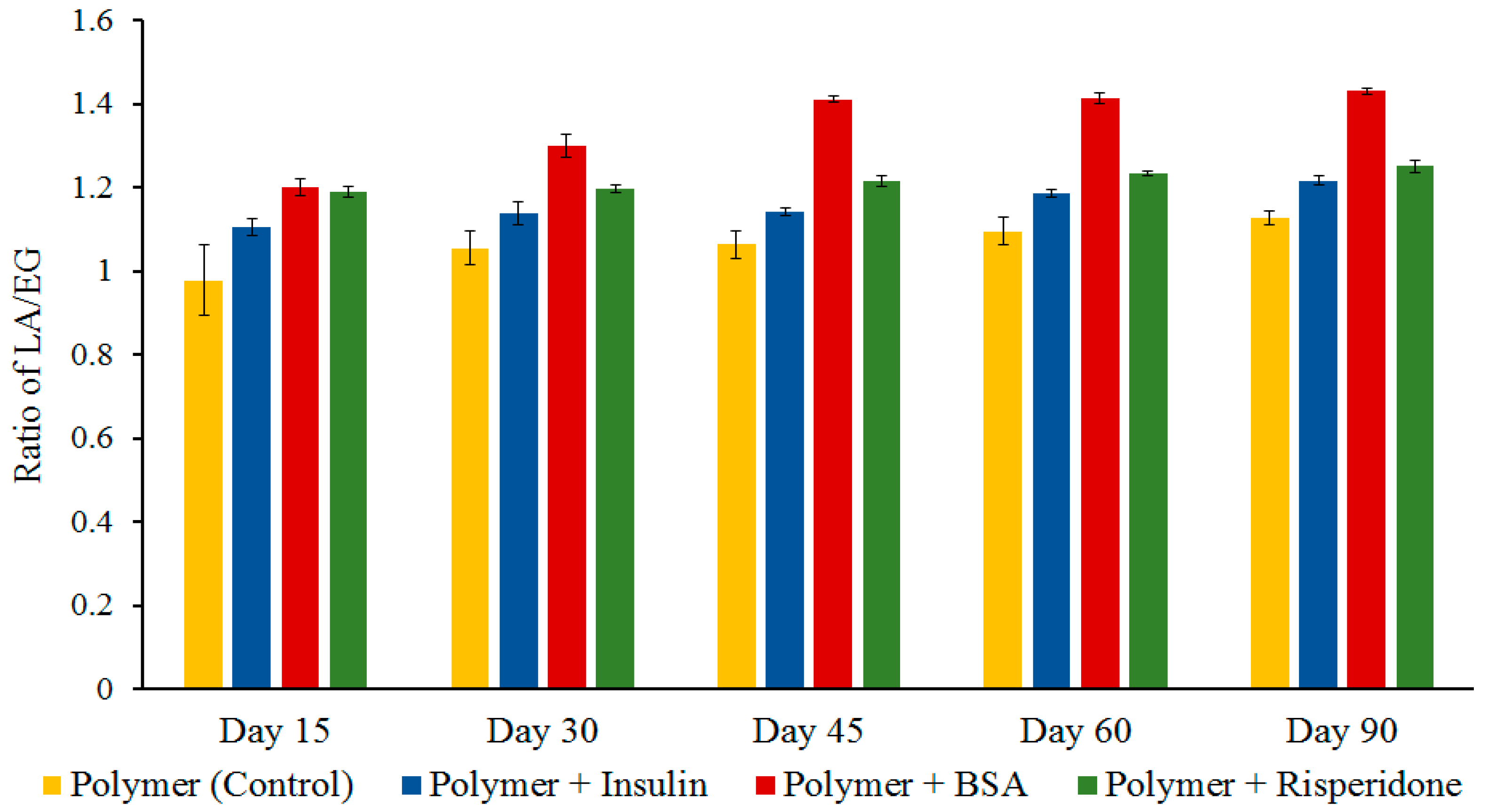
The changes in the relative amount of LA and EG content of the copolymer after degradation in PBS (pH 7.4) at 37 °C can be seen in
Figure 3. The change in the peak height of –CH
3 of PLA (1.55 ppm) and –CH
2 of PEG (3.65 ppm) in proton NMR spectra was used to evaluate the content of lactide (LA) and ethylene glycol (EG), respectively. In the case of BSA containing delivery system the ratio of LA and EG increased to 1.4 after 45 days of degradation, indicating preferential loss of hydrophilic PEG segments. The delivery systems containing insulin and risperidone showed gradual increase in LA/EG ratio.
The residual polymer appearance was visually compared and it was noted that the BSA loaded delivery systems appeared more porous. However, once the polymer degradation started and became predominant, larger pores were formed in the hydrogel resulting in widening the channels leading to increased release irrespective of the size of the molecule. Still, the release rate for risperidone was much lower, which was exclusively contributed to its high hydrophobicity, leading to slower degradation of the copolymers. It has been reported that the delivery systems containing larger proteins form larger pores on the surface which allows initial dissolution of protein present on the surface [
24]. The polymeric delivery system consisting of BSA (large hydrophilic protein, ~66,400 Da) might have formed larger channels near the surface during initial release, which led to rapid penetration of water molecules inside the gel. Interconnecting channel formation and solvation of protein in the previously formed channels within the polymer matrix along with the water penetration enhances polymer degradation [
24]. All of these combined effects resulted in the faster release of BSA.
Figure 3.
The change in LA/EG ratio of the delivery system during in vitro release (n = 4, mean ± SD). LA, lactide; EG, ethylene glycol.
Figure 3.
The change in LA/EG ratio of the delivery system during in vitro release (n = 4, mean ± SD). LA, lactide; EG, ethylene glycol.
Since the delivery systems containing a smaller protein (insulin ~5808 Da), and a hydrophobic molecule (risperidone, 410 Da) formed smaller channels in the gel matrix, they got entrapped in the polymer matrix resulting in low initial burst release and prolonged release duration. Though the delivery system containing small protein forms a higher percentage of small pores, this type of structure entraps protein within the delivery system due to the collapsing of channels [
24]. Some additional factors, such as affinity of protein for the polymer due to ionic and hydrophobic interactions, charge on the protein at physiological pH, and polymer degradation/erosion can also play an important role in protein release from the polymeric delivery systems [
25].
3.4. Hydrolytic Degradation Determined by GPC
GPC helped to determine the change in the molecular weight of copolymer during hydrolytic degradation. GPC results of polymer alone, and loaded with BSA, insulin, and risperidone analyzed over the release duration are summarized in
Table 1. The drug loaded delivery systems showed noticeable degradation after 30 days of
in vitro release. As the degradation proceeded, the chromatogram showed a bimodal distribution and a reduction in molecular weight (
Mn) of polymer illustrated by an increase in retention time (RT). Increase in the polydispersity index (PDI) indicated that the rate of degradation was fastest in the case of the polymeric delivery system containing BSA. The reduction in molecular weight was rapid after 30 days of incubation, and at the end of 60 days, most of the polymer was hydrolyzed into smaller segments indicated by the corresponding increase in RT. At the end of 90 days, no peak corresponding to original polymer was detected, while an increase in polymer degradation products with RT near 20 min was noticed.
Table 1.
Molecular weight of the polymer PLA–PEG–PLA (4500 Da) remaining after hydrolytic degradation in phosphate buffer saline (PBS), pH 7.4 at 37 °C.
Table 1.
Molecular weight of the polymer PLA–PEG–PLA (4500 Da) remaining after hydrolytic degradation in phosphate buffer saline (PBS), pH 7.4 at 37 °C.
| Sample | Time | Mn (1) | RT | PDI | Mn (2) | RT | PDI |
|---|
| Blank polymer (1500–1500–1500) | 0 day | 4,344 | 17.9 | 1.1 | – | – | – |
| 30 days | 3,177 | 18.0 | 1.3 | 335 | 20.8 | 1.2 |
| 60 days | 2,673 | 18.2 | 1.2 | 200 | 20.8 | 1.2 |
| 90 days | – | – | – | 188 | 20.9 | 1.2 |
| Polymer + Insulin | 0 day | 4,344 | 18.0 | 1.1 | – | – | – |
| 30 days | 3,157 | 18.0 | 1.3 | 317 | 20.6 | 1.2 |
| 60 days | 2,073 | 18.1 | 1.3 | 258 | 20.8 | 1.4 |
| 90 days | – | – | – | 200 | 20.8 | 1.2 |
| Polymer + Risperidone | 0 day | 4,344 | 18.0 | 1.1 | – | – | – |
| 30 days | 2,567 | 18.1 | 1.2 | – | – | – |
| 60 days | 1,902 | 18.2 | 1.3 | 200 | 20.8 | 1.2 |
| 90 days | – | – | – | 208 | 20.8 | 1.2 |
| Polymer + BSA | 0 day | 4,344 | 18.0 | 1.1 | – | – | – |
| 30 days | 2,431 | 18.0 | 1.3 | 225 | 20.7 | 1.2 |
| 60 days | – | – | – | 307 | 20.7 | 1.2 |
| 90 days | – | – | – | – | – | – |
3.5. Morphology of Polymeric Delivery System Determined by Cryo-SEM
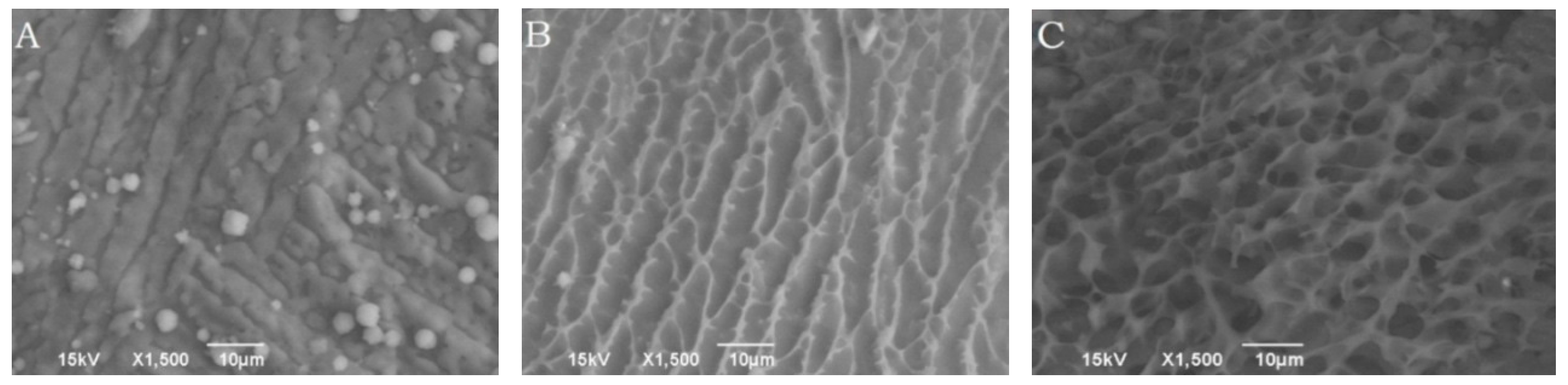
The surface morphologies of copolymer solution maintained at room temperature (25 °C) and body temperature (37 °C) visualized using Cryo-SEM are presented in
Figure 4, which shows a marked difference in their appearance. It can be seen in
Figure 4A,B, the copolymer solution which was maintained at 25 °C did not show any three dimensional surface structures in the freshly cut surface of copolymer solution. According to the approach orientation of the scalpel blade, the delivery surface exhibited striations oriented along the cut surface. Alternatively, copolymeric solution maintained at body temperature showed the presence of a three dimensional surface structures, which consisted of two distinct domains. The dense white area represented the polymer rich portion; whereas the dark empty spaces were observed to be the water filled pores (
Figure 4C).
Figure 4.
Cryo-SEM images visualizing the morphologies of freshly cut surfaces of the polymeric delivery systems maintained at 25 °C, (A): 0 min and (B) 5 min after sublimation; and (C) at 37 °C, 5 min after sublimation.
Figure 4.
Cryo-SEM images visualizing the morphologies of freshly cut surfaces of the polymeric delivery systems maintained at 25 °C, (A): 0 min and (B) 5 min after sublimation; and (C) at 37 °C, 5 min after sublimation.
3.6. Morphology of the Delivery Systems Determined by Cryo-SEM
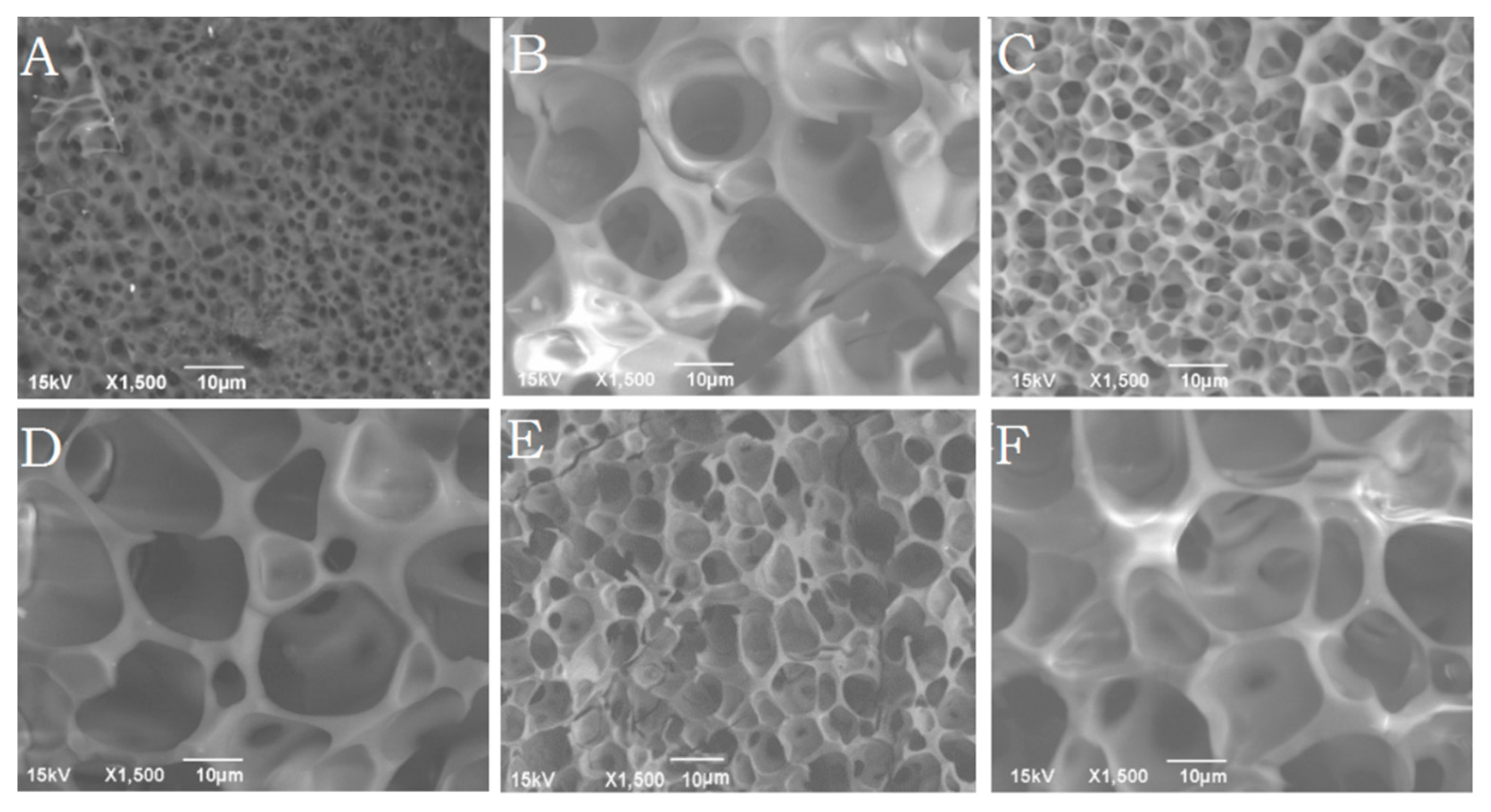
Figure 5A–F shows the morphology of the delivery systems after incorporation of insulin, BSA, and risperidone. It was observed that the size of pores formed in the delivery system was dependent on the type of drug incorporated.
Table 2 shows the average size of pores formed in the delivery systems at various drug loading. By the end of day 30, the hydrogel containing BSA showed significantly bigger (
p < 0.05) pores (4.0 ± 1.5 μm) than insulin (2.5 ± 0.9 μm), or risperidone (1.0 ± 0.3 μm) loaded hydrogels as shown in
Figure 5A,C,E. The smallest average pore size was observed in case of risperidone loading which was smaller than blank polymer solution. At the end of 60 days, pore size increased irrespective of drug loading and no significant difference was observed in the mean pore size (
Figure 5B,D,F). The BSA containing delivery system appeared more porous, indicating that polymer degradation took place faster in presence of a large hydrophilic protein. A striking difference in the porous morphologies of the delivery systems was seen during release of risperidone, BSA, and insulin. The results also suggested that the hydrophobic nature and solubility of the incorporated molecule considerably affected the porous structure of the hydrogel. BSA loaded delivery systems showed the presence of large pores on the surface which suggested that BSA might have escaped easily from the gel, creating bigger, open, water accessible pores as compared to risperidone and insulin.
Figure 5.
Cryo-SEM images of porous morphology of freshly cut surfaces of the polymeric delivery systems loaded with A,B: Risperidone (30 and 60 days), C,D: Insulin (30 and 60 days), and E,F: BSA (30 and 60 days), maintained at body temperature (5 min sublimation).
Figure 5.
Cryo-SEM images of porous morphology of freshly cut surfaces of the polymeric delivery systems loaded with A,B: Risperidone (30 and 60 days), C,D: Insulin (30 and 60 days), and E,F: BSA (30 and 60 days), maintained at body temperature (5 min sublimation).
Table 2.
Average size of pores formed in the delivery systems loaded with different molecules.
Table 2.
Average size of pores formed in the delivery systems loaded with different molecules.
| Incorporated Molecules | Day 30 (Average Pore Size) | Day 60 (Average Pore Size) |
|---|
| Blank (Polymer only) | 2.2 ± 0.4 μm | 10.8 ± 1.7 μm |
| Risperidone | 1.0 ± 0.3 μm | 9.7 ± 2.8 μm |
| Insulin | 2.5 ± 0.9 μm | 12.6 ± 2.6 μm |
| BSA | 4.0 ±1.5 μm | 15.3 ± 3.7 μm |