1. Introduction
Conventional treatment for psychosis and other neurological diseases has generally proved inadequate, mainly due to the restrictive blood-brain barrier (BBB). The systemic delivery of antipsychotic drugs results in minimal transport of these agents across the BBB and results in therapeutic failure [
1,
2,
3,
4,
5,
6]. Therapeutic failure of the majority of neurotherapeutic drugs relies on the fact that these drugs are only transported minimally across the BBB. Apart from this, the lack of innovation in product development to counteract these challenges is also a major contributing factor to a poor therapeutic outcome [
3,
7].
Nanotechnology provides many promising solutions to impediments arising from conventional neurotherapy. One of the major advantages of using nano-carriers is the fact that modification of the drug, intended for central nervous system (CNS) delivery, is not required. Furthermore, surface modification of these diminutive capsules may allow for brain entry of the drug molecules after an intravenous dose. This is achieved by receptor mediated transport [
8]. A number of studies have established the successful nano-encapsulation of antipsychotic drugs in order to optimize neurotherapy of psychotropic disorders [
9,
10,
11,
12,
13].
Benvegnú and co-workers (2011) successfully incorporated the antipsychotic drug, haloperidol into polysorbate nanocapsules. Extensive
in vivo testing on the Wistar rat model proved that the method was feasible in terms of improving the efficacy of haloperidol with antipsychotic effects of the drug being maintained for longer periods of time when compared to free haloperiodol. In addition, the acute and sub-acute extra-pyramidal motor disorders were also significantly reduced when compared to the administration of free haloperidol [
11]. Muthu and co-workers 2009 synthesized risperidone-loaded, poly(
d,
l-lactide-
co-glycolide) (PLGA) nanocapsules that were matricized in a thermo-responsive
in situ gel and administered subcutaneously in the Swiss albino mouse model. The antipsychotic effects of risperidone as well the marked lack of extra-pyramidal side effects were demonstrated by the system, thus confirming its success [
10]. A similar study by Silva and co-workers 2011, investigated the potential of entrapping risperidone in solid lipid nanocapsules (SLN), intended for oral administration. Results revealed highly stable risperidone-loaded SLNs with negligible toxicity when tested with Caco-2 cells using the (4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) assay [
13].
With regard to the design of nanocapsules in optimizing therapeutic efficacy and success, various parameters need to be considered. Factors such as lipophilicity, polarity, nanocapsule size, drug entrapment and drug release kinetics are all relevant. Popular methods for the design and development of nanocapsules include; the solvent evaporation method, spontaneous emulsification/solvent diffusion method, salting out/emulsification-diffusion method, supercritical fluid technology, ionic gelation and polymerization of monomers techniques [
14,
15,
16]. Most of these methods prove unsatisfactory and are related to many shortcomings such as; the inability to scale-up for large-scale pilot production, limited solubility of polymers in supercritical fluids and most of all, toxicity of using organic solvents. Apart from being environmentally hazardous, organic solvent impurities may remain in the nanocapsule post-synthesis and become physiologically dangerous and may even cause degradation of the active within the polymer matrix [
14].
This study uses this principle of adaptation and implements a melt dispersion technique for the preparation of CPZ-loaded nanocapsules taking into consideration the chemical and molecular properties of the polymer to be used, poly-ε-caprolactone (PCL) and the active water-soluble model antipsychotic drug Chlorpromazine (CPZ). Entrapment within the nanocapsules would control its release of the entrapped drugand enhance site-specific delivery, thereby reducing dosing amount and frequency and minimizing systemic side effects. PCL is a hydrophobic, semi-crystalline, thermoplastic polymer with five non-polar methylene groups in its repeating unit and a relatively polar ester group. In physiological conditions, PCL undergoes very slow biodegradation compared to other biodegradable polyesters (e.g., polyglycolic acid, poly-
l-lactide and their copolymers) by hydrolysis of its ester linkages and is biocompatible with surrounding tissues. This has sparked widespread interest in the polymer for use in long-term drug delivery systems lasting greater than a year [
17,
18,
19]. The use of PCL nanocapsules to successfully entrap a variety of pharmacological actives to improve their therapeutic efficacy has been well documented. Some of these actives include but are not limited to; anti-cancer drugs, anti-inflammatories, beta blockers, anti-infective agents, insulin, immune modulators, proteins and psychotropic agents [
17,
19]. The majority of PCL nano-carriers are synthesized by nanoprecipitation, solvent displacement and solvent evaporation [
19]. As previously discussed, these methods utilize hazardous and potentially physiologically dangerous organic solvents. The aim of this study was to design, synthesize and optimize CPZ-loaded, PCL based nanocapsules using a melt-dispersion technique that is non-arduous, inexpensive and devoid of any hazardous organic solvents, thereby eliminating the limitations of conventional nanocapsule synthesis, in particular, PCL-based nanocapsule synthesis. The study takes advantage of the thermoplastic nature of PCL which allows it to become soft and pliable once heated passed its melting point and then subjected to extreme shear whilst cooling to room temperature, thereby reforming the PCL and maintaining its integrity. From a formulatory aspect, Polysorbate 80 was included in the nanocapsule system for its stabilizing and cryoprotectant properties, thus counteracting agglomeration of the PCL nanoparticles (e.g., on centrifugation) and as a cryoprotectant to prevent freezing damage to the PCL polymer on lyophilisation of the nanoparticulate suspension. Mechanistically, Polysorbate 80 has been demonstrated to enhance endocytosis across the BBB via enhanced adsorption of the plasma apolipoprotein E (Apo E) on the surface of these particles. The Apo E assists in the transport of lipids into the brain by the low-density lipoprotein (LDL) receptors [
8].
Following optimization, the cytotoxicity of the optimized nanocapsule system on a neuronal PC12 cell line was ascertained. The optimized nanocapsules developed were subsequently incorporated within an implantable polymeric membrane system and implanted into the frontal lobe of the brain of the New Zealand Albino rabbit model with evaluation of CPZ levels locally and systemically compared to intramuscular CPZ administration; as well as histomorphological evaluation of the effect of the implanted system on surrounding brain tissue.
2. Experimental Section
2.1. Materials
Poly-ε-caprolactone (PCL), chlorpromazine hydrochloride, Glycerol B.P., Polysorbate 80, hexamethylenediamine (HMD) (Mw = 116.2 g/mol), sebacoyl chloride (SC) (Mw = 239.1 g/mol), anhydrous n-hexane, ethylcellulose (EC), dialysis tubing, anhydrous potassium bromide, isoniazid (INH), and anhydrous sodium hydroxide pellets were purchased from Sigma Aldrich (St. Louise, MO, USA). De-ionized water was obtained from a Milli-Q water purification system (Milli-Q, Millipore, Billerica, MA, USA). All solvents utilized for UPLC-UV detection were of UPLC (Ultra-performance liquid chromatography) grade and other reagents were of analytical grade and used as purchased. The CytoTox96® Non-Reactive Cytotoxic Assay which measures cell viability was purchased from Promega Corporation (Madison, WI, USA). Mammalian Pheochromocytoma (PC12) neuronal cells were purchased from the Health Science Research Resources Bank (HSRRB, Osaka, Japan).
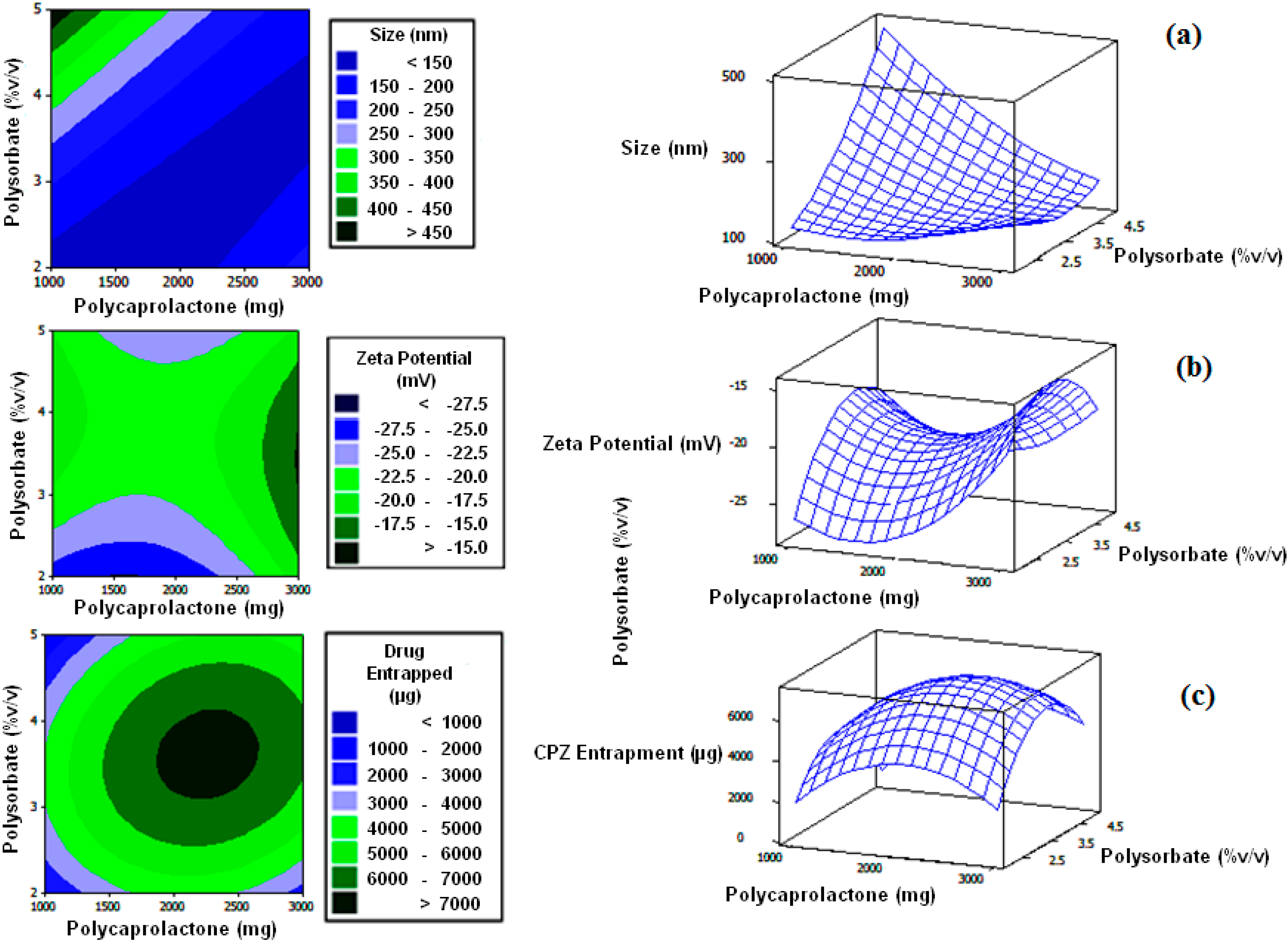
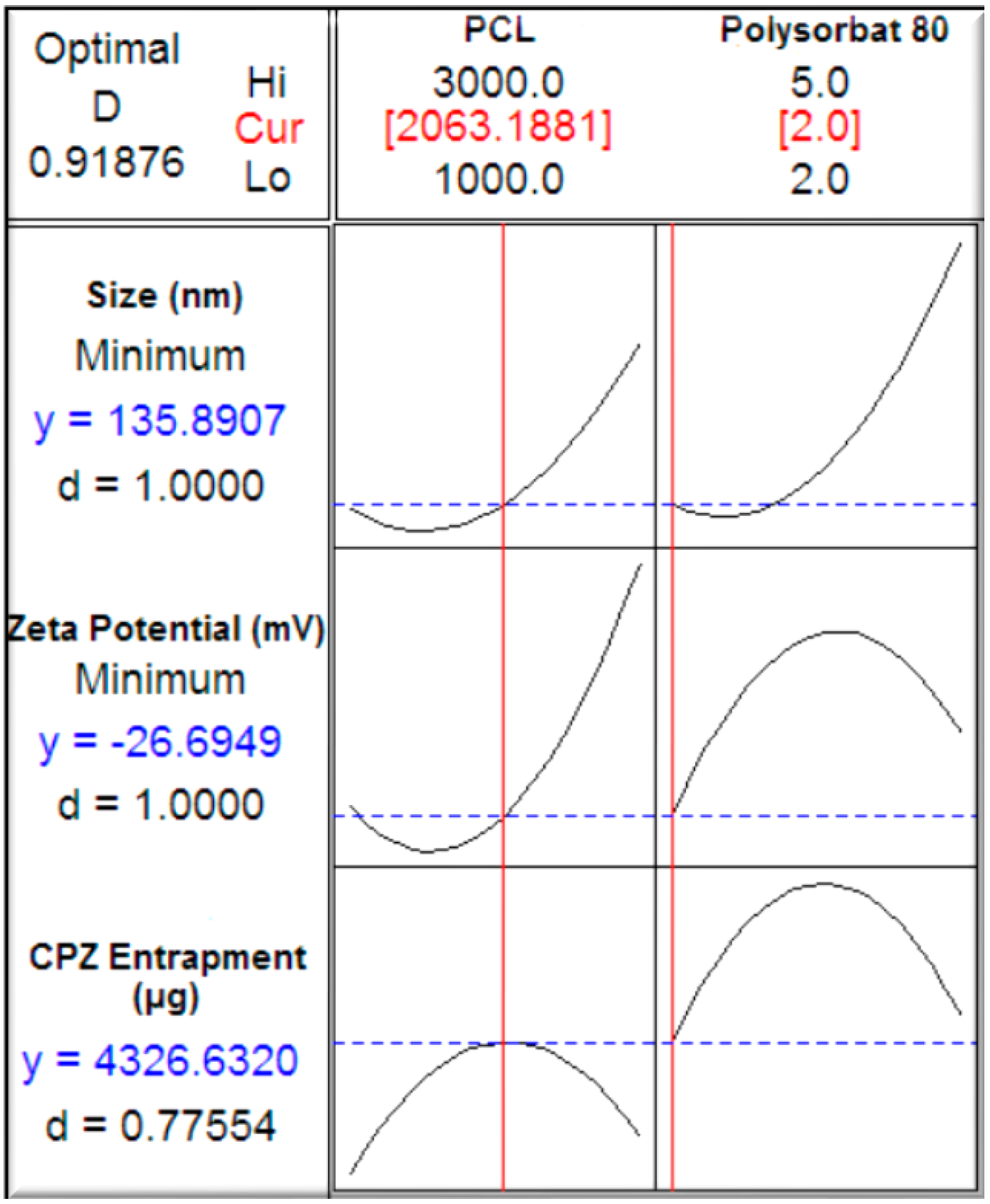
2.2. Construction of a Face-Centered Central Composite Design
A central composite design was used to determine the direct additive effects of the study variables on the physicochemical and physicomechanical properties and interactive effects on polycaprolactone nanocapsule formulation (
Table 1). Experimental trials were performed on 13 statistically derived formulations of various combinations of polymers and stabilizers. PCL (1000–3000 mg) and Polysorbate 80 (2%–5%
v/
v) were selected as the independent formulation variables. Nanocapsule size, zeta potential and drug entrapment efficiency were selected as the formulation responses. A statistical model incorporating interactive and polynomial terms was utilized to evaluate the responses. Response surface plots were constructed to visually represent the influence of the stabilizer and polymeric concentrations on the nanocapsule size, zeta potential as well as chlorpromazine hydrochloride encapsulation efficiency. As a control, non-drug loaded nanocapsules were also prepared and assessed.
Table 1.
Face-centered central composite design template with statistically generated formulations.
Table 1.
Face-centered central composite design template with statistically generated formulations.
| Formulation No. | PCL (mg) | Polysorbate 80 (% v/v) | Glycerol (mL) | Stirring Speed (rpm) | CPZ (% w/v) |
|---|
| 1 | 2000 | 3.5 | 65 | 1500 | 5 |
| 2 | 3000 | 5 | 65 | 1500 | 5 |
| 3 | 3000 | 2 | 65 | 1500 | 5 |
| 4 | 2000 | 3.5 | 65 | 1500 | 5 |
| 5 | 1000 | 3.5 | 65 | 1500 | 5 |
| 6 | 3000 | 3.5 | 65 | 1500 | 5 |
| 7 | 2000 | 3.5 | 65 | 1500 | 5 |
| 8 | 1000 | 2 | 65 | 1500 | 5 |
| 9 | 2000 | 2 | 65 | 1500 | 5 |
| 10 | 2000 | 5 | 65 | 1500 | 5 |
| 11 | 2000 | 3.5 | 65 | 1500 | 5 |
| 12 | 1000 | 5 | 65 | 1500 | 5 |
| 13 | 2000 | 3.5 | 65 | 1500 | 5 |
2.3. Preparation of the PCL Nanocapsules
Nanocapsules were prepared by a novel hot melt dispersion technique according to the design template in
Table 1 and are outlined in
Figure 1.
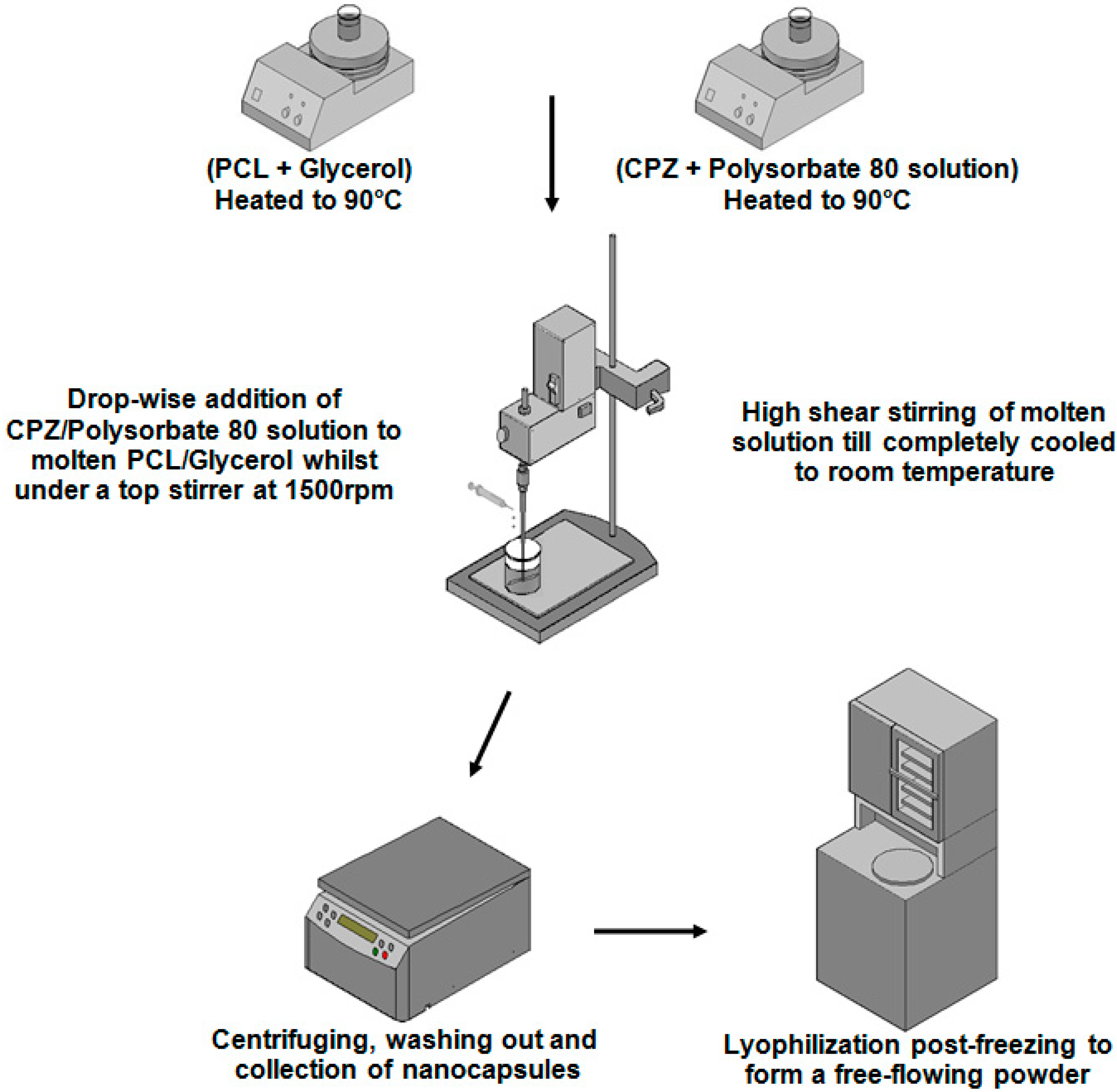
Figure 1.
Schematic depicting the synthesis process of the chlorpromazine hydrochloride (CPZ)-loaded, poly-ε-caprolactone (PCL) based nanocapsules by the novel melt-dispersion technique.
Figure 1.
Schematic depicting the synthesis process of the chlorpromazine hydrochloride (CPZ)-loaded, poly-ε-caprolactone (PCL) based nanocapsules by the novel melt-dispersion technique.
PCL (1000–3000 mg) was firstly dispersed in 65mL of glycerol and this was subjected to heating to 90 °C and magnetic stirring at 200 rpm while on a heat modulated magnetic stirrer. A 5% w/v aqueous CPZ solution containing Polysorbate 80 was also heated to 90 °C. Following adequate melting of PCL in the glycerol melting base, the molten solution was transferred to a high speed, high shear top stirrer and made to rotate at 1500 rpm. This was followed by the drop-wise addition of the heated CPZ (5–15 mL), maintaining the polymer:drug ratio at 4:1, and Polysorbate 80 solution to the molten solution. The solution was allowed to stir at 1500 rpm on the top stirrer for 45 min until cooled to room temperature. Nanocapsules were allowed to cure for 15 min and were processed by centrifuging at 3000 rpm for 10 min, removing the supernatant, re-dispersing in de-ionized water, with repetition of this centrifugation cycle three times. Due to the high viscosity of glycerol, de-ionized water was added to the nanocapsule solution just prior to centrifuging. This was followed by collection of the nanocapsule sediment, which was suspended in water with re-addition of glycerol (20% v/v), which was frozen at −75 °C at a fairly slow freezing rate of −1.25 °C/min (to minimize nanocapsule rupture) and maintained at this temperature for 48 h and, subsequently lyophilization at −70 °C at 25 mtorr for a further 48 h (FreeZone® 2.5, Labconco®, Kansas City, MO, USA). It should be noted that the freezing temperature of the solution must be performed at, or below the glass transition temperature of the maximally cryo-concentrated solution for freezing to go to completion (thus at least at or below the Tg of PCL of −60 °C). The inclusion of Polysorbate 80 prevented the agglomeration of the nanocapsules on centrifugation and on freezing, while glycerol, a commonly-used freeze-drying excipient served as a cryoprotectant and tonicity adjustor, controlling the osmotic pressure.
2.4. Analysis of Polymer Structure and Thermal Transition for Stability of Nanocapsule Constituents
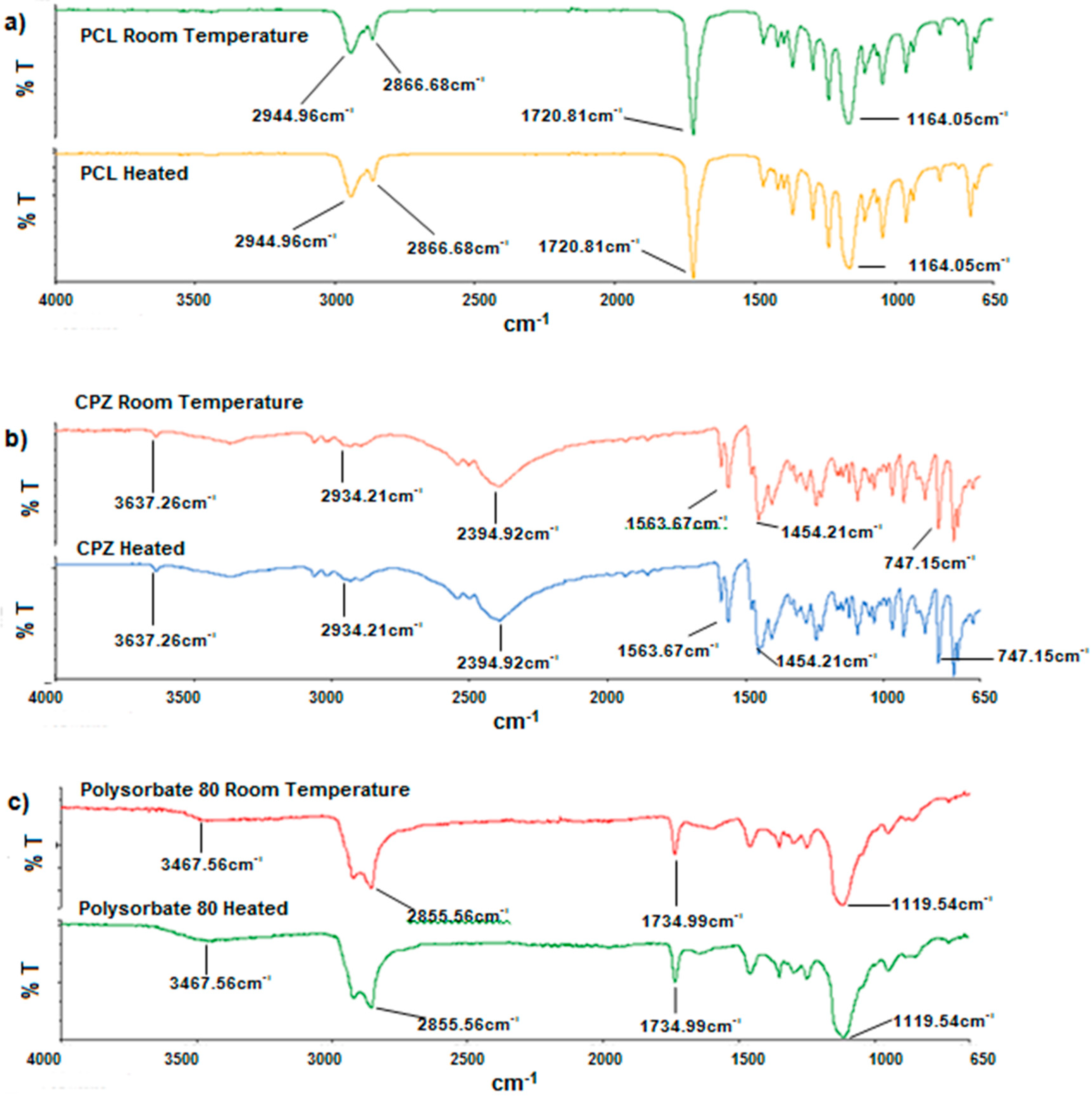
Owing to the fact that the melt dispersion technique is a process that uses relatively high temperatures, Fourier Transform Infrared (FTIR) spectroscopy, Differential Scanning Calorimetry (DSC) and Temperature-Modulated Differential Scanning Calorimetry (TMDSC) were performed to assess the possible presence of thermo-degradation.
2.4.2. Temperature-Modulated Differential Scanning Calorimetry
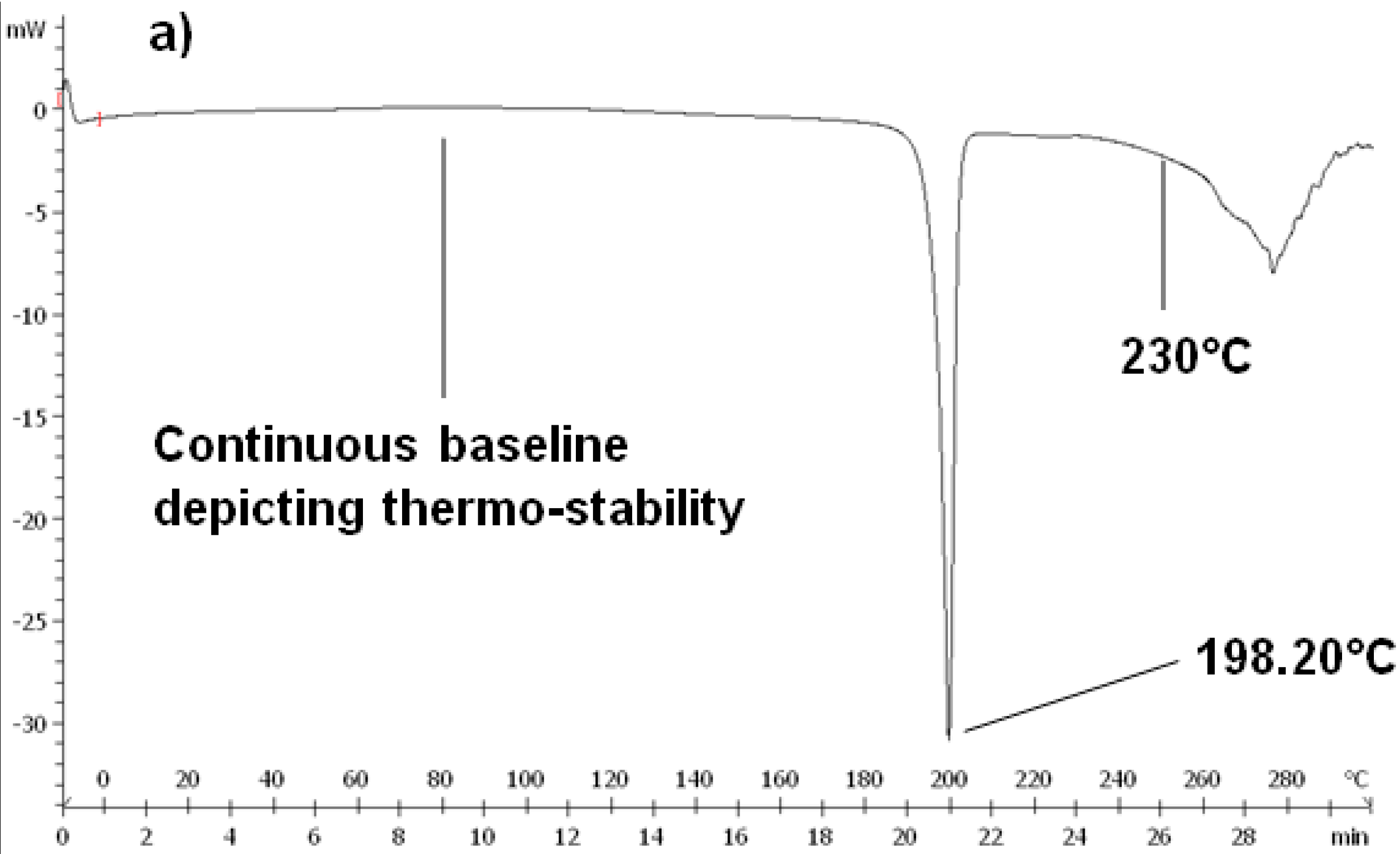
DSC and TMDSC (DSC 1 STARe Mettler Toledo system, Uster, Zürich, Switzerland) were performed on native CPZ, PCL and the CPZ-loaded, PCL nanocapsules to further assess the thermal behavior of the constituents and thus pinpoint any thermo-degradation at the temperatures used in the melt dispersion technique. DSC and TMDSC were also performed to determine the possibility of any interactions that may have occurred during the melt dispersion technique. Calibration of temperature and enthalpy on the instrument were done using indium and zinc. The heating rate was set at 10 °C·min−1 under a dry nitrogen atmosphere (Afrox, Germiston, Gauteng, South Africa) with a flow rate of 200 mL·min−1 acting as the purge gas in order to reduce oxidation. Samples sizes of 5–10 mg were accurately weighed into standard 40 μL aluminum open pans and sealed hermetically. This was followed by pinholes being punched on the lids of the aluminum pans. Samples were then heated from 0 to 300 °C and held at 300 °C for 3 min. This was done to evaporate any moisture in the sample and to eliminate any thermal history. The samples were then quenched from 300 to 0 °C at a rate of 20 °C·min−1. The midpoint melting point (Tm) obtained from the melting point depression was determined according to the peaks generated on the experimental DSC curves on heating the samples from 0 to 300 °C.
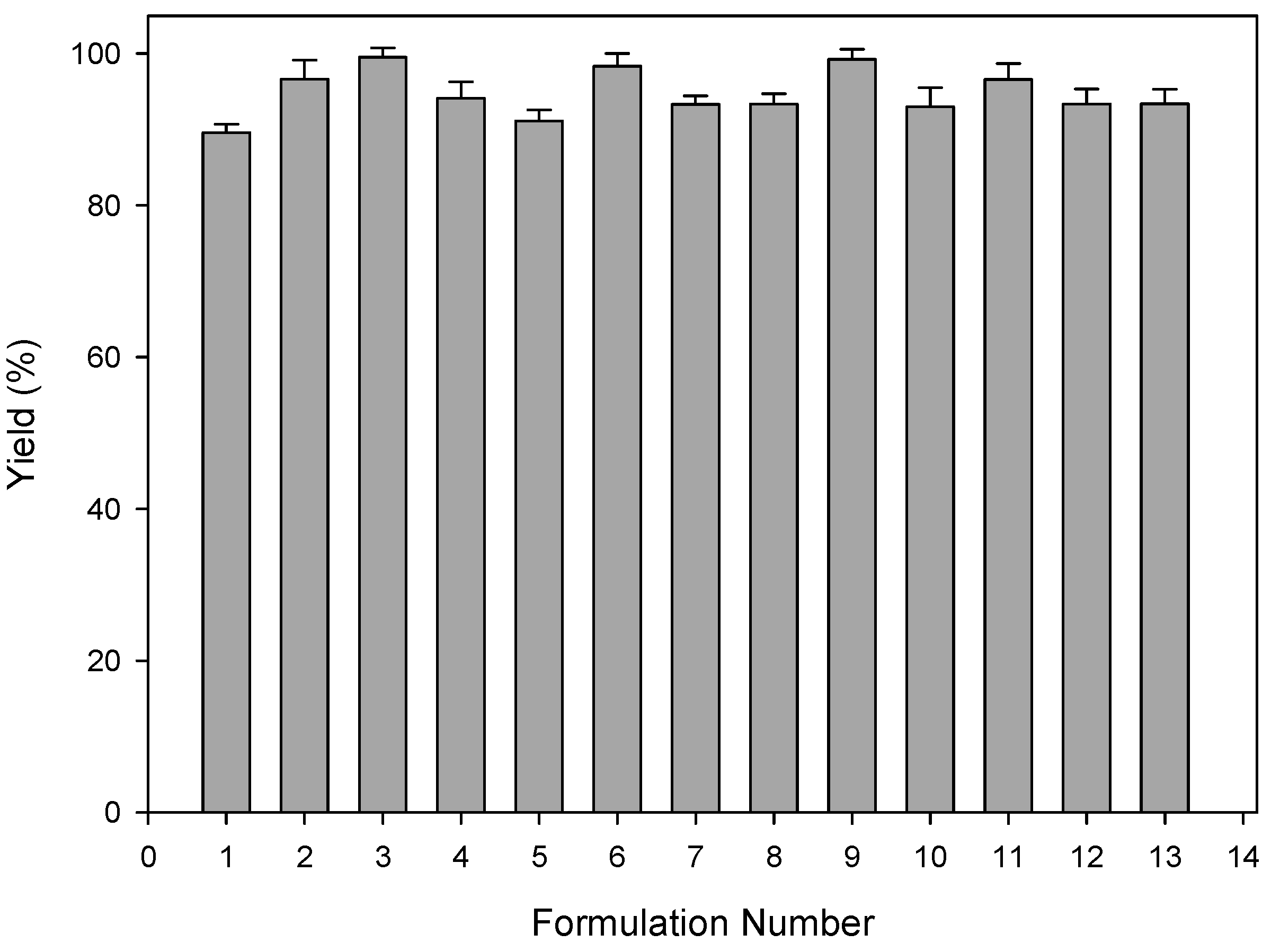
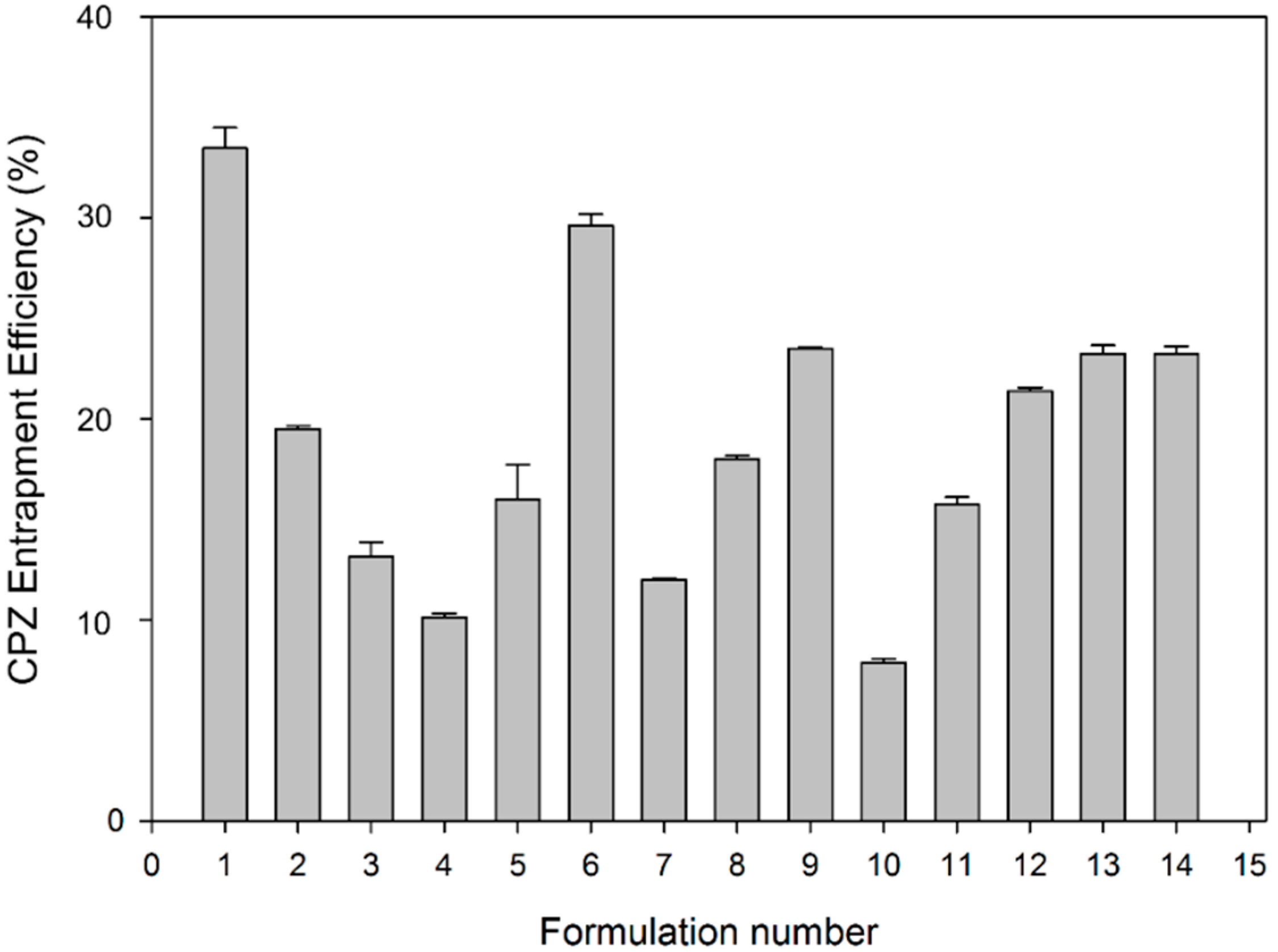
2.5. Drug Entrapment and Yield of the PCL Nanocapsules
The dry weight of the PCL nanocapsules was measured and compared to the dry weight of the initial formulation components. Chlorpromazine hydrochloride entrapment was determined by dissolving 100 mg nanocapsules in 950 mL of Phosphate Buffered Saline (PBS), pH 7.4, 37 °C, to which 5 mL of acetone was added. Chlorpromazine hydrochloride content (µg) was assessed in triplicate and determined by ultraviolet spectroscopy (Cecil CE 3021, Cecil Instruments Ltd., Milton, Cambridge, UK) at 319 nm against standard constructed curves. The entrapment efficiency was calculated as the actual percentage of drug entrapped compared to the original amount used in nanocapsule preparation.
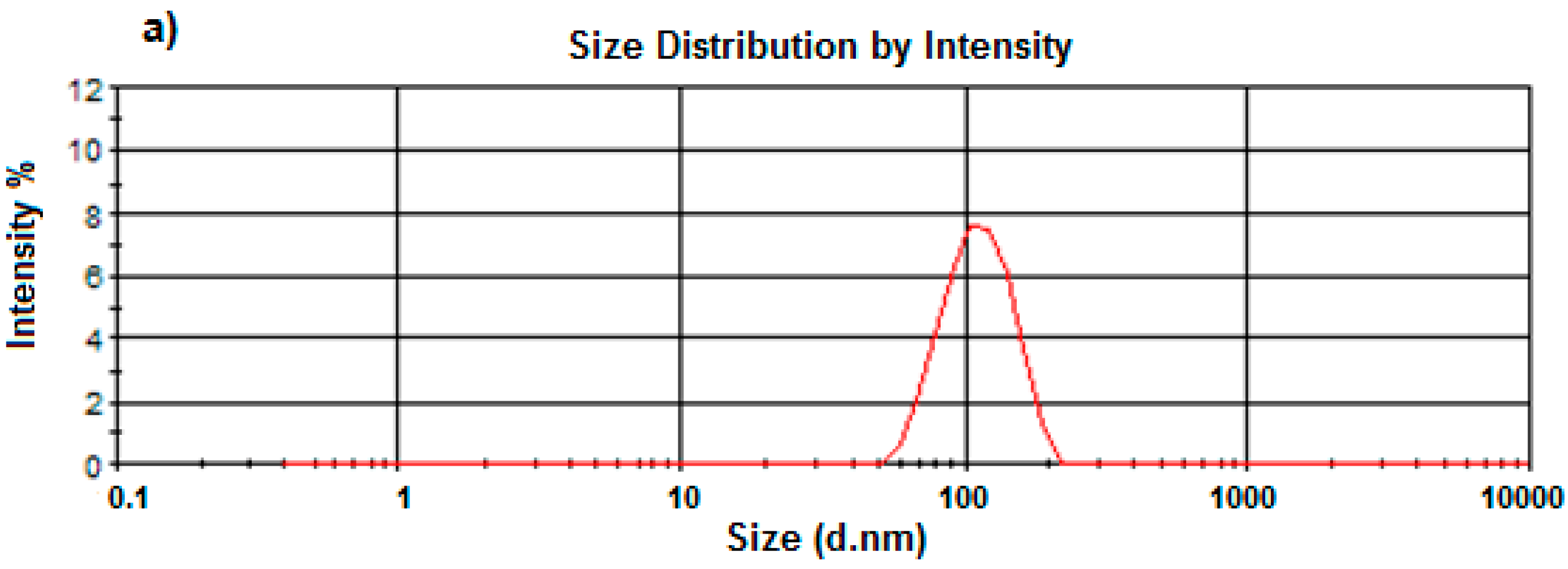
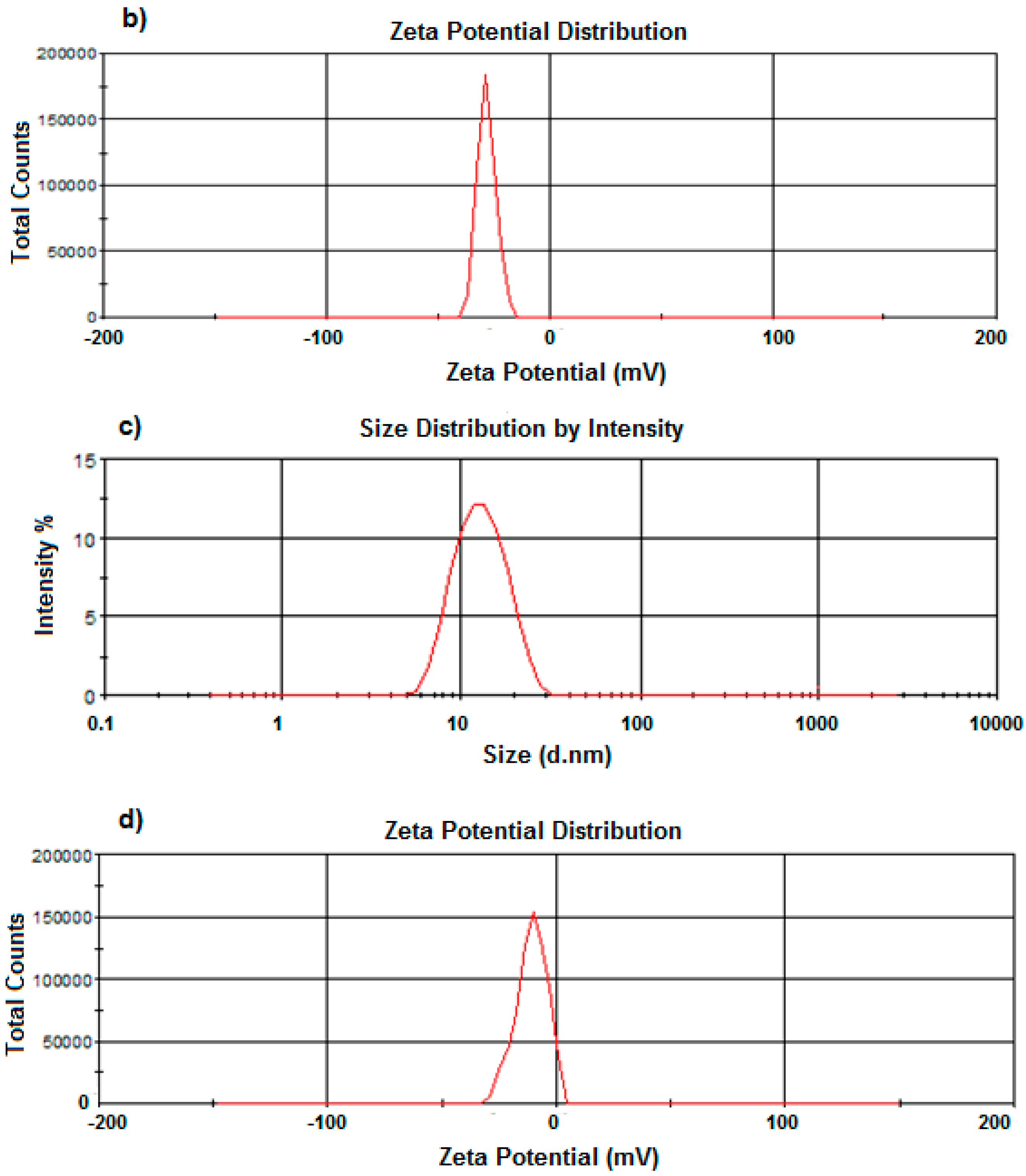
2.6. Size and Zeta-Potential Determination of the PCL Nanocapsules by Dynamic Light Scattering
A Zetasizer NanoZS (Malvern Instruments Ltd., Malvern, Worcestershire, UK) incorporating dynamic light scattering techniques at 37 °C at varying angles was used to determine the average sizes and size distribution of the nanocapsules produced, as well as their zeta potentials. A 1% w/v was appropriately diluted with de-ionized water, filtered using a 0.22 μm filter (Millipore Co., Billerica, MA, USA) and placed into disposal cuvettes (size) or capillary cells (zeta potential) (Malvern Instruments Ltd., Malvern, Worcestershire, UK). The viscosity and refractive index of the continuous phase were set to those specific to de-ionized water. Measurements were taken in triplicate to validate the reproducibility of the experiment.
2.7. Morphological Characterization of the PCL Nanocapsules
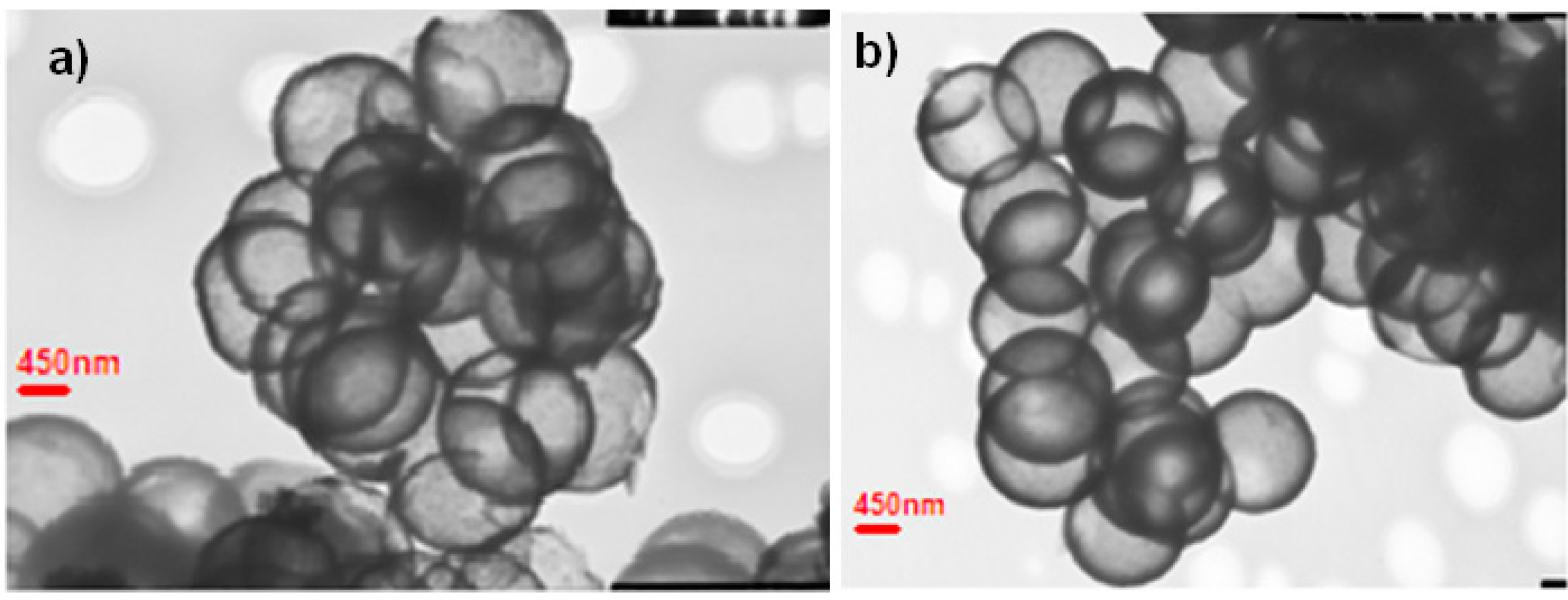
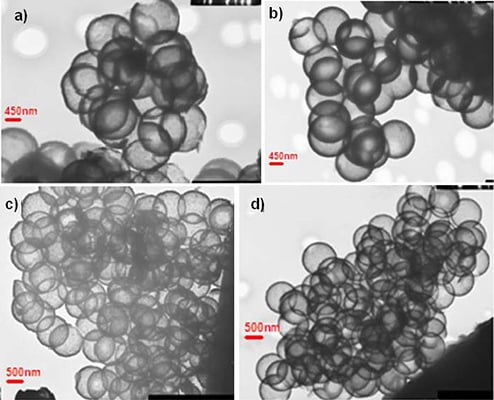
Nanocapsule size and shape was explored in great detail utilizing cryo-Transmission Electron Microscopy (TEM) (JEOL 1200 EX, Tokyo, Japan, 120 keV). Samples were prepared by placing a dispersion of nanocapsules in ethanol on a copper grid with a perforated carbon film followed by evaporation and viewing at room temperature.
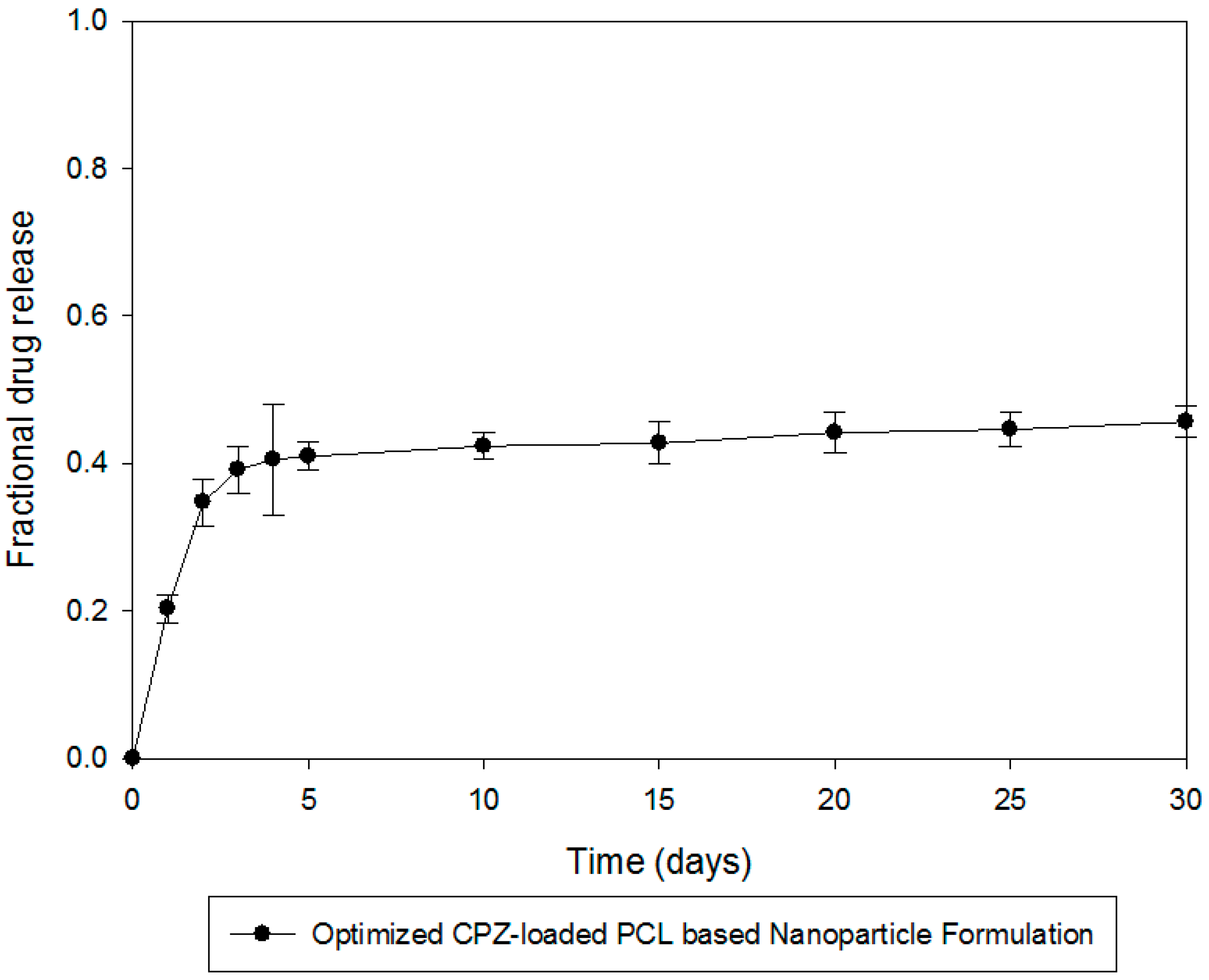
2.8. In Vitro Chlorpromazine Release Studies from the PCL Nanocapsules
In vitro release studies were performed on the CPZ-loaded PCL nanocapsule formulations utilizing an orbital shaker bath (Labex, Stuart SBS40
®, Gauteng, South Africa) set at 25 rpm. Due to the diminutive size of the nanocapsules and concerns of nanocapsule loss when sampling, one of the main methods reported for analysis of drug release from nanoparticulate systems was employed for
in vitro drug release analysis, namely, the dialysis bag diffusion method, which has been employed in various modifications for evaluating drug release from nanoparticulate systems for neurological drug delivery [
10,
12]. We employed the most commonly cited dialysis bag (regular dialysis) method. Capsules equivalent to 5 mg CPZ were placed in dialysis tubing membranes and then immersed in 100 mL phosphate buffered saline (PBS) (pH 7.4, 37 °C) contained in 150 mL glass jars. At predetermined time intervals 3 mL samples of the release media were removed, filtered through a 0.22 μm Cameo Acetate membrane filter (Millipore Co., Billerica, MA, USA) and analyzed by UV spectroscopy at a maximum wavelength of 319 nm for CPZ content analysis. Although the dialysis membrane should preclude their escape, the interference of nanocapsule remnants in filtered samples was accounted for, and found to be negligible, as ascertained by UV measurements at 319 nm on filtered samples obtained from drug-free nanocapsules. Further, preliminary experiments with 5 mg pure CPZ from dialysis tubing showed no impedance of the drug release employing this approach. CPZ release was quantified using a linear standard curve (
R2 = 0.99). To maintain sink conditions an equal volume of CPZ-free PBS was replaced into the release media. A model independent analysis, the time point approach, was used to analyze dissolution data to provide a more accurate analysis of CPZ release kinetics whereby mean dissolution time after 30 days (MDT
30) was calculated using Equation (1):
where
Mt is the fraction of dose released in time
ti = (
ti +
ti − 1)/2 and M
∞ corresponds to the loading dose.
2.10. In Vitro Cytotoxicity Analysis in a Neuronal Cell Line
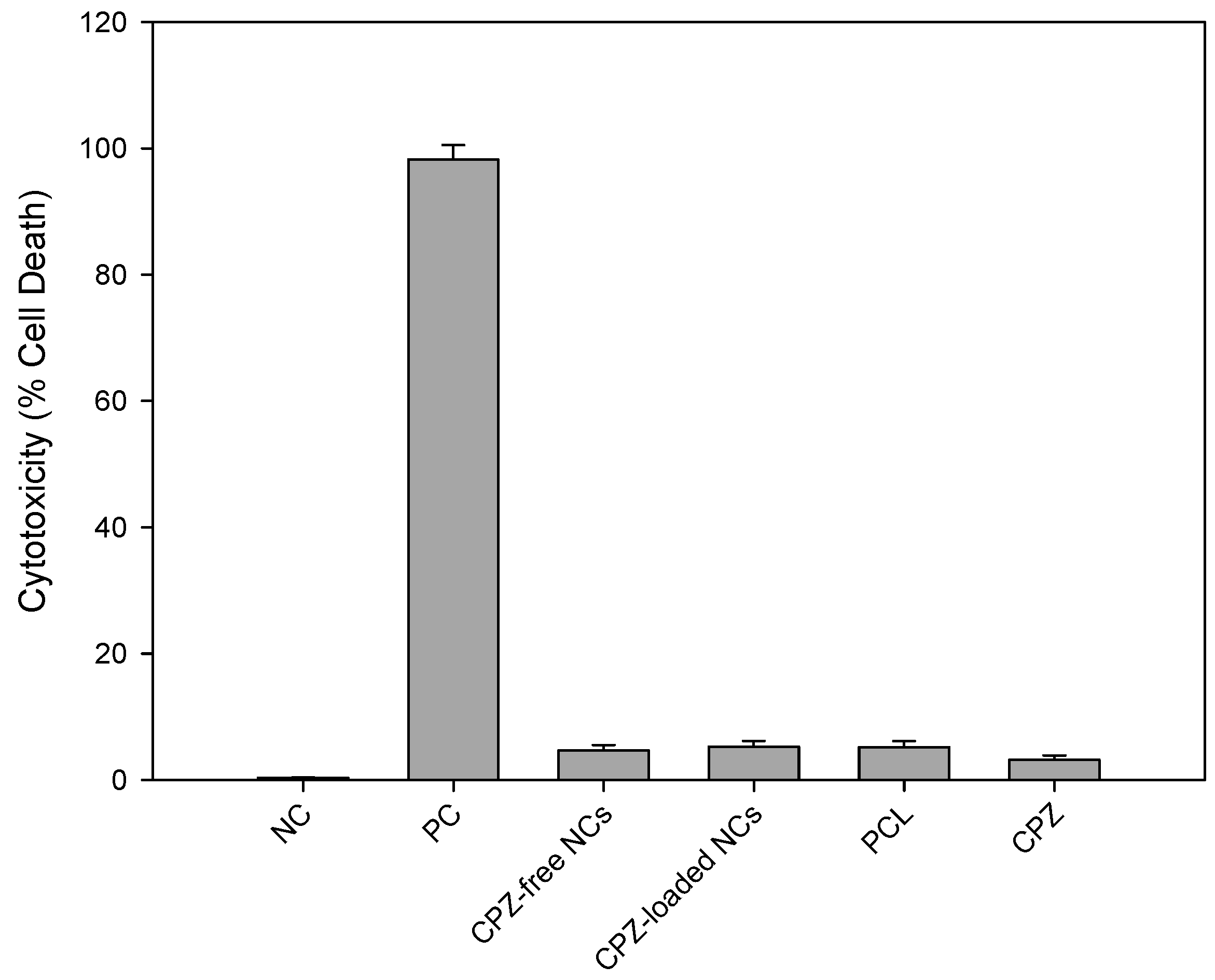
The release of intracellular substances can be quantified by either initially labeling the cells exogenously or the intracellular substances itself may be physiological cytoplasmic constituents. Lactate dehydrogenase (LDH) is a stable enzyme normally found in the cytosol of biological cells and is utilized in cytotoxicity assays as the quantification of LDH release from within the cell into the extracellular fluid (ECF) is proportional to cell death. A reduction in metabolic activity indicates incipient cell injury whereas LDH detection in the ECF represents more severe irreversible cell damage and implies death. The LDH assay assesses cell membrane integrity. Due to its advantages, the LDH assay method was employed in this study utilizing CytoTox 96® Non-Reactive Cytotoxic Assay. The effect of the nanocapsules and its componential elements were assayed to assess any cytotoxic effects on a neuronal cell line. PC12 neuronal cells were cultured in RPMI-1640 media comprising l-glutamine and sodium bicarbonate. The media was further enhanced with 5% fetal bovine serum (FBS), 10% horse serum (HS) and 1% penicillin/streptomycin (Sigma-Aldrich; St. Louise, MO, USA). The FBS and HS were both heat inactivated. The cells were then maintained in a laboratory incubator (RS Biotech Galaxy, Irvine, UK) with a humidified atmosphere of 5% medical CO2 at 37 °C. Preparation of the media and inoculation of cells were conducted using strict aseptic technique in a laminar flow unit.
PC12 neuronal cells were exposed to the following compounds for 48 h:
- a)
Negative control (NC)—untreated cells
- b)
Positive control (PC) cells treated with 2% lysis buffer
- c)
CPZ-loaded nanocapsules (20 mg)
- d)
CPZ-free (non-drug loaded) nanocapsules (10 mg)
- e)
PCL (10mg)
The released LDH in the culture supernatants were determined using a CytoTox96® Non-Radioactive Cytotoxicity Assay. The released LDH was quantified utilizing a 30 minute enzymatic coupled test ultimately resulting in INT being converted to red formazan and catalyzed by LDH. Briefly, PC12 neuronal cells were added to the experimental microplate wells. These were treated with the compounds to be tested. This along with an untreated control was incubated for 48 h at 37 °C. After incubation, a lysis solution (9% v/v) Triton® X-100) (10×), was added to all wells. The plate was then incubated at 37 °C for 45 min. LDH was quantified by firstly transferring 50 µL of the supernatant of each well to another 96-well assay plate. The substrate mix was reconstituted using assay buffer and 50 µL of this reconstituted substrate was added to each well. The assay plate was covered and incubated at room temperature, stored away from light, for 30 min. This was followed by the addition of 50 µL of stop solution to each well of the microplate. This was performed to stop the enzymatic reaction. Absorbance values at 490 nm were recorded utilizing a luminometer (Victor™X3, Perkin Elmer Inc., Waltham, MA, USA). The amount of LDH released and red color formed was directly proportional to the number of dead cells.
In order to relate the number of dead cells formed to the number of living cells and determine the cytotoxicity of the tested compounds, a target cell maximum LDH release control had to be conducted. A known quantity or density of PC12 neuronal cells were added to the culture medium in three wells of the microplate so that the final volume and concentration was the same as that utilized in the experimental assay above. In order to determine the known number of PC12 neuronal cells, the number of cells per mL was determined by utilizing a tryptan blue exclusion assay as well as a hemocytometer. The counting was based on the fact that viable cells will remain opaque and non-viable cells will stain blue and this would be quantified utilizing a hemocytometer and light microscope. Briefly, 200 µL of the cell suspension was diluted with 300 µL PBS and added to 500 µL tryptan blue thereby creating a dilution factor of 5. The dilution factor was relatively high so as to easily identify individualized cells. The suspension was mixed thoroughly and allowed to stand for 10 min. A Pasteur pipette was used to transfer a miniscule quantity of the suspension to the chamber of the hemocytometer. The cells in the 1mm square and four corners were counted. Each square of the hemocytometer is equal to a volume of 0.1 mm
3/10
−4 cm
3 and since 1 cm
3 = 1 mL, the cell count per mL could be calculate utilizing Equation (2). Only cell suspensions with a viability in excess of 95% were used in the assay:
Once the cells were counted and pipetted onto a microplate, 10 µL of lysis solution per 100 µL of culture medium was added and the cells together with the lysis solution were incubated at 37 °C for 45 min. This resulted in complete lysis and death of the PC12 neuronal cells. The supernatants were harvested as described above and the ultraviolet reading at 490 nm for complete cell destruction was computed. Volume correction as well as culture medium controls was performed to cater for the volume changes by addition of lysis buffer as well as correcting LDH activity contributed by serum in culture medium and the phenol red in the culture medium respectively. Furthermore, a target-cell spontaneous LDH release control was also performed to correct spontaneous LDH release from cells. The cytotoxicity of the compounds towards the PC12 neuronal cells were calculated using Equation (3):
The statistical significance of the cytotoxic effects of the various compounds was evaluated wherein a probability limit of p < 0.05 was considered to be significant.
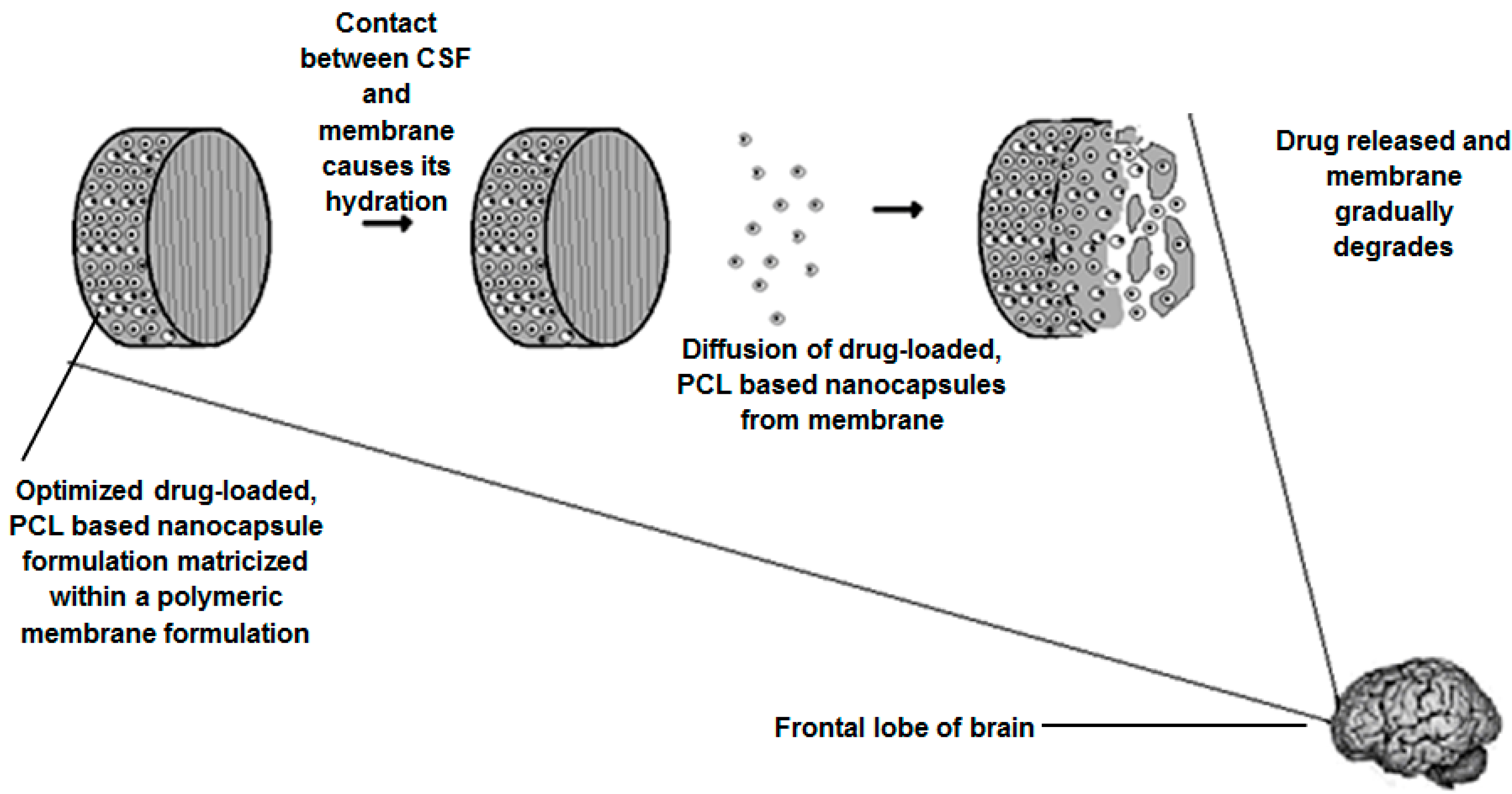
2.11. Assimilation of the Optimized Nanocapsules into a Composite Polymeric Membrane for Implantation
The assembly of a nanocapsule-loaded polymeric membrane (NCPM) was carried out by incorporating and evenly dispersing the optimized CPZ-loaded, PCL-based nanoparticle formulation into a polyamide (PA) 6,10-ethylcellulose (EC) composite membrane, derived from a polymeric slurry, with subsequent molding to obtain a composite membrane. The PA 6,10 utilized in this study is a novel polymer synthesized by Kolowale and co-workers [
20]. For preparation of the membrane slurry, PA 6,10 was dissolved in 2 mL Formic Acid (FA). The solution was heated to 60 °C to aid the complete solubilization of the PA 6,10 in the FA. A separate solution was formulated and comprised of EC dissolve in 1 mL of acetone (ACN). Once the PA 6,10 and the EC were completely solubilized in their respective solvents, the PA 6,10-FA solution was the gradually added to the EC-ACN solution with stirring at 500 rpm. This led to the formation of a white, murky slurry of consistent texture. The slurry was then removed and added to a dialysis tubing membrane which was then suspended in 5 L of de-ionized water. As water permeated through the dialysis tubing and came into contact with the slurry, it led to the formation of a white precipitate. Once all FA was removed, half of the PA 6,10-EC slurry post dialysis was placed in the liquid-paraffin lubricated molds. This was followed by the addition of 500 mg of optimized nanoparticles and the further addition of the remainder of the slurry. The molds were then placed under a fume hood and air-dried for 24 h. The final NCPM was sterilized via gamma irradiation at 20 kGy.
2.12. In Vivo Analysis of the Nanocapsule-Loaded Polymeric Membrane in a Rabbit Model
2.12.1. Implantation of the Nanocapsule-Loaded Polymeric Membrane into a Rabbit Model
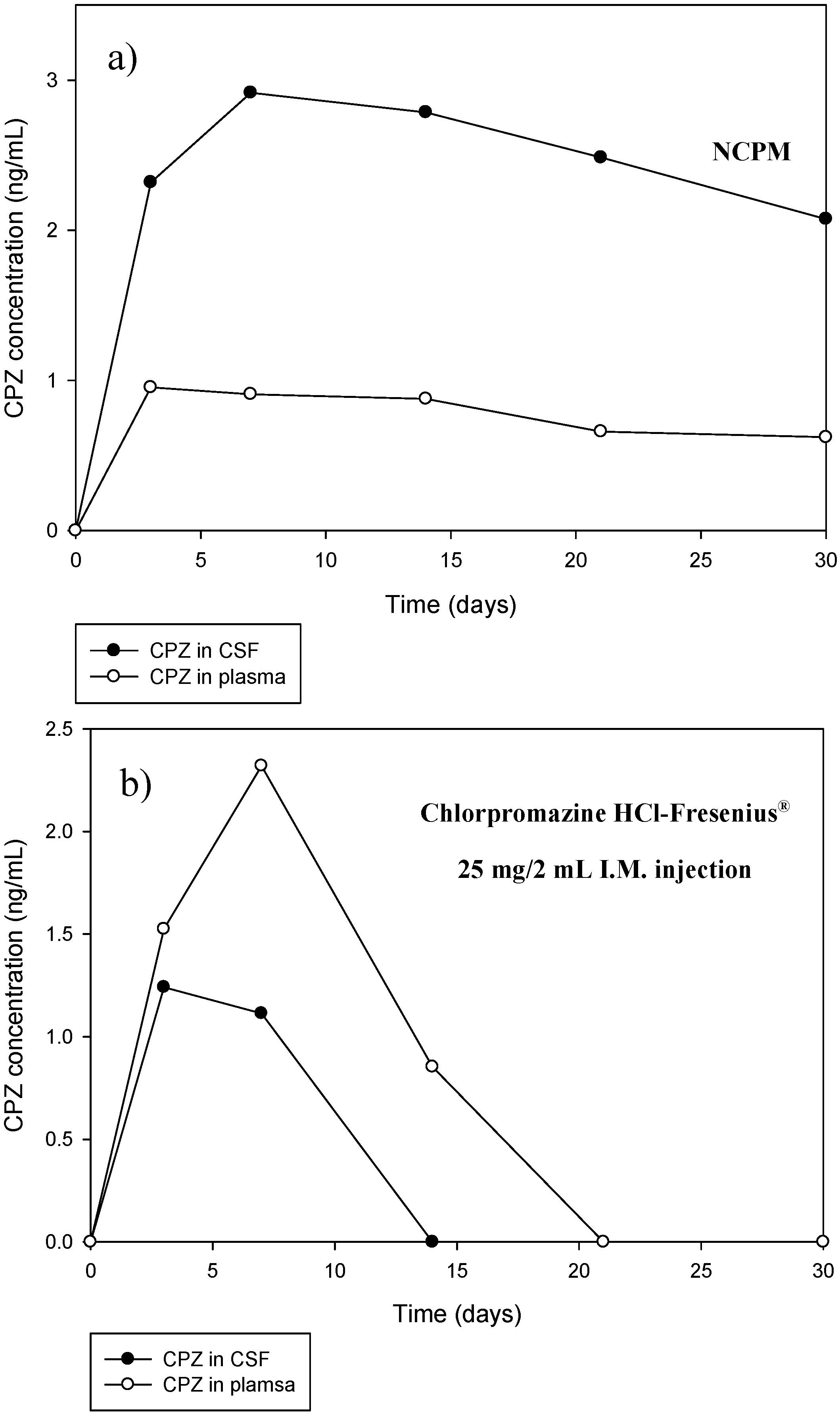
New Zealand White Rabbits were utilized in this study and were obtained as per the protocols of the Central Animal Services at the University of the Witwatersrand. Ethics clearance from the Animal Ethics Screening Committee (AESC) of the University of the Witwatersrand was obtained for this study (No. 2011/51/04 Eight rabbits weighing 4.5–5 kg were housed in single cages, i.e., one rabbit per cage and were provided with a standard rabbit diet and water ad libitum under a controlled temperature (20–24 °C) and a 12 h light/dark cycle.
In vivo assessment was conducted on 6 rabbits. Two rabbits were assigned to 3 sub-groups where the first sub-group was implanted with a drug-loaded NCPM (Group 1), the second with a non drug-loaded NCPM (for baseline comparative purposes) (Group 2) and the third with an intramuscular parenteral dose of commercially available CPZ (Chlorpromazine HCl-Fresenius® 25 mg/mL) (Group 3). The dose of the intramuscular CPZ injection was 1 mg/kg of body weight. This dose was attained by the veterinary formulary of the University of Minesota. One rabbit from each group was euthanized approximately halfway through the study (Day 14) and was subjected to histolomorphological toxicity studies. The remaining rabbits were euthanized at the end of the study (Day 30) and underwent further histolomorphological toxicity studies.
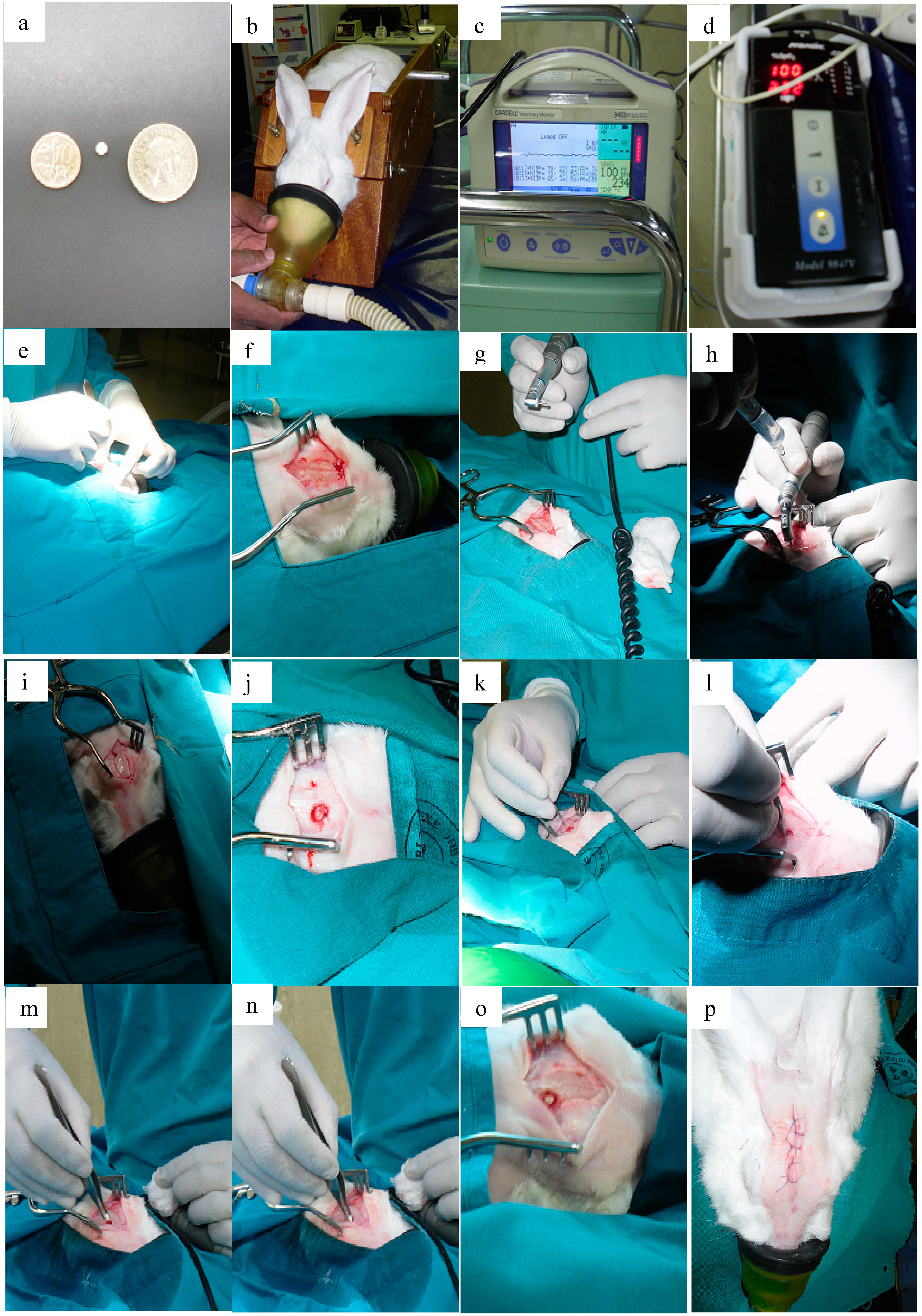
The implantation was conducted on live, healthy, rabbits. Rabbits were initially given half the recommended dose of midazolam to as a sedating agent followed by anaesthesia with a mixture of Ketamine (50 mg/kg) and Xylazine (5 mg/kg). The implantation procedure was adapted from Brem and co-workers [
21] and is briefly outlined; the heads of the animals were shaven and appropriately prepared. A midline incision was made and this was followed by the removal of subcutaneous tissue and periosteum. A unilateral burrhole of approximately 5 mm was made using a dental drill. The burrhole was located anterior to the coronal suture and lateral to the saggital suture. The dura mater and cortex were pierced with minimal resultant bleeding. The burrhole was flushed with saline and the implant was placed intraparenchymally. The scalp insertion was then appropriately closed with a single layer of non-absorbable suture.
2.12.2. Histomorphological Evaluation of the Brain Post-Implantation
Histomorphological analysis was conducted to assess the biocompatibility of the NCPM and its immune response in terms of its inflammation response, whether or not it is toxic or causes excessive bleeding. Rabbits were euthanized midway through the study (Day 14) and at the end of the study (Day 30) with sodium pentobarbitone and decapitated. The entire brain was removed and fixed in 10% v/v neutral buffered formalin solution. Following the normal fixation period, the brain specimens were cut by cross section in the anterior third region where the NCPM was implanted or where areas of lysis could be identified. Additional sections in the mid-brain and cerebellum were also examined to assess any effects of nanocapsules, drug or the NCPM that may have translocated to these areas as a result of biodegradation. These selected blocks were then processed in an automated histological processor overnight. After complete tissue processing, wax blocks were prepared and histological sections of 6 µm were cut on a microtome. The slides produced were stained with haematoxylin and eosin (H & E) in an automated stainer prior to mounting for histomorphological evaluation.
2.12.3. Cerebrospinal Fluid Sampling
Cerebrospinal fluid (CSF) samples (500 µL) were be drawn by cisternal puncture by a method. Briefly, the rabbits were anesthetized and placed in lateral recumbency, with the head flexed at 90° to the neck. The fur was then shaved on the dorsal head and neck and the site was appropriately disinfected. A 23 gauge hypodermic needle was inserted into the dorsal midline between the external occipital protuberance and the craniodorsal tip of the dorsal spine of the C2 vertebra, cranial to the cranial wings of the C1 vertebra. The needle then gently entered the subarachnoid space and CSF was passively withdrawn into 1 mL syringes and stored in Eppendorf tubes at −80 °C till further analysis. Any contaminated CSF samples were centrifuged at 1300 relative centrifugal force (RCF) and the clear supernatant was collected and stored immediately at −80 °C in a laboratory freezer. Blank CSF for base-line data was withdrawn from the rabbit 1 week prior to implantation with the membrane.
2.12.4. Blood Sampling
Due to the fact that the link between CSF and the venous blood flow are the arachnoid granulations, substances within the CSF can enter the blood stream via the arachnoid granulations [
22]. Blood samples were therefore taken to assess the potential levels of CPZ that may have enter the systemic circulation due to the minute size of the nanocapsules and its potential ability to pass through the arachnoid granulations. Blood samples were withdrawn at specific time intervals through the marginal ear vein of the rabbit on days 0, 3, 7, 14, 21 and 30. An intravenous catheter (22 gauge) was used to draw approximately 1% of the animal’s body weight,
i.e., 3 mL of blood from a 3 kg rabbit and 4.5 mL of blood from a 4.5 kg rabbit. These guidelines were attained from “The Guidelines for Collection of Blood from Experimental Animals”, Research Animal Resources, University of Minnesota. Blood sampling was conducted on anesthetized rabbits. Blank blood for base-line data was withdrawn from the rabbit 1 week prior to implantation with the membrane. The blood samples were collected into heparinised vacutainer tubes and gently mixed to prevent clotting. The samples were centrifuged at 1300 RCF soon after. The supernatant, containing the plasma, was carefully aspirated and transferred into a clean appropriate collection tube and frozen at −80 °C immediately till further analysis.
2.12.5. Analysis of CSF and Blood Samples
UPLC was performed utilizing a Waters Acquity® UPLC system (Waters Corp., Milford, MA, USA). The system contains a binary solvent manager, sample manager and a photodiode array (PDA) detector, all connected to a Waters Empower 2 data station. An Acquity UPLC Ethylene Bridged Hybrid (BEH) C18 column (50 mm × 2.1 mm, i.d., 1.7 μm particle size, Waters) was used at 40 °C to achieve separation. The machine was primed with the appropriate solvents mentioned above for 10 cycles each of 5 min. The method for separation, identification and quantification was isocratic whereby the mobile phase comprised ammonium acetate buffer and acetonitrile (AA–ACN 65%–35% v/v), the flow rate through the column was 0.50 mL/min, the injection volume was 2 µL and the run time was 3 min. Between injections, the machine was allowed to equilibrate for 3 min so as to attain a constant pressure and more reliable results. Upon completion of analysis, ACN was gradually injected into the system to remove any residue until the entire column was immersed in 100% ACN. Isoniazid (INH) was employed as the internal standard (IS). The PDA detector was set at 254 nm for CPZ and INH detection. Standards were prepared and analyzed (ensuring precision and accuracy) for calibration curve construction.
2.12.6. Plasma and CSF Extraction of CPZ
An uncomplicated and effective liquid–liquid plasma extraction procedure was developed and applied to CPZ in blood samples. Aliquots of plasma (500 μL) of sample were transferred into polypropylene tubes followed by the addition of 1 mL of ACN in order to precipitate the plasma proteins. The samples were subsequently vortexed for 1 min followed by the addition of 500 μL of ACN. The mixture was vortexed again for 1 min and was then centrifuged at 1300 RCF (Nison Instrument Limited, Shanghai, China) for 5 min. The supernatant was removed and filtered through 0.22 μm Cameo Acetate membrane filters. The filtrate was then spiked with a known amount of the IS, INH. The final solution was transferred into Waters® certified UPLC vials for analysis. Due to the fact that CFS does normally contain proteins and that CPZ is highly protein bound, the same extraction procedure was applied to CSF samples.