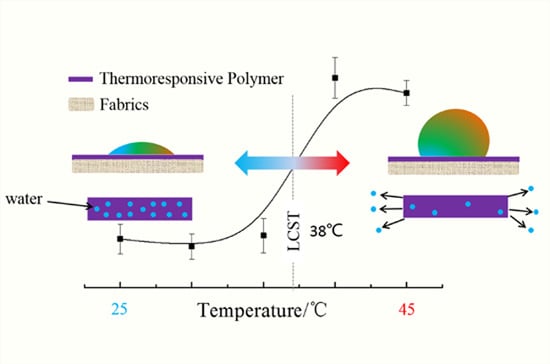
Synthesis and Thermosensitive Behavior of Polyacrylamide Copolymers and Their Applications in Smart Textiles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Analytical and Physical Methods
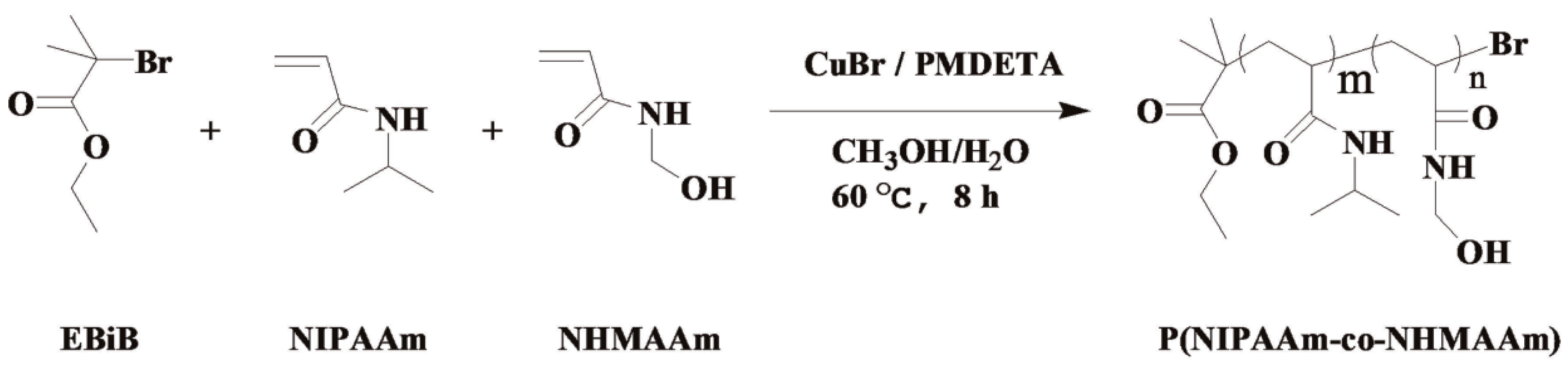
2.3. Synthesis of P(NIPAAm-co-NHMAAm)
2.4. Determination of Copolymers’ LCST in Aqueous Solutions
2.5. Determination of Thermosensitive Properties of Copolymer Films
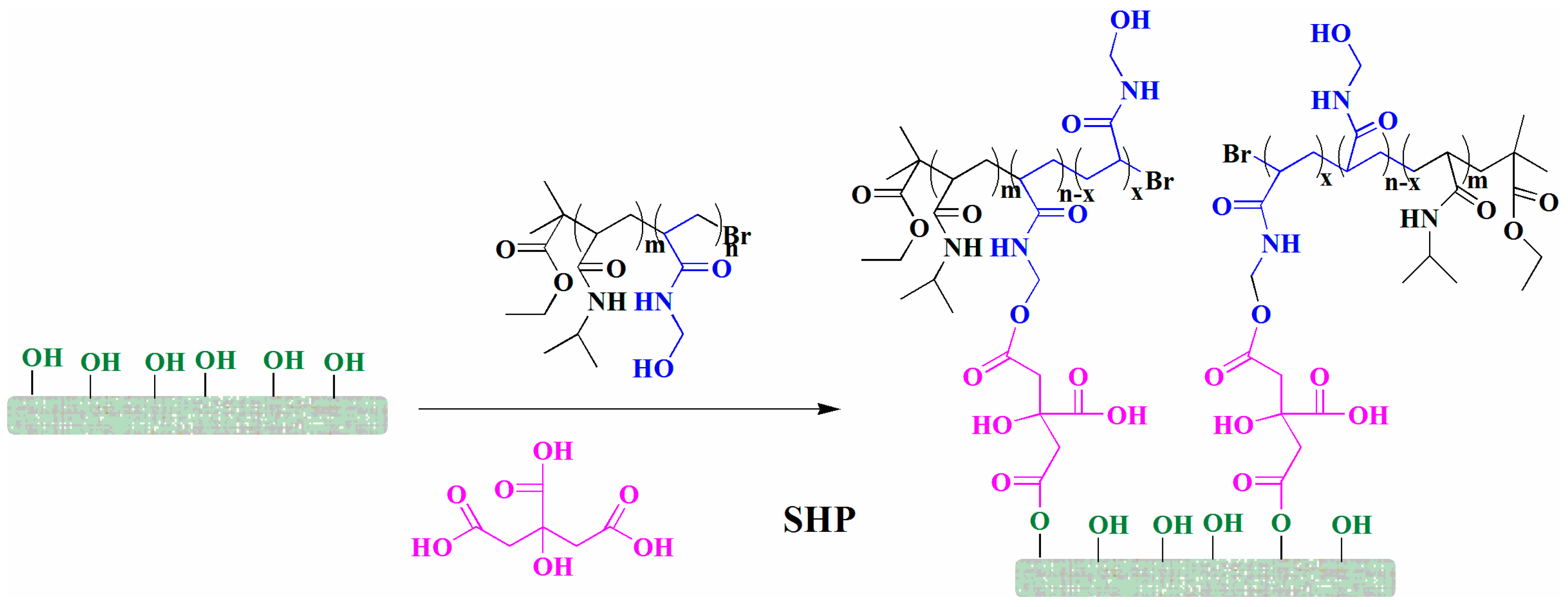
2.6. Grafting of Copolymers onto Cotton Fabrics
| Material | wt% |
|---|---|
| Polymer | 15 |
| CA | 2 |
| SHP | 3 |
| Water | 80 |
3. Results and Discussion
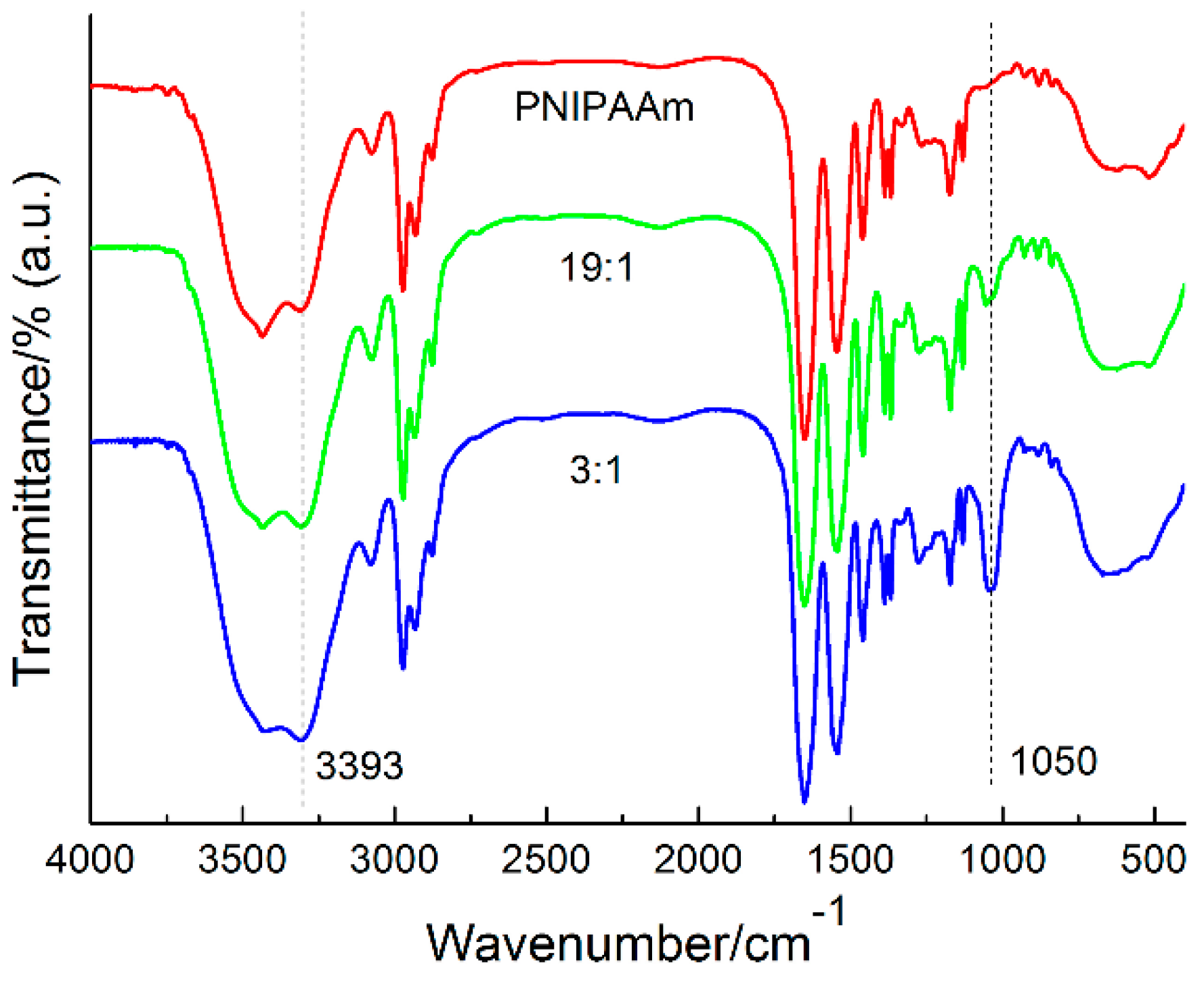
3.1. Synthesis and Structural Characterization of Copolymers
| Entry | Sample Name | NHMAAm Ratio (%) a | Mn b | LCST (°C) c |
|---|---|---|---|---|
| 1 | PNIPAAm | 0 | 10,913 | 33 |
| 2 | 29:1 | 3.1 | 13,852 | 36 |
| 3 | 19:1 | 3.8 | 10,200 | 37 |
| 4 | 9:1 | 10.4 | 10,738 | 52 |
| 5 | 3:1 | 23.5 | 12,561 | 87 |
3.2. TGA Analysis
3.3. Thermosensitive Behavior of Copolymers
3.3.1. Aqueous Solutions
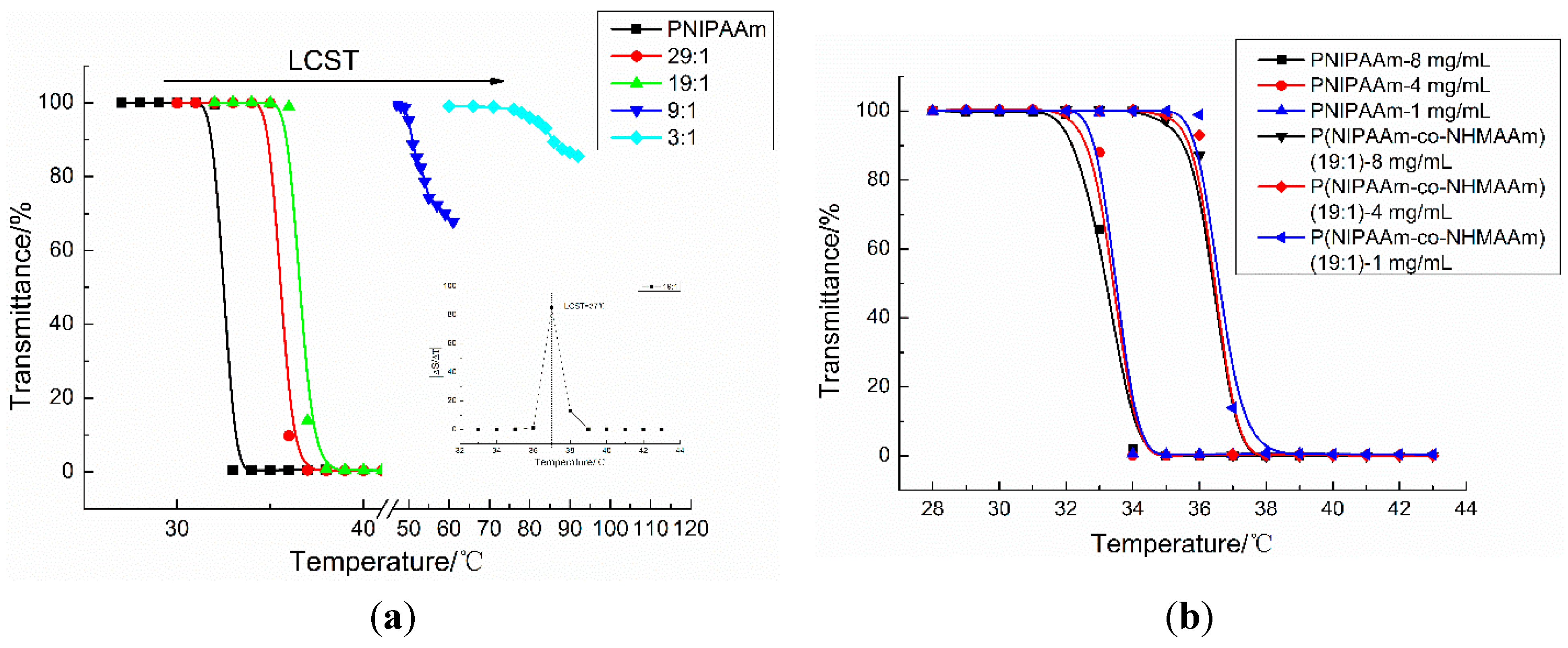
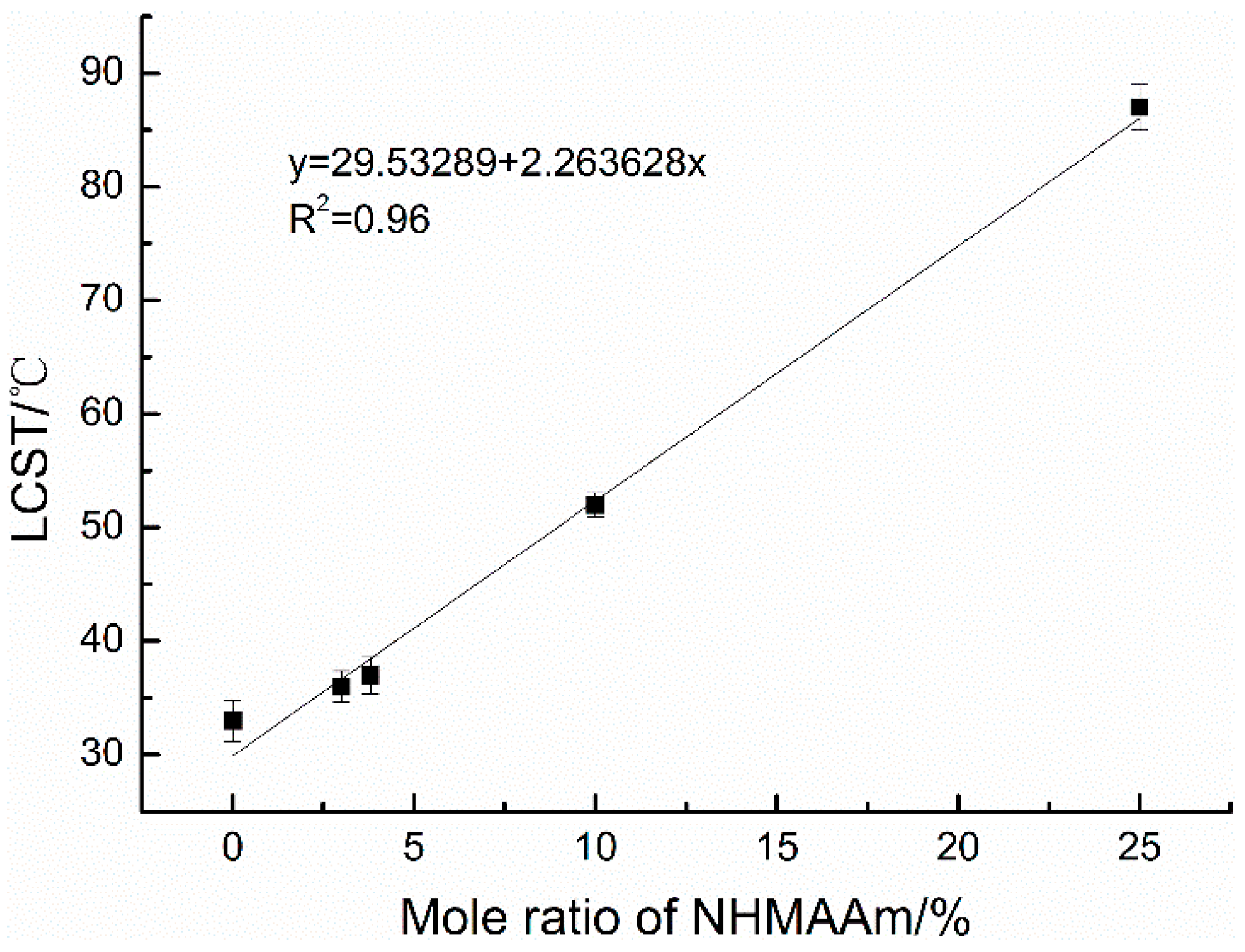
3.3.2. Copolymer Films
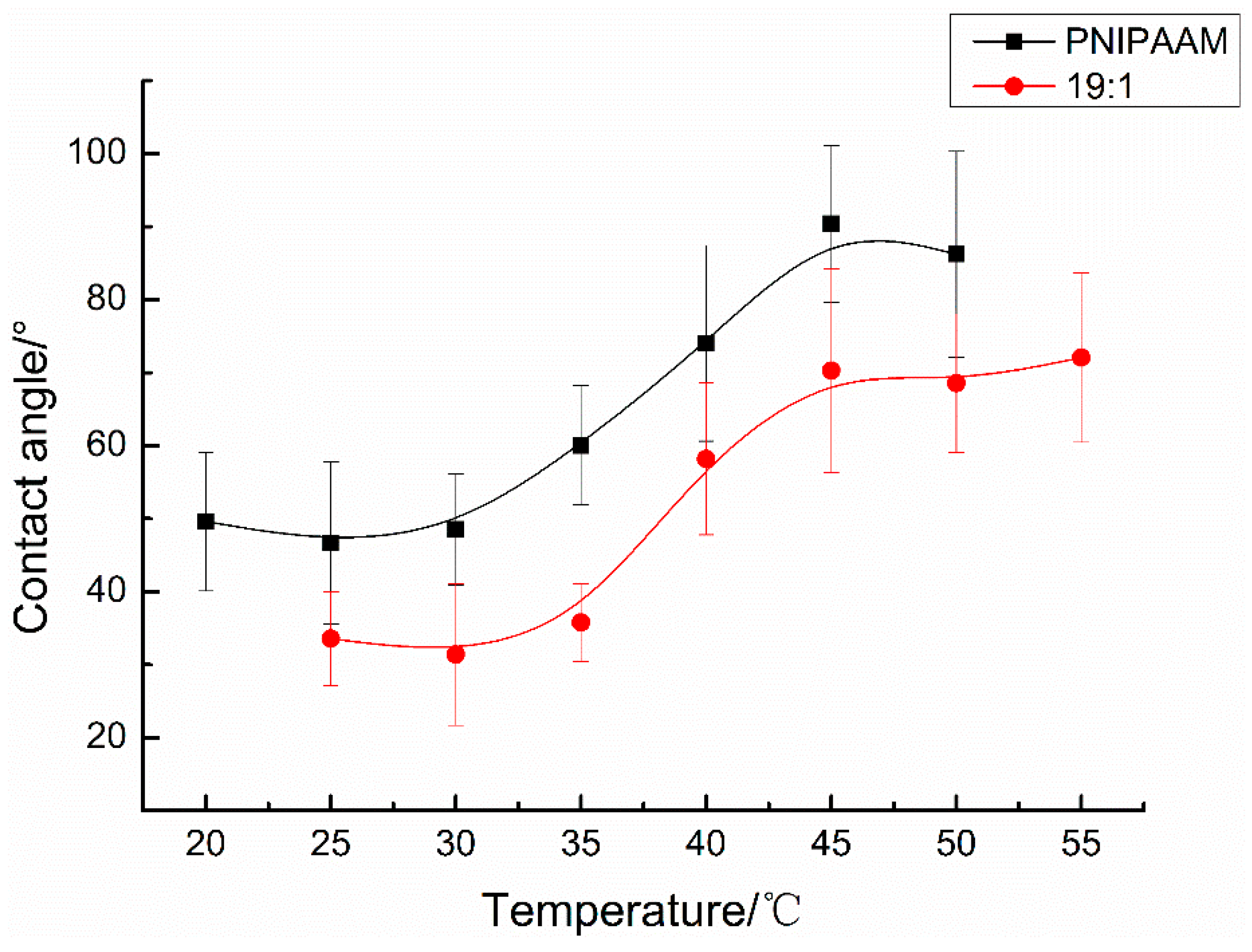
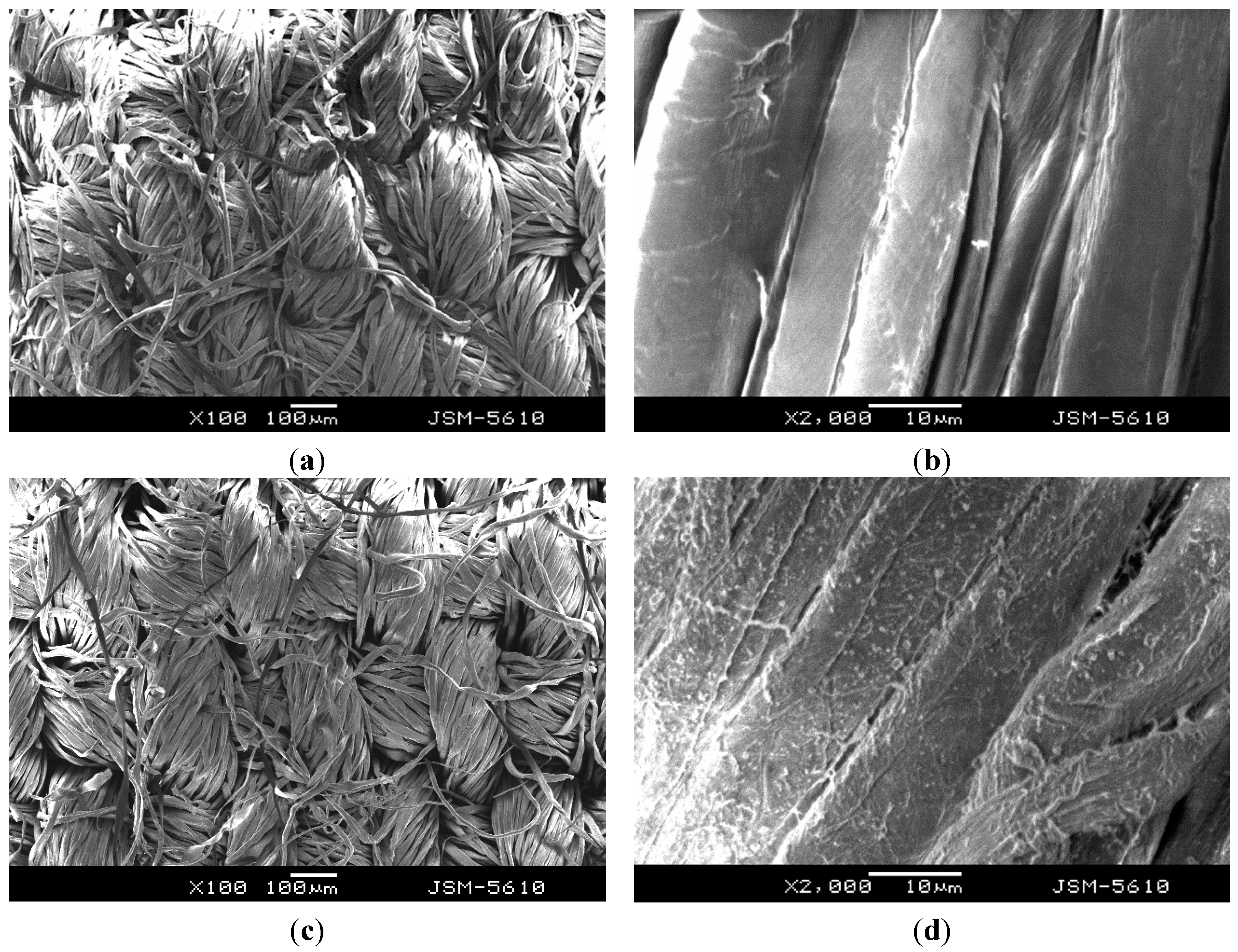
3.4. Grafting Copolymers onto Cotton Fabric
| Polymer | Liquid Retention (%) | Weight Gain (%) |
|---|---|---|
| PNIPAAm | 170 | −1.8 |
| P(NIPAAm-co-NHMAAm) (29:1) | 144 | 2.6 |
| P(NIPAAm-co-NHMAAm) (19:1) | 120 | 12.5 |

4. Conclusions
Acknowledgments
Supplementary Information
Author Contributions
Conflicts of Interest
References
- Roy, I.; Gupta, M.N. Smart polymeric materials: Emerging biochemical applications. Chem. Biol. 2003, 10, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Minko, S. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, S.; Kwon, D.; Khang, G.; Lee, H.; Kim, M. Thermo-responsive injectable mpeg-polyester diblock copolymers for sustained drug release. Polymers 2014, 6, 2670–2683. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Hu, S.-H.; Liu, D.-M.; Chen, I.-W. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today 2009, 4, 52–65. [Google Scholar] [CrossRef]
- Galaev, I.Y.; Mattiasson, B. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends biotechnol. 1999, 17, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Maharjan, P.; Woonton, B.W.; Bennett, L.E.; Smithers, G.W.; DeSilva, K.; Hearn, M.T.W. Novel chromatographic separation—The potential of smart polymers. Innov. Food Sci. Emerg. Technol. 2008, 9, 232–242. [Google Scholar] [CrossRef]
- Gil, E.; Hudson, S. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Q.; Wang, T. Thermoresponsive PNIPAAm-modified cotton fabric surfaces that switch between superhydrophilicity and superhydrophobicity. Appl. Surf. Sci. 2012, 258, 4888–4892. [Google Scholar] [CrossRef]
- Pretsch, T. Review on the functional determinants and durability of shape memory polymers. Polymers 2010, 2, 120–158. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Thermo-responsive amphiphilic di- and triblock copolymers based on poly(N-isopropylacrylamide) and poly(methoxy diethylene glycol acrylate): Aggregation and hydrogel formation in bulk solution and in thin films. Prog. Colloid Polym. Sci. 2013, 140, 15–34. [Google Scholar]
- Ishizone, T.; Seki, A.; Hagiwara, M.; Han, S.; Yokoyama, H.; Oyane, A.; Deffieux, A.; Carlotti, S. Anionic polymerizations of oligo(ethylene glycol) alkyl ether methacrylates: Effect of side chain length and ω-alkyl group of side chain on cloud point in water. Macromolecules 2008, 41, 2963–2967. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Progr. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Alexander, C.; Shakesheff, K.M. Responsive polymers at the biology/materials science interface. Adv. Mater. 2006, 18, 3321–3328. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Trillo, F.; van Hest, J.C.; Thies, J.C.; Michon, T.; Weberskirch, R.; Cameron, N.R. Fine-tuning the transition temperature of a stimuli-responsive polymer by a simple blending procedure. Chem. Commun. 2008, 19, 2230–2232. [Google Scholar] [CrossRef]
- Yang, H.; Esteves, A.C.C.; Zhu, H.; Wang, D.; Xin, J.H. In-situ study of the structure and dynamics of thermo-responsive PNIPAAm grafted on a cotton fabric. Polymer 2012, 53, 3577–3586. [Google Scholar] [CrossRef]
- Choi, W.; Tuteja, A.; Chhatre, S.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. Fabrics with tunable oleophobicity. Adv. Mater. 2009, 21, 2190–2195. [Google Scholar] [CrossRef]
- Becer, C.R.; Hahn, S.; Fijten, M.W.M.; Thijs, H.M.L.; Hoogenboom, R.; Schubert, U.S. Libraries of methacrylic acid and oligo(ethylene glycol) methacrylate copolymers with LCST behavior. J. Polym. Sci. A Polym. Chem. 2008, 46, 7138–7147. [Google Scholar] [CrossRef]
- Sosnik, A.; Cohn, D. Reverse thermo-responsive poly(ethylene oxide) and poly(propylene oxide) multiblock copolymers. Biomaterials 2005, 26, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations. Bioconjugate Chem. 1993, 4, 341–346. [Google Scholar] [CrossRef]
- Wang, W.; Metwalli, E.; Perlich, J.; Papadakis, C.M.; Cubitt, R.; Müller-Buschbaum, P. Cyclic switching of water storage in thin block copolymer films containing poly(N-isopropylacrylamide). Macromolecules 2009, 42, 9041–9051. [Google Scholar] [CrossRef]
- Wang, W.; Troll, K.; Kaune, G.; Metwalli, E.; Ruderer, M.; Skrabania, K.; Laschewsky, A.; Roth, S.V.; Papadakis, C.M.; Cubitt, R.; et al. Thin films of poly(N-isopropylacrylamide) end-capped with N-butyltrithiocarbonate. Macromolecules 2008, 41, 3209–3218. [Google Scholar] [CrossRef]
- Kujawa, P.; Winnik, F.M. Volumetric studies of aqueous polymer solutions using pressure perturbation calorimetry: A new look at the temperature-induced phase transition of poly(N-isopropylacrylamide) in water and D2O. Macromolecules 2001, 34, 4130–4135. [Google Scholar] [CrossRef]
- Taylor, L.D.; Cerankowsky, L.D. Preparation of films exhibiting a balanced temperature dependence to permeation by aqueous solutions—A study of lower consolute behavior. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Bivigou-Koumba, A.M.; Kristen, J.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Synthesis of symmetrical triblock copolymers of styrene and N-isopropylacrylamide using bifunctional bis(trithiocarbonate)s as RAFT agents. Macromol. Chem. Phys. 2009, 210, 565–578. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Fang, Q.; Zhong, Q.; Chen, Y.; Wang, J. Synthesis and Thermosensitive Behavior of Polyacrylamide Copolymers and Their Applications in Smart Textiles. Polymers 2015, 7, 909-920. https://doi.org/10.3390/polym7050909
Chen T, Fang Q, Zhong Q, Chen Y, Wang J. Synthesis and Thermosensitive Behavior of Polyacrylamide Copolymers and Their Applications in Smart Textiles. Polymers. 2015; 7(5):909-920. https://doi.org/10.3390/polym7050909
Chicago/Turabian StyleChen, Tao, Qisheng Fang, Qi Zhong, Yangyi Chen, and Jiping Wang. 2015. "Synthesis and Thermosensitive Behavior of Polyacrylamide Copolymers and Their Applications in Smart Textiles" Polymers 7, no. 5: 909-920. https://doi.org/10.3390/polym7050909