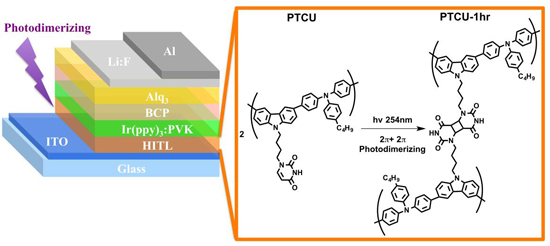
Photo-Crosslinking of Pendent Uracil Units Provides Supramolecular Hole Injection/Transport Conducting Polymers for Highly Efficient Light-Emitting Diodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
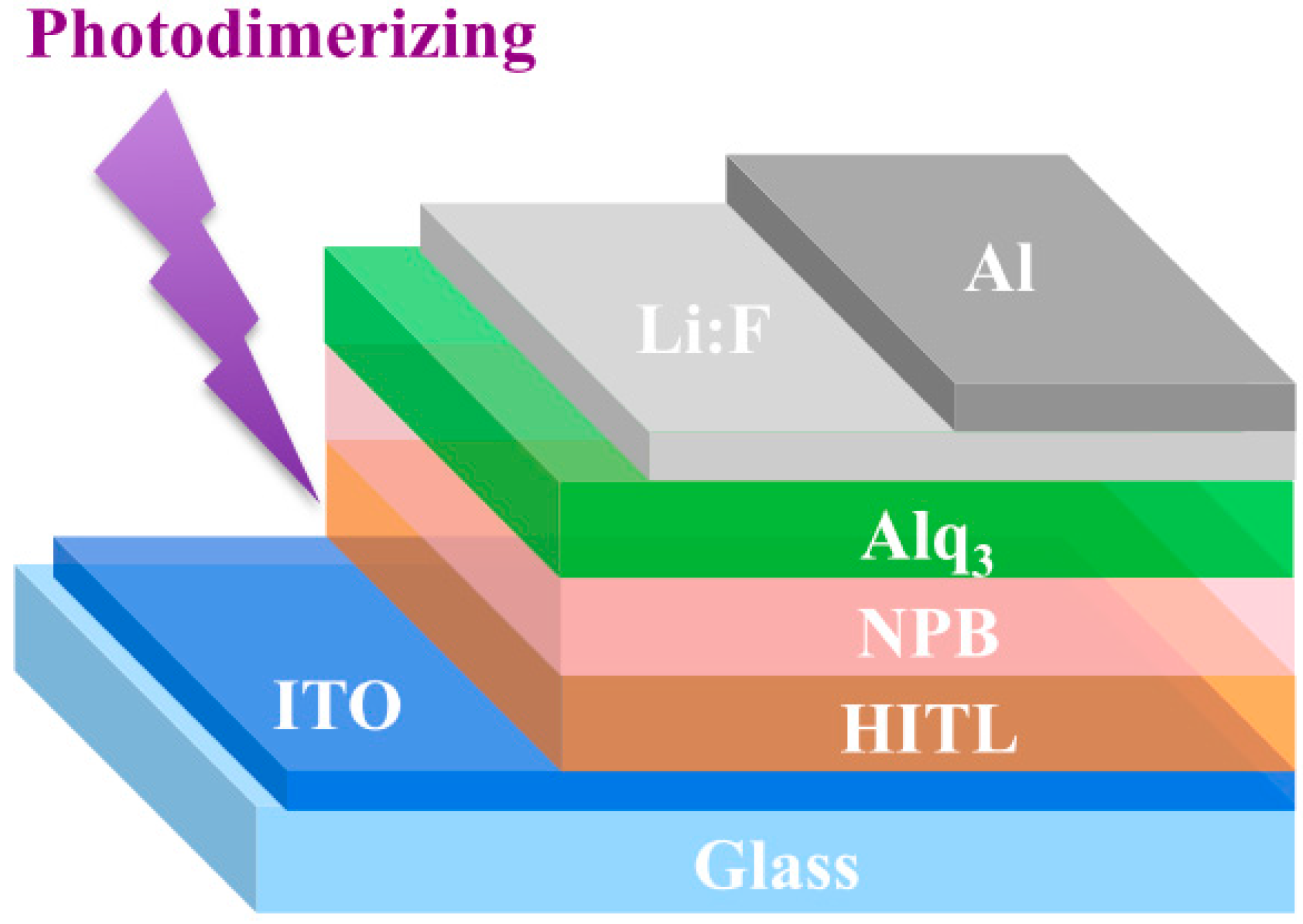
2.3. Fabrication of OLEDs
3. Results and Discussion
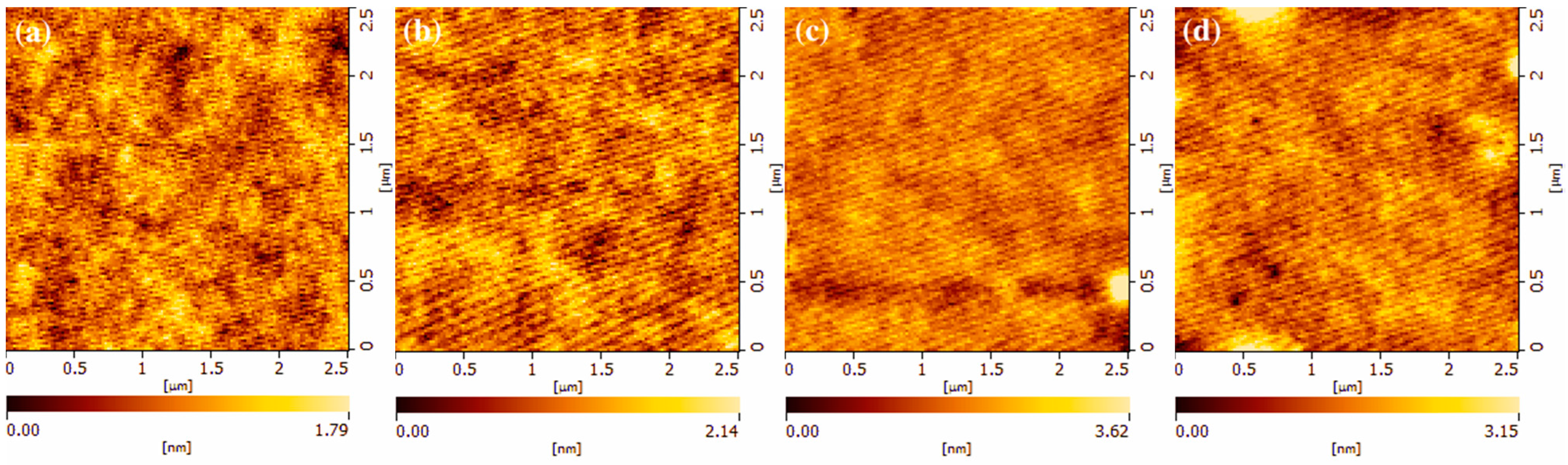
3.1. Physical and Electrical Properties
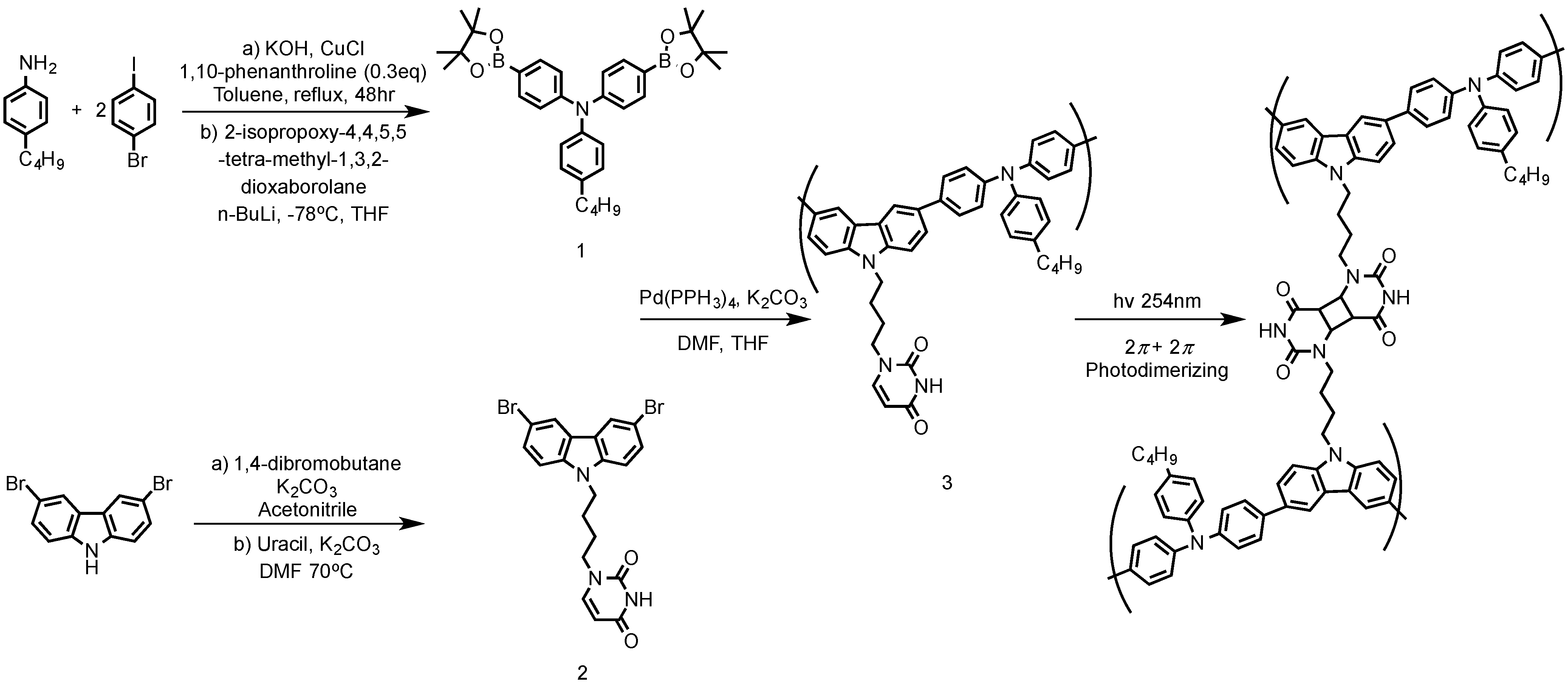
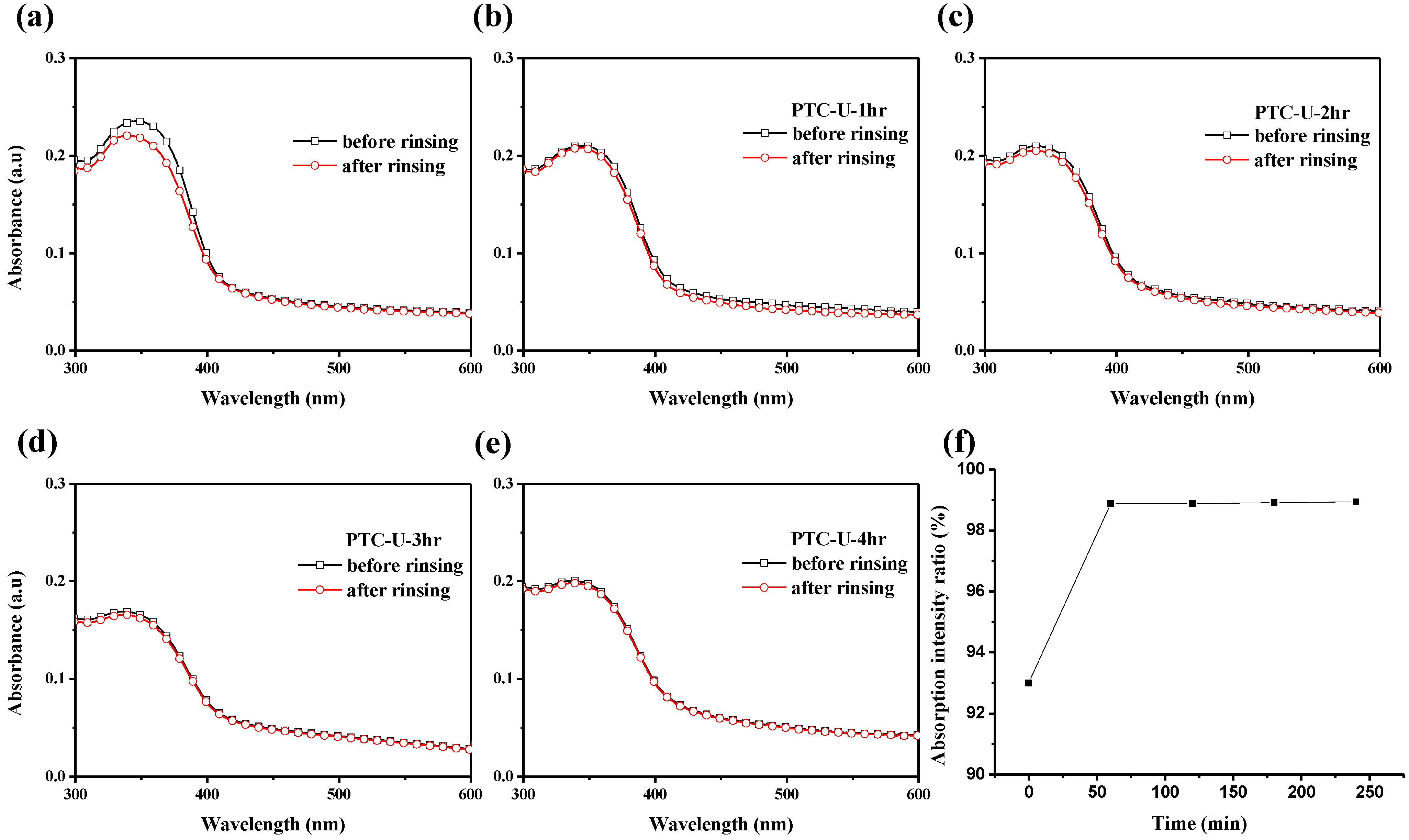
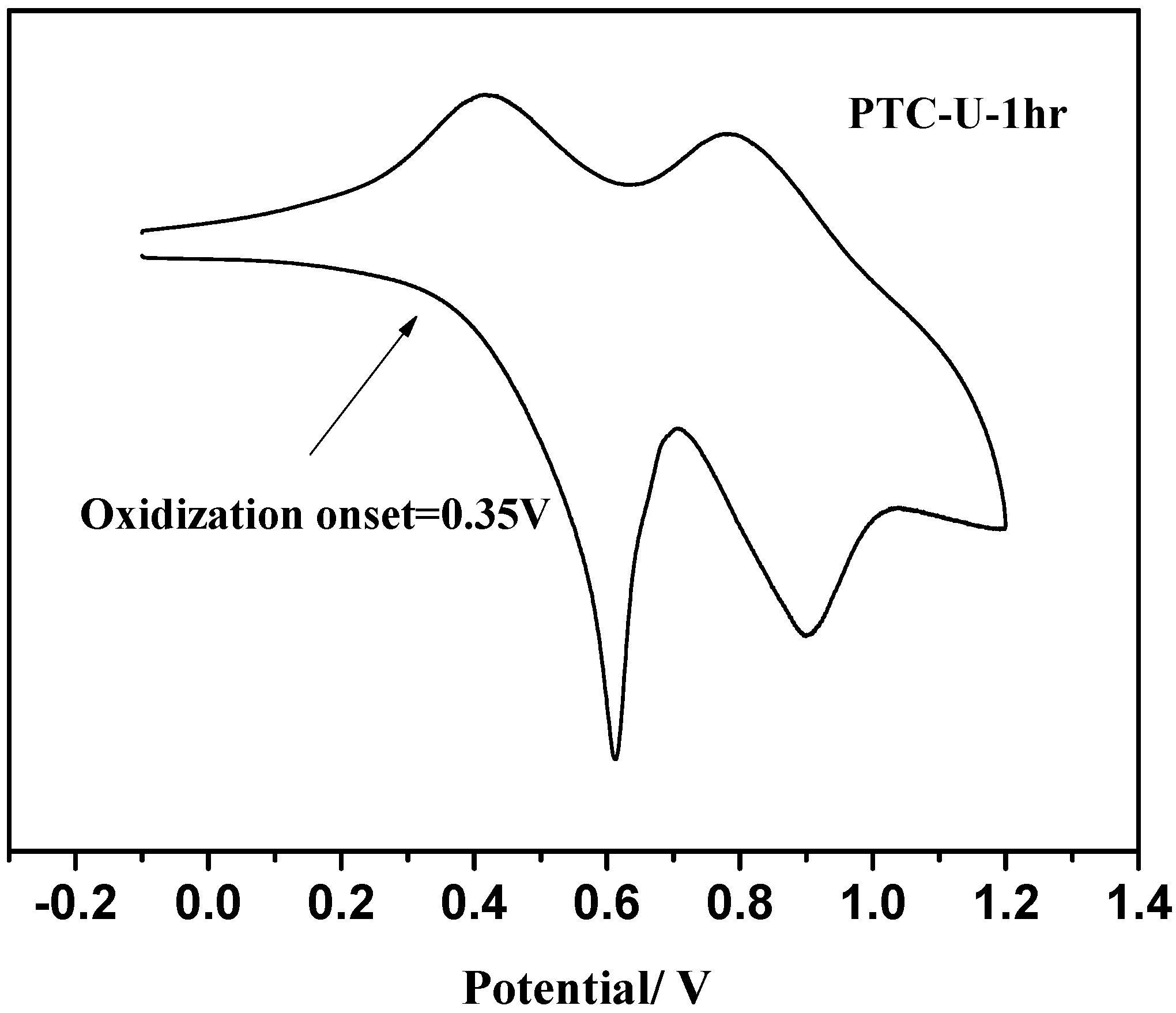
3.2. Florescence OLED Device
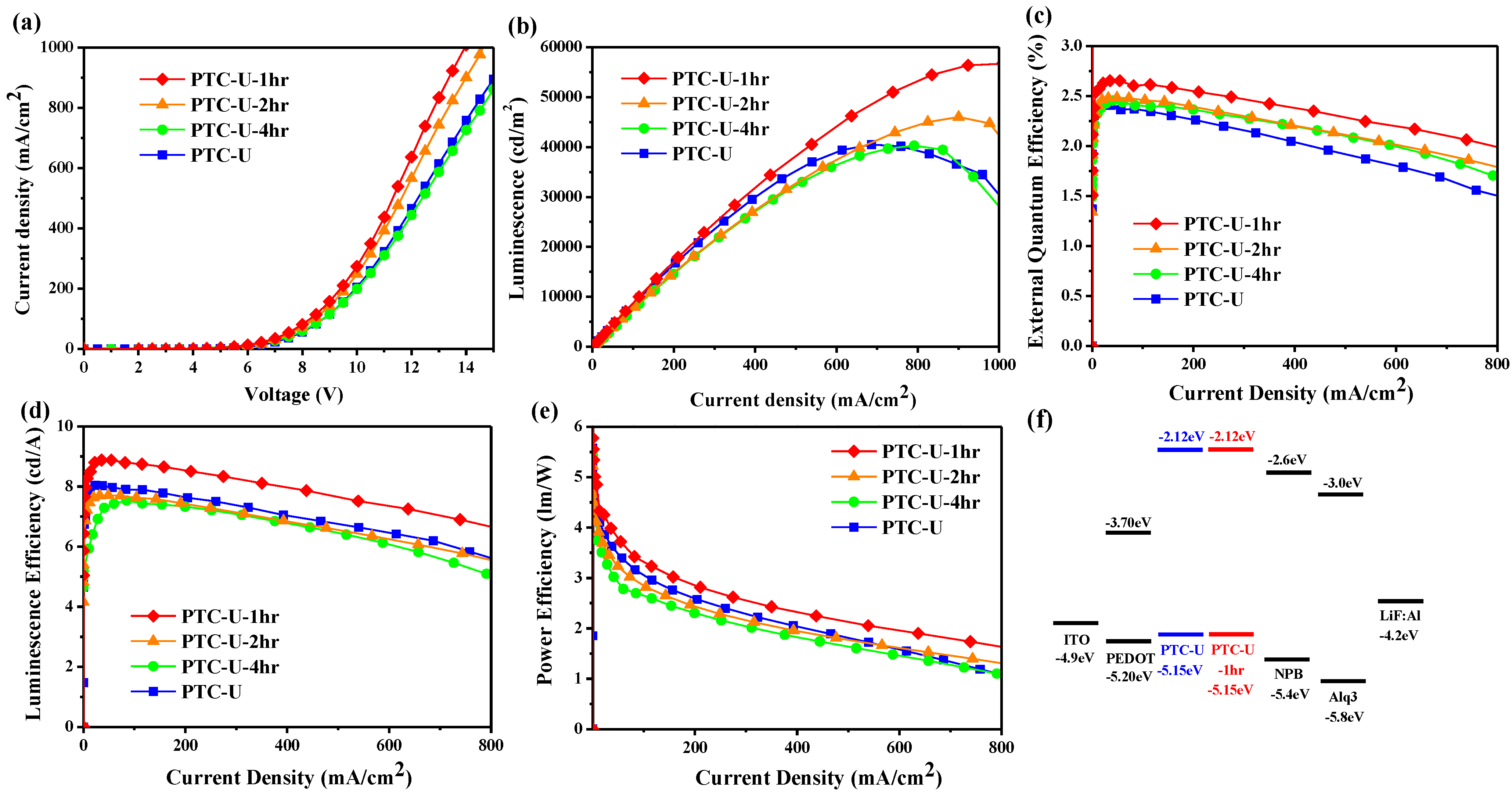
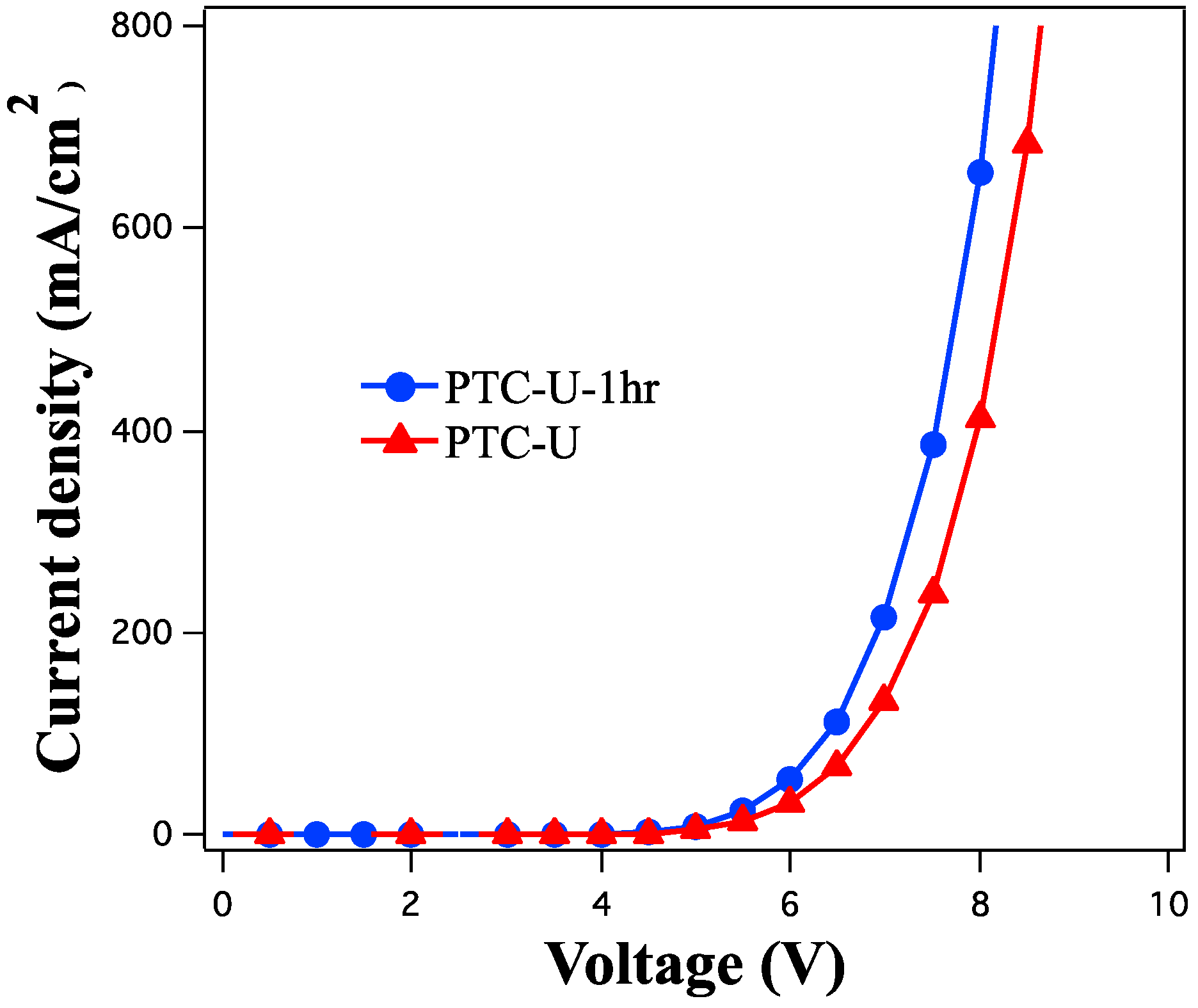
| HITL | Von (V) | Qmax (%) | LEmax (cd/A) | ηEmax (lm/W) | Bmax (cd/m2) |
|---|---|---|---|---|---|
| PTC-U | 3.5 | 2.40 | 8.0 | 4.98 | 40,490 |
| PTC-U-1hr | 3.6 | 2.65 | 8.9 | 5.55 | 56,704 |
| PTC-U-2hr | 3.7 | 2.48 | 7.7 | 4.65 | 46,027 |
| PTC-U-4hr | 3.7 | 2.45 | 7.5 | 4.65 | 40,288 |
| PEDOT a | 2.4 | 1.46 | 4.7 | 3.57 | 41,948 |
3.3. Phosphorescence OLED Device
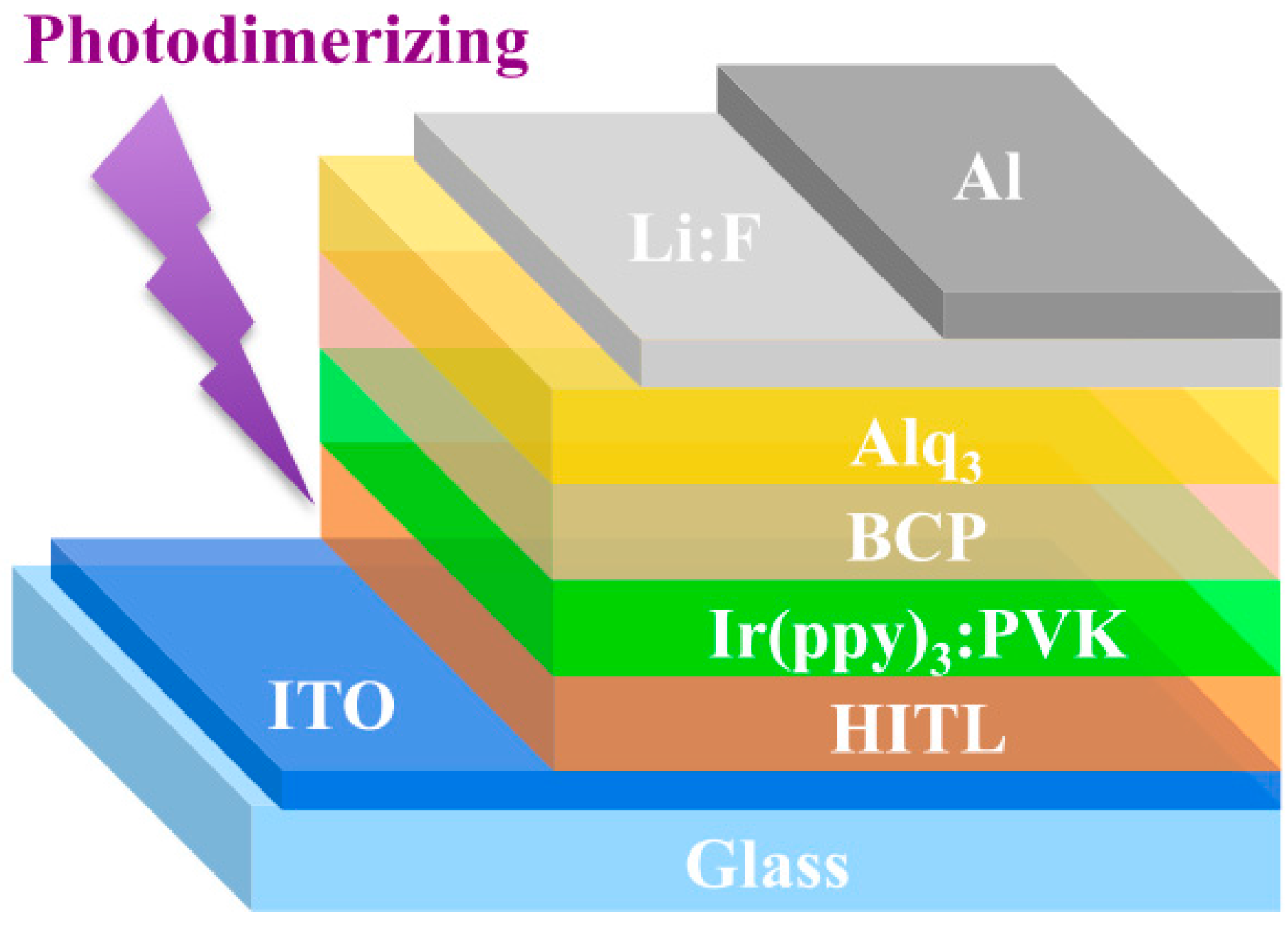
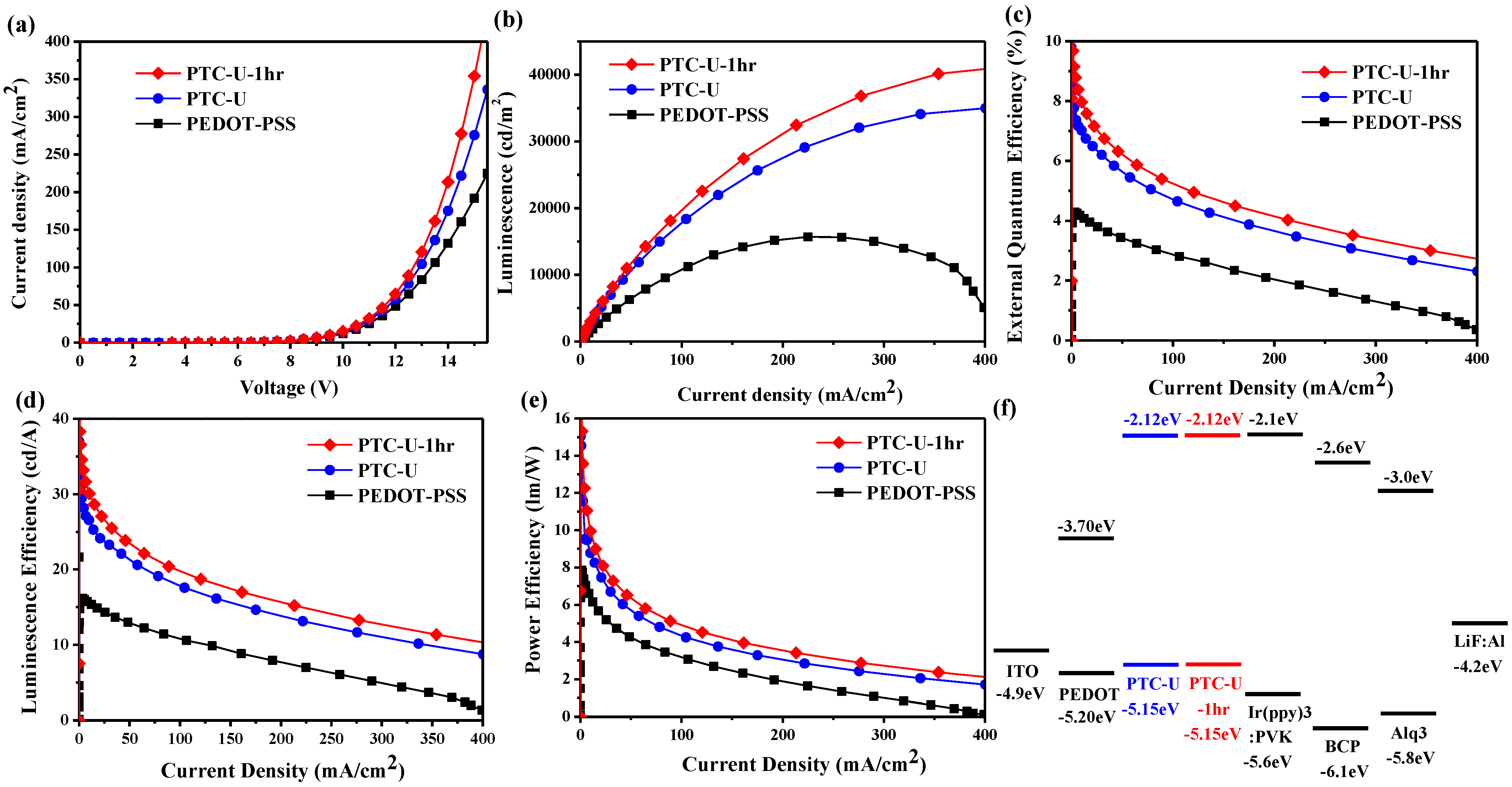
| HITL | Von (V) | Qmax (%) | LEmax (cd/A) | ηEmax (lm/W) | Bmax (cd/m2) |
|---|---|---|---|---|---|
| PTC-U-1hr | 7.13 | 9.68 | 36.6 | 15.32 | 41,466 |
| PTC-U | 7.13 | 8.35 | 30.8 | 11.65 | 34,978 |
| PEDOT | 7.33 | 4.29 | 16.2 | 7.85 | 15,678 |
4. Conclusions
Acknowledgments
Author Contributions
Supplementary Information
References
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.A.; Liu, B.; Donoghue, E.P.; Kravchenko, I.; Kim, D.Y.; So, F.; Rinzler, A.G. Low-voltage, low-power, organic light-emitting transistors for active matrix displays. Science 2011, 332, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Shin, D.C.; Park, S.H. Large-area OLED lightings and their applications. Semicond. Sci. Technol. 2011, 26. [Google Scholar] [CrossRef]
- Kim, J.Y.; Joo, C.W.; Lee, J.; Woo, J.-C.; Oh, J.-Y.; Baek, N.S.; Chu, H.Y.; Lee, J.-I. Save energy on OLED lighting by a simple yet powerful technique. RSC Adv. 2015, 5, 8415–8421. [Google Scholar] [CrossRef]
- Strukelj, M.; Papadimitrakopoulos, F.; Miller, T.M.; Rothberg, L.J. Design and application of electron-transporting organic materials. Science 1995, 267, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Ji, W.; Wang, J.; Zeng, Q.; Su, Z.; Sun, Z. Highly efficient organic light-emitting devices by introducing traps in the hole-injection layer. RSC Adv. 2013, 3, 14616–14621. [Google Scholar] [CrossRef]
- Granström, M.; Berggren, M.; Inganäs, O. Micrometer- and nanometer-sized polymeric light-emitting diodes. Science 1995, 267, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.K.H.; Kim, J.S.; Burroughes, J.H.; Becker, H.; Li, S.F.Y.; Brown, T.M.; Cacialli, F.; Friend, R.H. Molecular-scale interface engineering for polymer light-emitting diodes. Nature 2000, 404, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.A.; Angelopoulos, M.; Karg, S.; Brock, P.J.; Scott, J.C. Polymeric anodes for improved polymer light-emitting diode performance. Appl. Phys. Lett. 1997, 70, 2067–2069. [Google Scholar] [CrossRef]
- De Jong, M.P.; van Ijzendoorn, L.J.; de Voigt, M.J.A. Stability of the interface between indium-tin-oxide and poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) in polymer light-emitting diodes. Appl. Phys. Lett. 2000, 77, 2255–2257. [Google Scholar] [CrossRef]
- Baranoff, E.; Curchod, B.F.E.; Frey, J.; Scopelliti, R.; Kessler, F.; Tavernelli, I.; Rothlisberger, U.; Graẗzel, M.; Nazeeruddin, Md.K. Acid-induced degradation of phosphorescent dopants for OLEDs and its application to the synthesis of tris-heteroleptic iridium(III) bis-cyclometalated complexes. Inorg. Chem. 2012, 51, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Schaer, M.; Nüesch, F.; Berner, D.; Leo, W.; Zuppiroli, L. Water vapor and oxygen degradation mechanisms in organic light emitting diodes. Adv. Funct. Mater. 2001, 11, 116–121. [Google Scholar] [CrossRef]
- Hittinger, E.; Kokil, A.; Weder, C. Synthesis and characterization of cross-linked conjugated polymer milli-, micro-, and nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 1808–1811. [Google Scholar] [CrossRef]
- Weder, C. Synthesis, processing and properties of conjugated polymer networks. Chem. Commun. 2005, 43, 5378–5389. [Google Scholar] [CrossRef]
- Niu, Y.H.; Liu, M.S.; Ka, J.W.; Bardker, J.; Zin, M.T.; Schofield, R.; Chi, Y.; Jen, A.K.Y. Crosslinkable hole-transport layer on conducting polymer for high-efficiency white polymer light-emitting diodes. Adv. Mater. 2007, 19, 300–304. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Liu, M.S.; Zhang, Y.; Niu, Y.; Huang, F.; Ka, J.W.; Yip, H.L.; Tian, Y.; Jen, A.K.Y. Thermally cross-linkable hole-transporting materials on conducting polymer: Synthesis, characterization, and applications for polymer light-emitting devices. Chem. Mater. 2008, 20, 413–422. [Google Scholar] [CrossRef]
- Su, W.F.; Chen, Y. Synthesis and optoelectronic properties of a luminescent fluorene derivative containing hole-transporting triphenylamine terminals. Polymer 2011, 52, 3311–3317. [Google Scholar] [CrossRef]
- Cha, S.J.; Cho, S.N.; Lee, W.H.; Chung, H.S.; Kang, I.N.; Suh, M.C. Thermally cross-linkable hole transport polymers for solution-based organic light-emitting diodes. Macromol. Rapid Commun. 2014, 35, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Thomas, K.R.J.; Jou, J.-H.; Kumar, S.; Chen, Y.-L.; Jou, Y.-C. Deep-blue emitting pyrene-benzimidazole conjugates for solution processed organic light-emitting diodes. RSC Adv. 2015, 5, 8727–8738. [Google Scholar] [CrossRef]
- Bacher, E.; Bayerl, M.; Rudati, P.; Reckefuss, N.; Müller, C.D.; Meerholz, K.; Nuyken, O. Synthesis and characterization of photo-cross-linkable hole-conducting polymers. Macromolecules 2005, 38, 1640–1647. [Google Scholar] [CrossRef]
- Jungermann, S.; Riegel, N.; Müller, C.D.; Meerholz, K.; Nuyken, O. Novel photo-cross-linkable hole-transporting polymers: Synthesis, characterization, and application in organic light emitting diodes. Macromolecules 2006, 39, 8911–8919. [Google Scholar] [CrossRef]
- Solomeshch, O.; Yu, Y.-J.; Medvedev, V.; Razin, A.; Blumer-Ganon, B.; Eichen, Y.; Jin, J.-I.; Tessler, N. Wide band gap cross-linkable semiconducting polymer LED. Synth. Met. 2007, 157, 841–845. [Google Scholar] [CrossRef]
- Solomeshch, O.; Medvedev, V.; Mackie, P.R.; Cupertino, D.; Razin, A.; Tessler, N. Electronic formulations—Photopatterning of luminescent conjugated polymers. Adv. Funct. Mater. 2006, 16, 2095–2102. [Google Scholar] [CrossRef]
- Kohnen, A.; Riegel, N.; Kremer, J.H.-W.M.; Lademann, H.; Müller, D.C.; Meerholz, K. The simple way to solution-processed multilayer OLEDs—Layered block-copolymer networks by living cationic polymerization. Adv. Mater. 2009, 21, 879–884. [Google Scholar] [CrossRef]
- Cheng, C.C.; Yen, Y.C.; Ye, Y.S.; Chang, F.C. Biocomplementary interaction behavior in DNA-like and RNA-like polymers. J. Polym. Sci. Polym. Chem. 2009, 47, 6388–6395. [Google Scholar] [CrossRef]
- Chu, Y.L.; Cheng, C.C.; Yen, Y.C.; Chang, F.C. A new supramolecular hole injection/transport material on conducting polymer for application in light-emitting diodes. Adv. Mater. 2012, 24, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Kwon, Y.; Park, J.J.; Pu, L.; Hayakawa, T.; Kakimoto, M.-A. Novel hyperbranched phthalocyanine as a hole injection nanolayer in organic light-emitting diodes. Macromol. Rapid Commun. 2007, 28, 1657–1662. [Google Scholar] [CrossRef]
- Lee, T.W.; Park, J.J.; Kwon, Y.; Hayakawa, T.; Choi, T.L.; Park, J.H.; Das, R.R.; Kakimoto, M.-A. Spin-assembled nanolayer of a hyperbranched polymer on the anode in organic light-emitting diodes: The mechanism of hole injection and electron blocking. Langmuir 2008, 24, 12704–12709. [Google Scholar] [CrossRef] [PubMed]
- Setlow, R.B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science 1966, 153, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.M.; Davies, R.J.H. The structure of thymine photo-dimer. J. Chem. Soc. C 1966, 23, 2239–2244. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cheng, C.C.; Ye, Y.S.; Yen, Y.C.; Chang, F.C. Bioinspired photo-cross-linked nanofibers from uracil-functionalized polymers. ACS Macro Lett. 2012, 1, 159–162. [Google Scholar] [CrossRef]
- Tominaga, M.; Konishi, K.; Aida, T. A Photocrosslinkable dendrimer consisting of a nucleobase. Chem. Lett. 2000, 29, 374–375. [Google Scholar] [CrossRef]
- Thangthong, A.-M.; Prachumrak, N.; Tarsang, R.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Blue light-emitting and hole-transporting materials based on 9,9-bis(4-diphenylaminophenyl)fluorenes for efficient electroluminescent devices. J. Mater. Chem. 2012, 22, 6869–6877. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Hreha, R.D.; Jabbour, G.E.; Kippelen, B.; Peyghambarian, N.; Marder, S.R. Photo-crosslinkable polymers as hole-transport materials for organic light-emitting diodes. J. Mater. Chem. 2002, 12, 1703–1708. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, Y.J.; Zhang, Y.; Liu, M.S.; Jen, A.K.Y. Crosslinkable hole-transporting materials for solution processed polymer light-emitting diodes. J. Mater. Chem. 2008, 18, 4495–4509. [Google Scholar] [CrossRef]
- Lee, J.; Han, H.; Lee, J.; Yoon, S.H.; Lee, C. Utilization of “thiol-ene” photo cross-linkable hole-transporting polymers for solution-processed multilayer organic light-emitting diode. J. Mater. Chem. C 2014, 2, 1474–1481. [Google Scholar] [CrossRef]
- Baldo, M.A.; Lamansky, S.; Burrows, P.E.; Thompson, M.E.; Forrest, S.R. Very high-efficiency green organic light-emitting devices based on electrophosphorescence. Appl. Phys. Lett. 1999, 75, 4–6. [Google Scholar] [CrossRef]
- Lee, C.L.; Lee, K.B.; Kim, J.J. Polymer phosphorescent light-emitting devices doped with tris(2-phenylpyridine) iridium as a triplet emitter. Appl. Phys. Lett. 2000, 77, 2280–2282. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yanagida, S.; Forrest, S.R. Energy transfer in polymer electrophosphorescent light emitting devices with single and multiple doped luminescent layers. J. Appl. Phys. 2002, 92, 87–93. [Google Scholar] [CrossRef]
- Gong, X.; Robinson, M.R.; Ostrowski, J.C.; Moses, D.; Bazan, G.C.; Heeger, A.J. High-efficiency polymer-based electrophosphorescent devices. Adv. Mater. 2002, 14, 581–585. [Google Scholar] [CrossRef]
- Lee, C.C.; Yeh, K.M.; Chen, Y. New host homopolymers containing pendant triphenylamine derivatives: Synthesis, optical, electrochemical properties and its blend with Ir(ppy)3 for green phosphorescent organic light-emitting devices. J. Polym. Sci. Polym. Chem. 2008, 46, 7960–7971. [Google Scholar] [CrossRef]
- Yeh, K.M.; Lee, C.C.; Chen, Y. Vinyl polymer containing 1,4-distyrylbenzene chromophores: synthesis, optical, electrochemical properties and its blend with PVK and Ir(ppy)3. Synth. Met. 2008, 158, 411–416. [Google Scholar] [CrossRef]
- Yang, M.J.; Tsutsui, T. Use of poly(9-vinylcarbazole) as host material for iridium complexes in high-efficiency organic light-emitting devices. Jpn. J. Appl. Phys. 2000, 39, 828–829. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, H.-K.; Chen, Y.-H.; Chu, Y.-L.; Cheng, C.-C.; Chang, F.-C.; Zhu, C.-Y.; Kuo, S.-W. Photo-Crosslinking of Pendent Uracil Units Provides Supramolecular Hole Injection/Transport Conducting Polymers for Highly Efficient Light-Emitting Diodes. Polymers 2015, 7, 804-818. https://doi.org/10.3390/polym7050804
Shih H-K, Chen Y-H, Chu Y-L, Cheng C-C, Chang F-C, Zhu C-Y, Kuo S-W. Photo-Crosslinking of Pendent Uracil Units Provides Supramolecular Hole Injection/Transport Conducting Polymers for Highly Efficient Light-Emitting Diodes. Polymers. 2015; 7(5):804-818. https://doi.org/10.3390/polym7050804
Chicago/Turabian StyleShih, Hsi-Kang, Yi-Han Chen, Yu-Lin Chu, Chih-Chia Cheng, Feng-Chih Chang, Chao-Yuan Zhu, and Shiao-Wei Kuo. 2015. "Photo-Crosslinking of Pendent Uracil Units Provides Supramolecular Hole Injection/Transport Conducting Polymers for Highly Efficient Light-Emitting Diodes" Polymers 7, no. 5: 804-818. https://doi.org/10.3390/polym7050804