Effects of RAFT Agent on the Selective Approach of Molecularly Imprinted Polymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
2.3. Preparation of MAA-β-CD and HEMA-β-CD Monomers
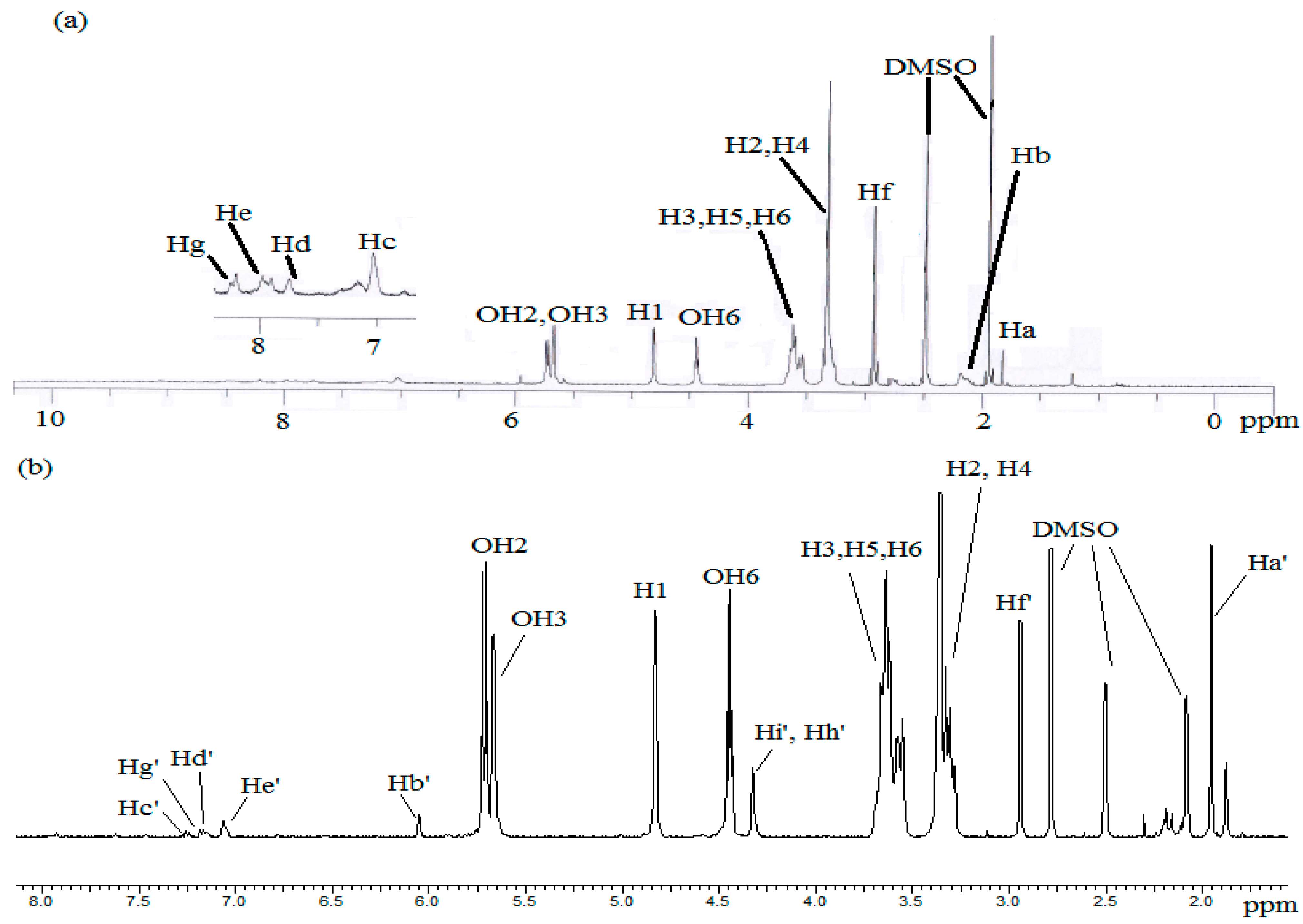
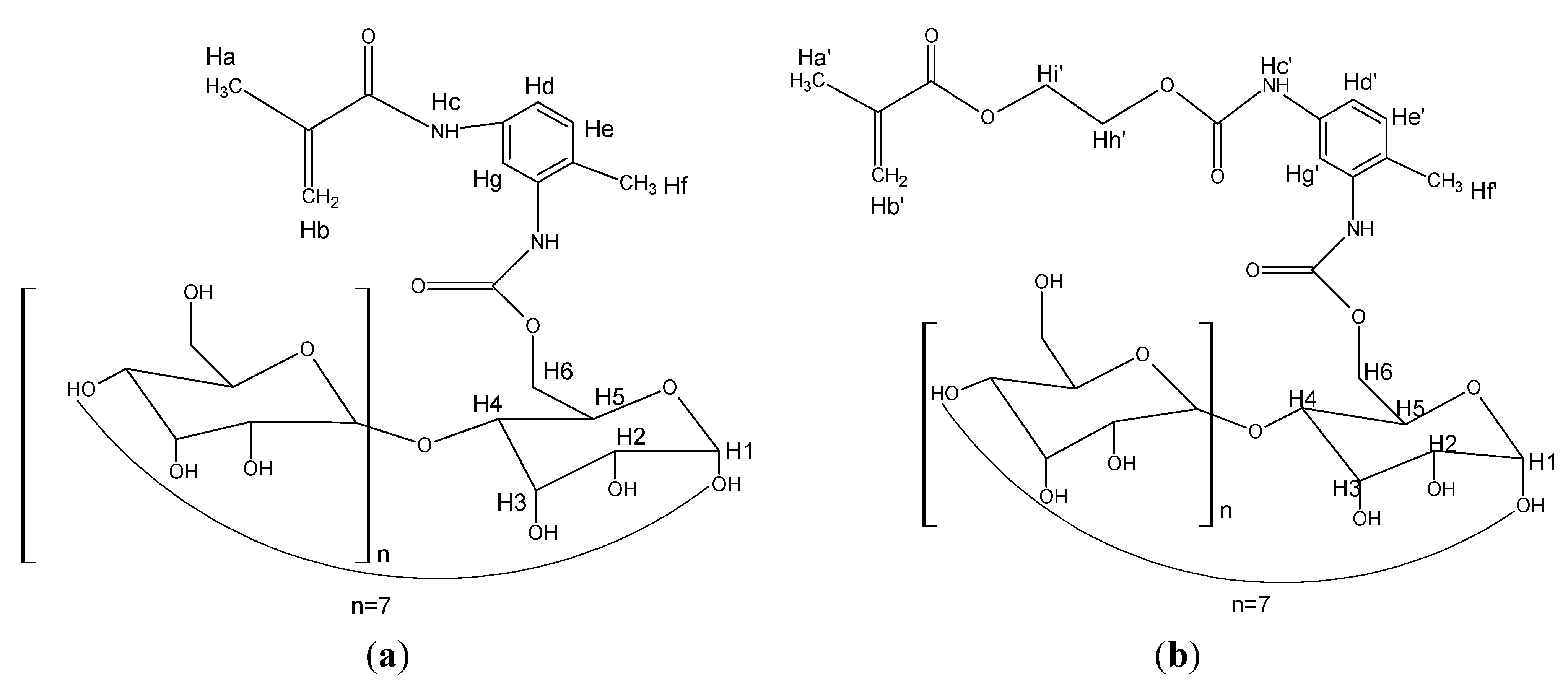
2.4. Synthesis of RAFT-MIPs/RAFT-NIPs
2.5. Rebinding Experiments
3. Results and Discussion
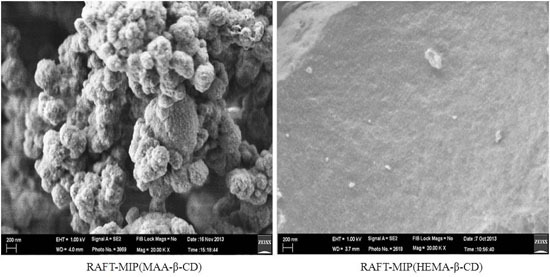
3.1. Characterization of RAFT-MIPs and MIPs
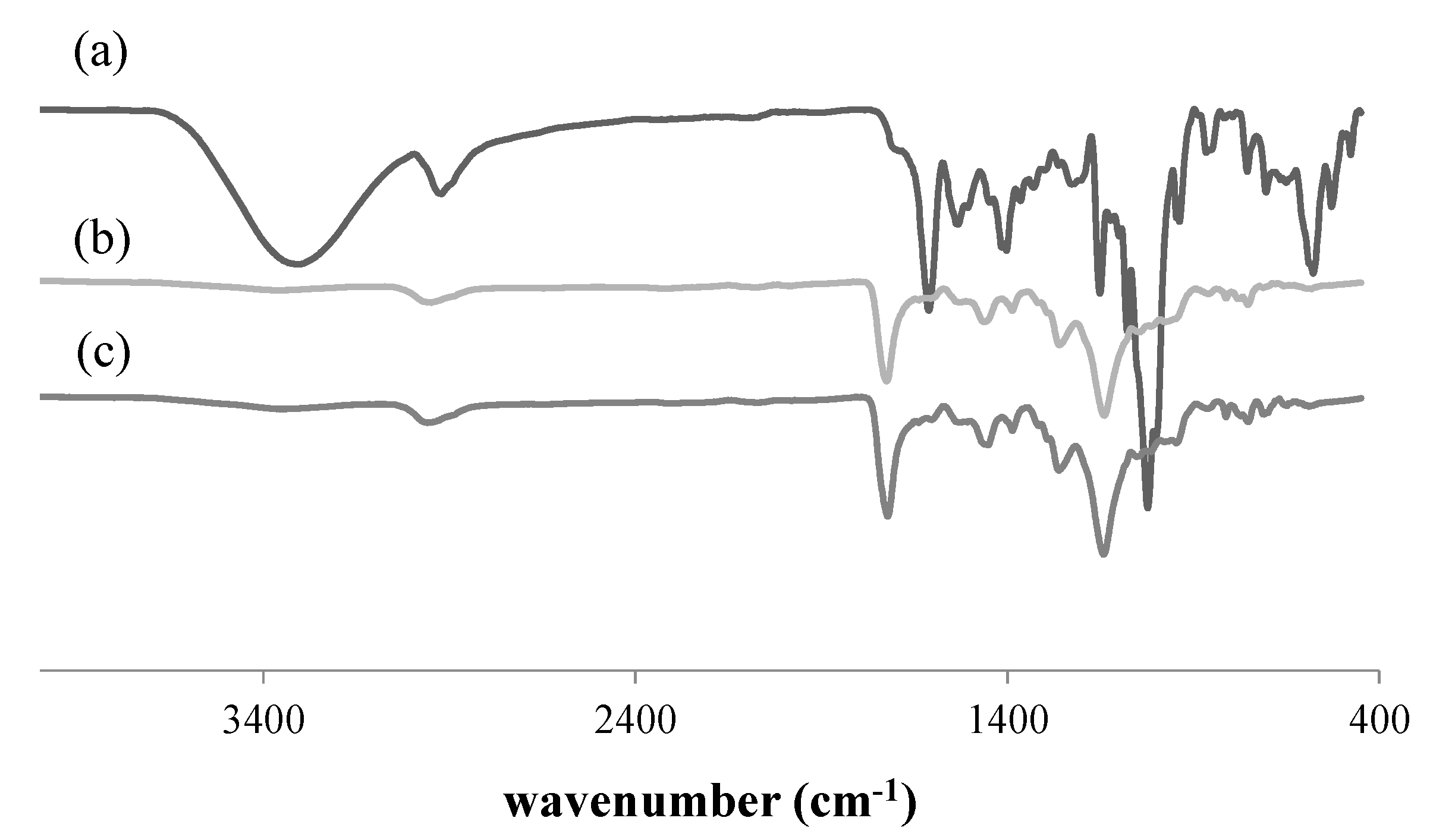
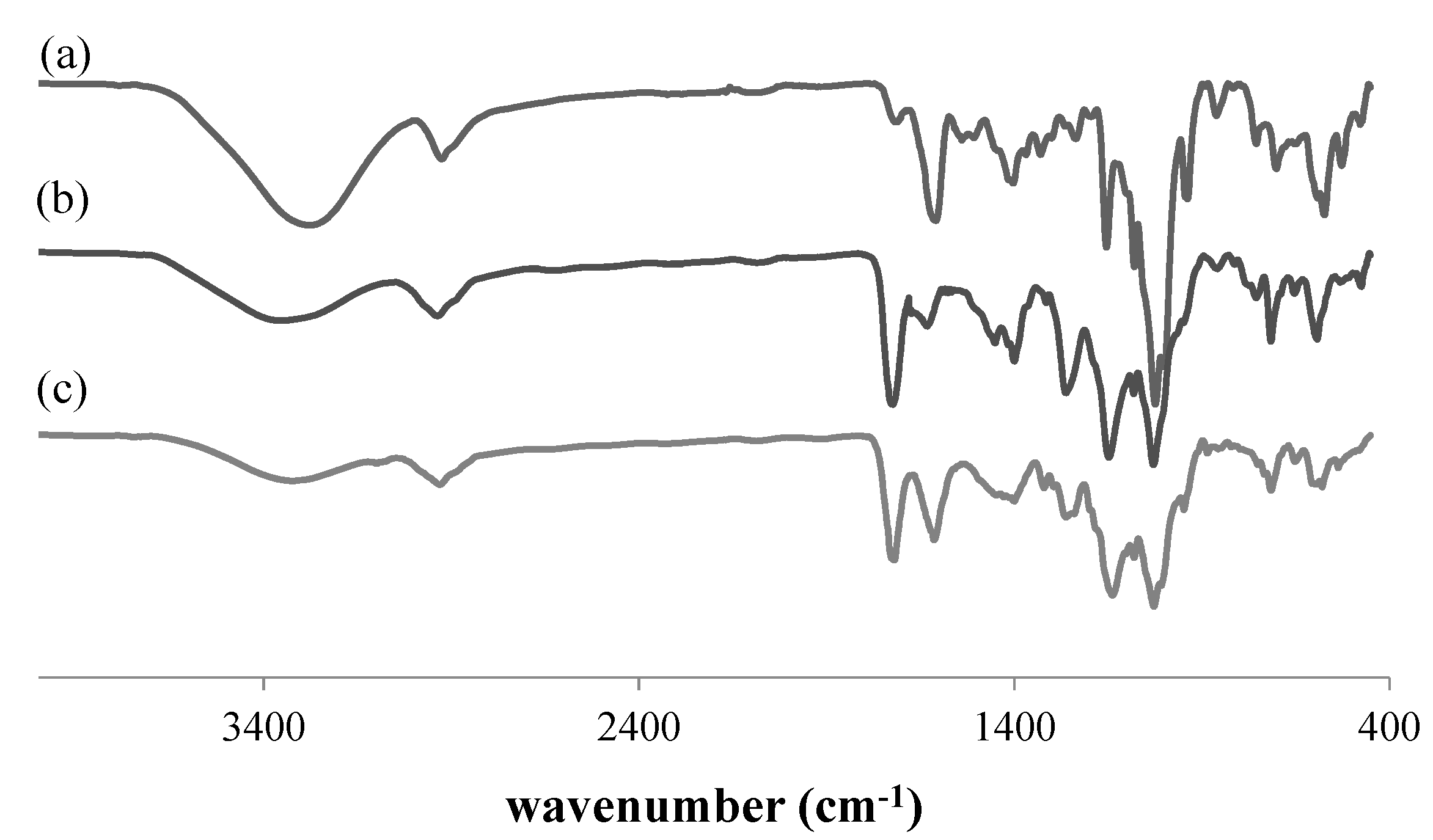
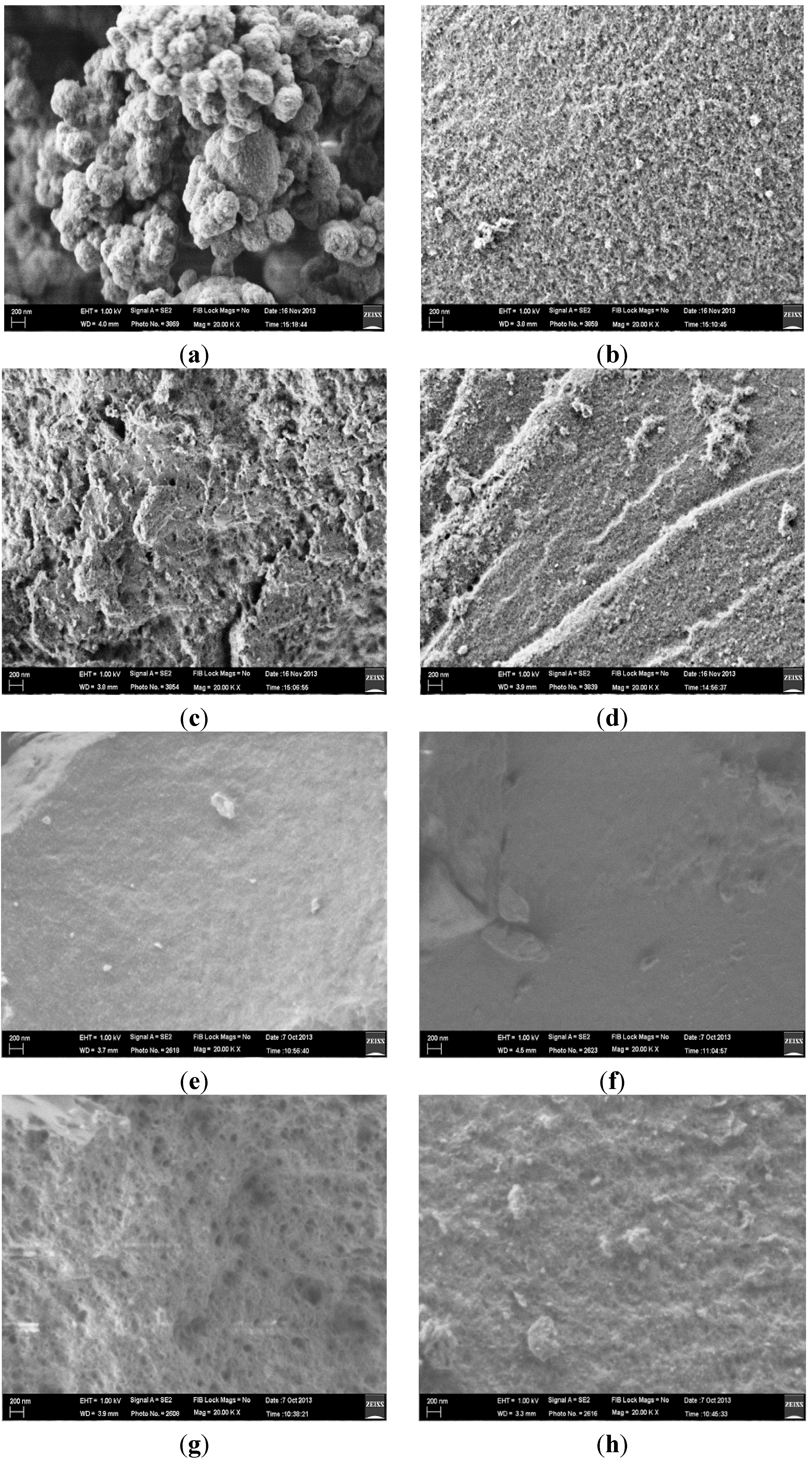
| Polymers | Surface Area, S (m2/g) | Pore Diameter, dp (nm) | Pore Volume, Vp (cm3/g) |
|---|---|---|---|
| RAFT-MIP1 | 11.31 | 8.67 | 4.20 × 10−4 |
| RAFT-NIP1 | 2.22 | 8.21 | 5.75 × 10−4 |
| MIP1 | 1.91 | 3.79 | 7.76 × 10−4 |
| NIP1 | 1.57 | 6.74 | 5.36 × 10−4 |
| RAFT-MIP2 | 0.66 | 6.03 | 3.86 × 10−4 |
| RAFT-NIP2 | 0.14 | 7.29 | 4.12 × 10−4 |
| MIP2 | 47.68 | 3.32 | 6.05 × 10−3 |
| NIP2 | 5.73 | 3.51 | 1.89 × 10−3 |
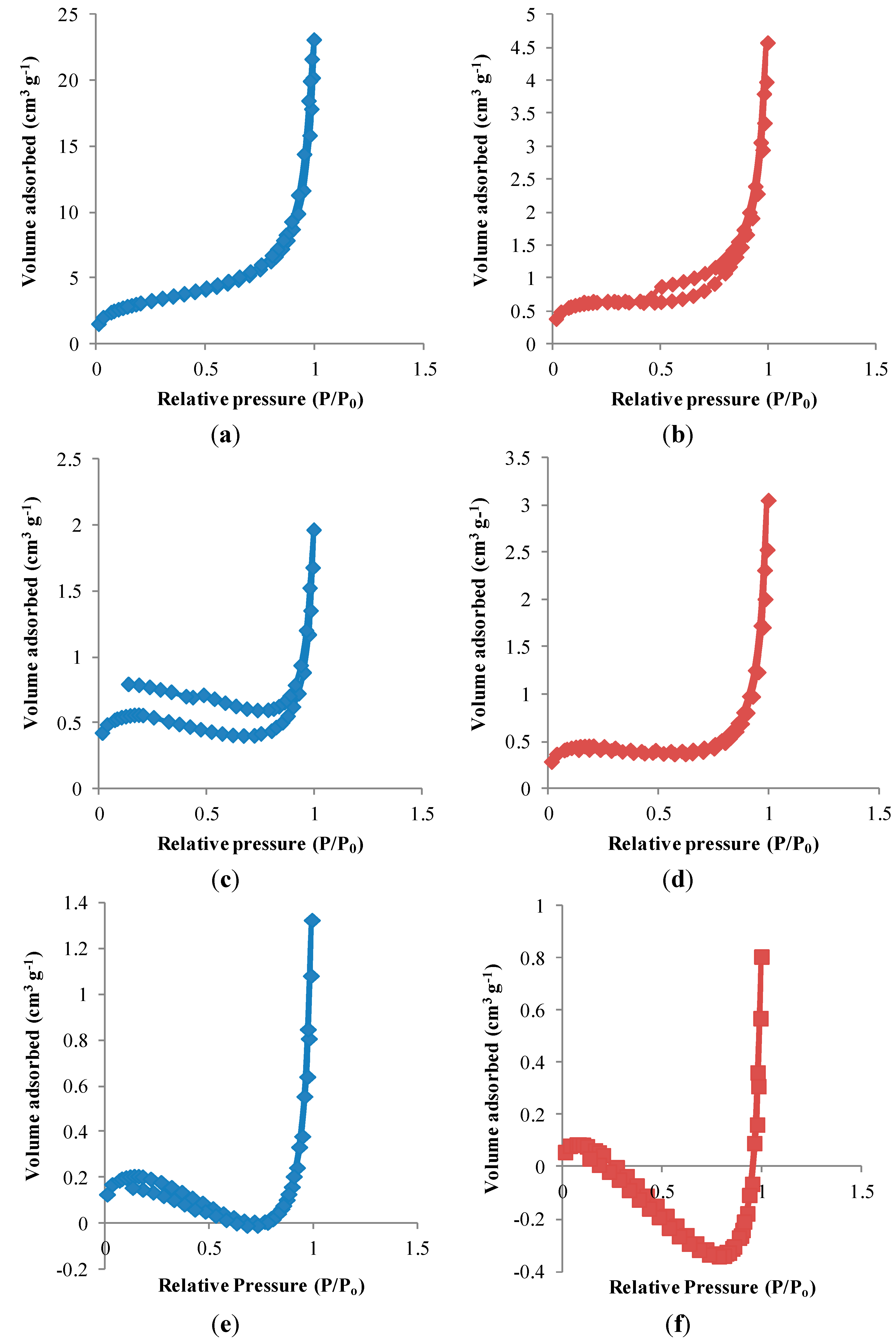
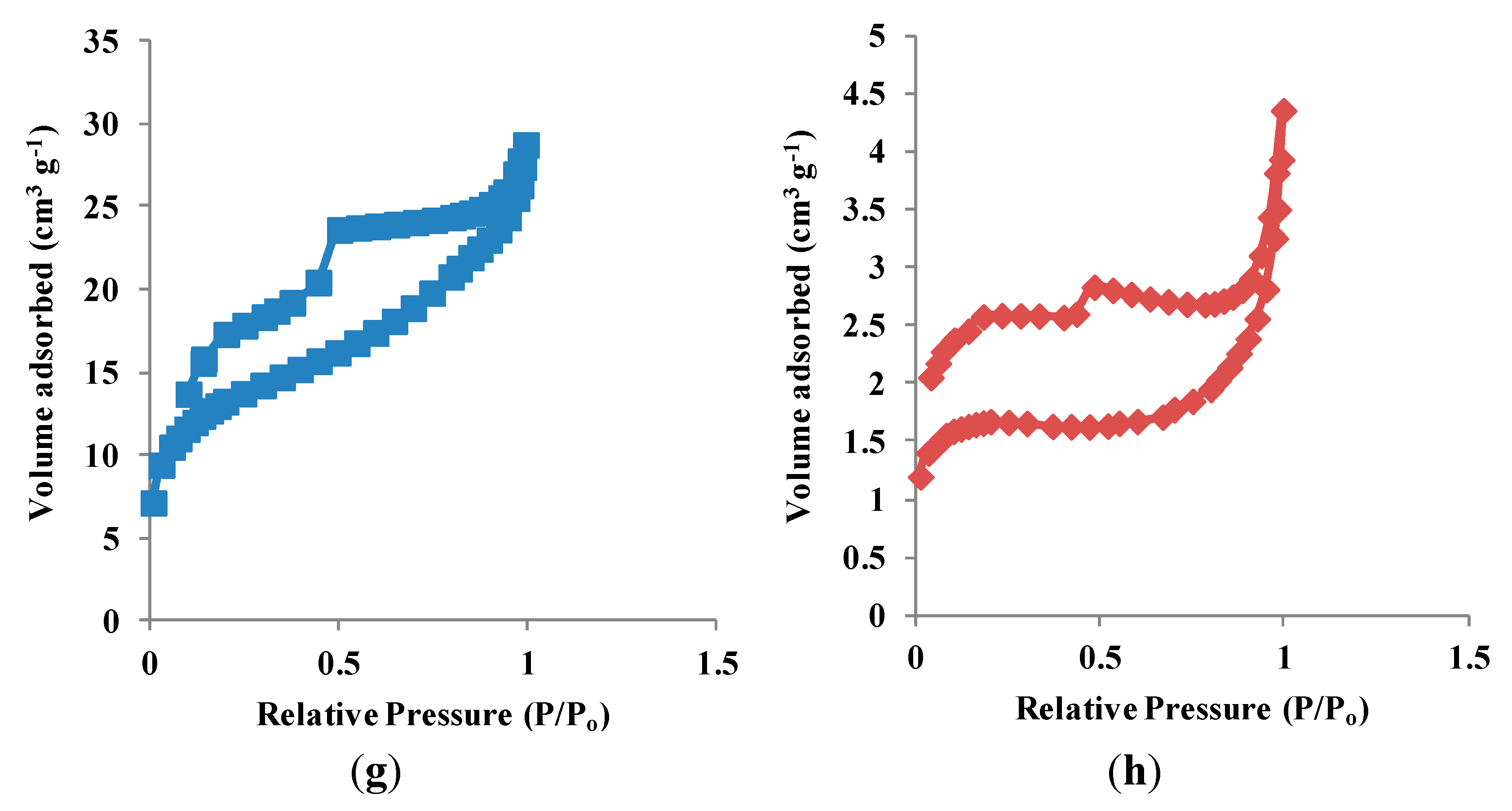
3.2. Binding Characteristics of the Polymers for BzP
| Polymers | Cp (mg/g) | Kd (L/g) | IF |
|---|---|---|---|
| RAFT-MIP1 | 4.12 | 2.03 | 7.86 |
| RAFT-NIP1 | 1.92 | 0.26 | – |
| MIP1 | 3.54 | 2.73 | 1.41 |
| NIP1 | 3.36 | 1.94 | – |
| RAFT-MIP2 | 3.10 | 1.13 | 1.31 |
| RAFT-NIP2 | 2.74 | 0.86 | – |
| MIP2 | 3.68 | 1.33 | 1.44 |
| NIP2 | 3.34 | 0.93 | – |
| Parabens | RAFT-MIP1 | RAFT-NIP1 | IF | MIP1 | NIP1 | IF | ||||
| Cp (mg/g) | Kd(L/g) | Cp (mg/g) | Kd(L/g) | Cp (mg/g) | Kd(L/g) | Cp (mg/g) | Kd(L/g) | |||
| BzP | 4.12 | 2.03 | 1.96 | 0.26 | 7.86 | 3.54 | 2.73 | 3.36 | 1.94 | 1.41 |
| BuP | 3.62 | 0.68 | 1.58 | 0.11 | 5.99 | 2.97 | 1.09 | 2.86 | 0.96 | 1.15 |
| PrP | 3.07 | 1.05 | 1.60 | 0.19 | 5.54 | 2.22 | 0.29 | 1.88 | 0.22 | 1.31 |
| EtP | 2.63 | 0.39 | 0.59 | – | – | 1.35 | 0.20 | 1.24 | 0.18 | 1.13 |
| MeP | 1.62 | 0.17 | – | – | – | 0.53 | 0.04 | 0.44 | 0.03 | 0.83 |
| Parabens | RAFT-MIP2 | RAFT-NIP2 | IF | MIP2 | NIP2 | IF | ||||
| Cp (mg/g) | Kd(L/g) | Cp (mg/g) | Kd(L/g) | Cp (mg/g) | Kd(L/g) | Cp (mg/g) | Kd(L/g) | |||
| BzP | 3.10 | 1.13 | 2.74 | 0.86 | 1.25 | 3.68 | 1.33 | 3.34 | 0.93 | 1.44 |
| BuP | 2.74 | 0.79 | 2.71 | 0.77 | 1.04 | 3.49 | 1.08 | 3.39 | 0.81 | 1.33 |
| PrP | 2.72 | 0.78 | 2.64 | 0.71 | 1.09 | 3.41 | 1.13 | 3.14 | 0.85 | 1.34 |
| EtP | 2.61 | 0.76 | 2.33 | 0.83 | 0.92 | 2.95 | 0.50 | 2.64 | 0.39 | 1.27 |
| MeP | 2.55 | 0.62 | 2.49 | 0.58 | 1.07 | 2.55 | 0.58 | 2.53 | 0.58 | 1.01 |

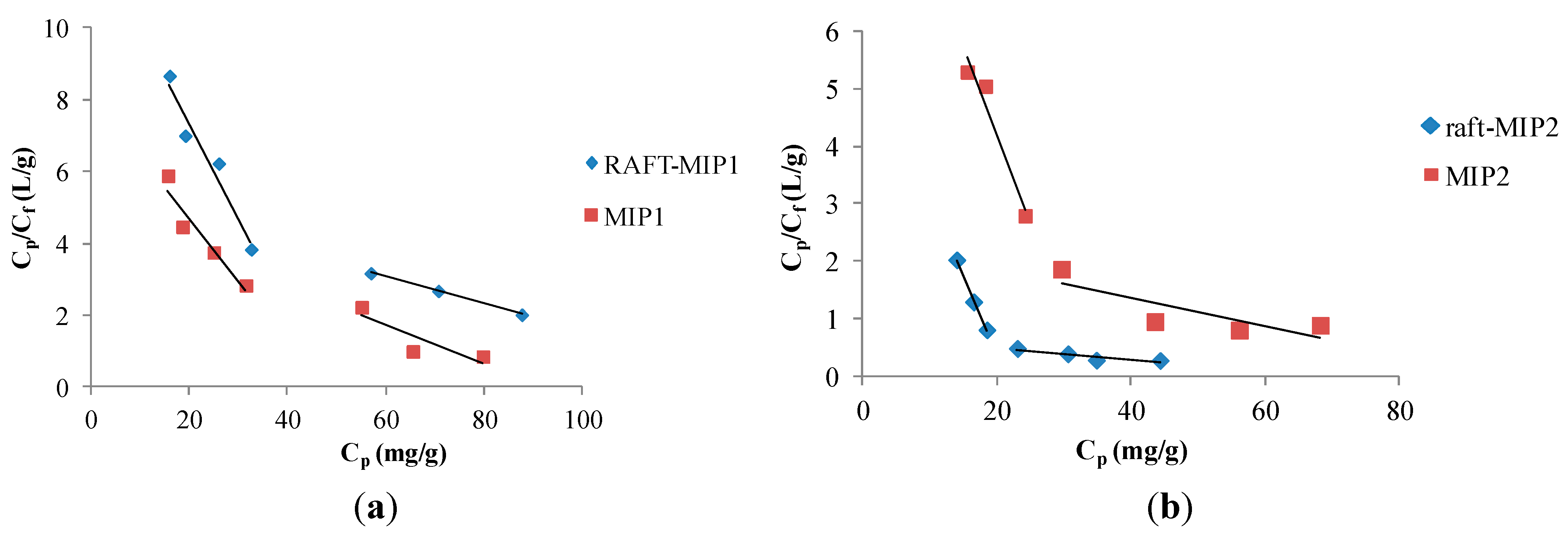
| MIPs | The Linear Regression Equation | R2 | Cpmax(mg/g) | Kd |
|---|---|---|---|---|
| RAFT-MIP1 | (high binding affinity) Cp/Cf = −0.2648Cp + 12.643 | 0.9478 | 47.75 | 3.78 |
| (low binding affinity) Cp/Cf = −0.0376Cp + 5.3206 | 0.9994 | 141.51 | 26.60 | |
| MIP1 | (high binding affinity) Cp/Cf = −0.1741Cp + 8.1928 | 0.9108 | 47.03 | 5.74 |
| (low binding affinity) Cp/Cf = −0.0532Cp + 4.9131 | 0.7557 | 92.35 | 18.80 | |
| RAFT-MIP2 | (high binding affinity) Cp/Cf = −0.2698Cp + 5.7916 | 0.9976 | 21.47 | 3.71 |
| (low binding affinity) Cp/Cf = −0.1030Cp + 0.7028 | 0.8273 | 6.82 | 9.71 | |
| MIP2 | (high binding affinity) Cp/Cf = −0.1691Cp + 8.0157 | 0.9824 | 47.40 | 5.91 |
| (low binding affinity) Cp/Cf = −0.0245Cp + 2.3405 | 0.6762 | 95.53 | 40.82 |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andersson, L.I.; Nicholls, I.A.; Mosbach, K. Molecular imprinting: The current status and future development of polymer-based recognition system. Adv. Mol. Cell Biol. 1996, 15, 651–670. [Google Scholar]
- Yan, H.; Row, K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Yusof, N.A.; Rahman, S.K.A.; Hussein, M.Z.; Ibrahim, N.A. Preparation and characterization of molecularly imprinted polymer as SPE sorbent for melamine isolation. Polymers 2013, 5, 1215–1228. [Google Scholar] [CrossRef]
- Wackers, G.; Vandenryt, T.; Cornelis, P.; Kellens, E.; Thoelen, R.; Ceuninck, W.D.; Losada-Pérez, P.; Grinsven, B.V.; Peeters, M.; Wagner, P. Array formatting of the heat-transfer method (HTM) for the detection of small organic molecules by molecularly imprinted polymers. Sensors 2014, 14, 11016–11030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, C.; Davidson, L.; Hayes, W. Imprinted polymers: Artificial molecular recognition materials with applications in synthesis and catalysis. Tetrahedron 2003, 59, 2025–2057. [Google Scholar] [CrossRef]
- Asman, S.; Yusof, N.A.; Abdullah, A.H.; Haron, M.J. Synthesis and characterization of hybrid molecularly imprinted polymer (MIP) membranes for removal of methylene blue. Molecules 2012, 17, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Zhu, S. Branching and gelatine in atom transfer radical polymerization of methyl methacrylate and ethylene glycol dimethacrylate. Polym. Eng. Sci. 2005, 5, 720–727. [Google Scholar] [CrossRef]
- Watabe, Y.; Hosoya, K.; Tanaka, N.; Kubo, T.; Morita, M. Novel surface modified molecularly imprinted polymer focused on the removal of interference in environmental water samples for chromatographic determination. J. Chromatorgr. A 2005, 1073, 363–370. [Google Scholar] [CrossRef]
- Pan, G.; Zu, B.; Guo, X.; Zhang, Y.; Li, C.; Zhang, H. Preparation of molecularly imprinted polymer microspheres via reversible addition-fragmentation chain transfer precipitation polymerization. Polymer 2009, 50, 2819–2825. [Google Scholar] [CrossRef]
- Goto, A.; Kukuda, T. Kinetics of living radical polymerization. Prog. Polym. Sci. 2004, 29, 329–385. [Google Scholar] [CrossRef]
- Hawker, C.J. Living free radical polymerization: A unique technique for the preparation of controlled macromolecular architectures. Acc. Chem. Res. 1997, 30, 373–382. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Zu, B.; Pan, G.; Guo, X.; Zhang, Y.; Zhang, H. Preparation of Molecularly imprinted polymer microspheres via atom transfer radical precipitation polymerization. J. Polym. Sci. A Polym. Chem. 2009, 47, 3257–3270. [Google Scholar] [CrossRef]
- Peeters, M.; Kobben, S.; Jiménez-Monroy, K.L.; Modesto, L.; Kraus, M.; Vandenryt, T.; Gaulke, A.; van Grinsven, B.; Ingebrandt, S.; Junkers, T.; et al. Thermal detection of histamine with a graphene oxide based molecularly imprinted polymer platform prepared by reversible addition–fragmentation chain transfer polymerization. Sens. Actuators B Chem. 2014, 203, 527–535. [Google Scholar] [CrossRef]
- Hu, X.; Fan, Y.; Zhang, Y.; Dai, G.; Cai, Q.; Cao, Y.; Guo, C. Molecularly imprinted polymer coated solid-phase microextraction fiber prepared by surface reversible addition-fragmentation chain transfer polymerization for monitoring of Sudan dyes in chilli tomato sauce and chilli pepper samples. Anal. Chim. Acta 2012, 731, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.; Gong, S. Preparation of molecularly imprinted polymers for vanillin via reversible addition-fragmentation chain transfer suspension polymerization. Appl. Polym. Sci. 2012, 128, 2927–2932. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Chen, L. Molecularly imprinted polymers by reversible addition-fragmentation chain transfer precipitation polymerization for preconcentration of atrazine in food matrices. Talanta 2011, 85, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Pan, J.; Xu, L.; Li, X.; Zhou, Z.; Zhang, R.; Yan, Y. Preparation of molecularly imprinted nanoparticles with supermagnetic susceptibility through atom transfer radical emulsion polymerization for the selective recognition of tetracycline from aqueous medium. J. Hazard. Mater. 2012, 205–206, 179–188. [Google Scholar]
- Li, Y.; Li, X.; Chu, J.; Dong, C.; Qi, J.; Yuan, Y. Synthesis of core-shell magnetic molecular imprinted polymer by the surface RAFT polymerization for the fast and selective removal of endocrine disrupting chemicals from aqueous solutions. Environ. Pollut. 2010, 158, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Zhou, W.-H.; Han, B.; Yang, H.-H.; Chen, X.; Wang, X.-R. Surface-imprinted core-shell nanoparticles for sorbent assays. Anal. Chem. 2007, 79, 5457–5461. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.; Chen, L. Molecularly imprinted core-shell nanoparticles for determination of trace atrazine by reversible addition-fragmentation chain transfer surface imprinting. J. Mater. Chem. 2011, 21, 4345–4351. [Google Scholar]
- Boonpangrak, S.; Whitcombe, M.J.; Prachayasittikul, V.; Mosbach, K.; Ye, L. Preparation of molecularly imprinted polymers using nitroxide-mediated living radical polymerization. Biosen. Bioelectron. 2006, 22, 349–354. [Google Scholar] [CrossRef]
- Sasaki, S.; Ooya, T.; Takeuchi, T. Highly selective bisphenol A-imprinted polymers prepared by atom transfer radical polymerization. Polym. Chem. 2010, 1, 1684–1688. [Google Scholar] [CrossRef]
- Vaughan, A.D.; Sizemore, S.P.; Byrne, M.E. Enhancing molecularly imprinted polymer binding properties via controlled/living radical polymerization and reaction analysis. Polymer 2007, 48, 74–81. [Google Scholar] [CrossRef]
- Pérez-Moral, N.; Mayes, A.G. Comparative study of imprinted polymer particles prepared by different polymerisation methods. Anal. Chim. Acta 2004, 504, 15–21. [Google Scholar] [CrossRef]
- Zu, B.; Zhang, Y.; Guo, X.; Zhang, H. Preparation of molecularly imprinted polymers via atom transfer radical “bulk” polymerization. J. Polym. Chem. 2010, 48, 532–541. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, G.; Zhang, Y.; Guo, X.; Zhang, H. Comparative study of the molecularly imprinted polymers prepared by reversible addition–fragmentation chain transfer “bulk” polymerization. J. Mol. Recognit. 2013, 26, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Moad, G. RAFT (Reversible addition–fragmentation chain transfer) crosslinking (co)polymerization of multi-olefinic monomers to form polymer networks. Polym. Int. 2014, 64, 15–24. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayagunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical addition–fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O.; Stenzel, M.H.; Davis, T.P.; Barner-Kowollik, C.; Barner, L. Verification of controlled grafting of styrene from cellulose via radiation-induced RAFT polymerization. Macromolecules 2007, 40, 7140–7147. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; THang, S.H. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Harrisson, S.; Liu, X.; Ollagnier, J.-N.; Coutelier, O.; Marty, J.-D.; Destarac, M. RAFT polymerization of vinyl esters: Synthesis and applications. Polymers 2014, 6, 1437–1488. [Google Scholar] [CrossRef]
- Cormack, P.A.G.; Mehamod, F.S. Molecularly imprinted polymer synthesis using RAFT polymerization. Sains Malays. 2013, 42, 529–535. [Google Scholar]
- Wei, Z.; Hao, X.; Kambouris, P.A.; Gan, Z.; Hughes, T.C. One-pot synthesis of hyperbranched polymers using small molecule and macro RAFT inimers. Polymer 2012, 53, 1429–1436. [Google Scholar] [CrossRef]
- Wang, Z.; He, J.; Tao, Y.; Yang, L.; Jiang, H.; Yang, Y. Controlled chain branching by RAFT-based radical polymerization. Macromolecules 2013, 36, 7446–7452. [Google Scholar] [CrossRef]
- Carter, S.; Rimmer, S.; Sturdy, A.; Webb, M. Highly branched stimuli responsive Poly[(N-isopropyl acrylamide)-co-(1,2-propandiol-3-methacrylate)]s with protein binding functionality. Macromol. Biosci. 2005, 5, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kazlauciunas, A.; Guthrie, J.T.; Perrier, S. Influence of reaction parameters on the synthesis of hyperbranched polymers via reversible addition fragmentation chain transfer (RAFT) polymerization. Polymer 2005, 46, 6293–6299. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Sellergren, B. Thin molecularly imprinted polymer films via reversible addition-fragmentation chain transfer polymerization. Chem. Mater. 2006, 18, 1773–1779. [Google Scholar] [CrossRef]
- Southard, G.E.; van Houten, K.A.; Ott, E.W., Jr.; Murray, G.M. Luminescent sensing of organophosphates using europium (III) containing imprinted polymers prepared by RAFT polymerization. Anal. Chim. Acta 2007, 581, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, M.; Legge, T.M.; Perrier, S.; Patrickios, C.S. Poly(ethylene glycol)-based amphiphilic model conetworks: Synthesis by RAFT polymerization and characterization. J. Polym. Sci. A Polym. Chem. 2008, 46, 7556–7565. [Google Scholar] [CrossRef]
- Krasia, T.; Patrickios, C.S. Amphiphilic polymethacrylate model co-networks: Synthesis by RAFT radical polymerization and characterization of the swelling behaviour. Macromolecules 2006, 39, 2467–2473. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Xu, Z.; Kuang, D.; Liu, L.; Deng, Q. Selective adsorption of norfloxacin in aqueous media by an imprinted polymer based on hydrophobic and electrostatic interactions. J. Pharm. Biomed. Anal. 2007, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Asman, S.; Mohamad, S.; Sarih, N.M. Exploiting β-cyclodextrin in molecular imprinting for achieving recognition of benzylparaben in aqueous media. Int. J. Mol. Sci. 2015, 16, 3656–3676. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, K. Grafting of β-cyclodextrin-modified 2-hydroxyethyl methacrylate onto polyurethane. J. Appl. Polym. Sci. 1996, 60, 2245–2249. [Google Scholar] [CrossRef]
- Mohamad, S.; Yusof, N.H.M.; Asman, S. Effect of bifunctional isocyanate linker on adsorption of chromium (IV) diphenylcarbazide complex onto β-cyclodextrin. Asian J. Chem. 2013, 25, 2213–2220. [Google Scholar]
- Spivak, D.A. Optimization, evaluation and characterization of molecularly imprinted polymers. Adv. Drug Deliv. Rev. 2005, 57, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Kania, N.; Rio, S.; Monflier, E.; Ponchel, A. Cyclodextrin adsorbed onto activated carbons: Preparation, characterization and effect on the dispersibility of the particles in water. J. Colloid Interface Sci. 2012, 371, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and characterization of mesoporous silica functionalized with calix[4]arene derivatives. Int. J. Mol. Sci. 2012, 13, 13726–13736. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Jiao, Y.; Xia, Y.; Xia, L.; Wang, Z.; Zhang, W.; Wang, K.; et al. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012, 47, 1898–1904. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Surface area and pore structure properties of urethane- based copolymers containing β-cyclodextrin. J. Colloid Interface Sci. 2011, 357, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Asouhidou, D.D.; Triantafyllidis, K.S.; Lazaridis, N.K.; Matis, K.A. Adsorption of remazol red 3BS from aqueous solutions using APTES- and cyclodextrin-modified HMS-type mesoporous silicas. Colloids Surf. A 2009, 346, 83–90. [Google Scholar] [CrossRef]
- Mena-Duran, C.J.; Kou, M.S.; Lopez, T.; Azamar-Barrios, J.A.; Aguilar, D.H.; Domínguez, M.I.; Odriozola, J.A.; Quintana, P. Nitrate removal using natural clays modified by acid thermoactivation. Appl. Surf. Sci. 2007, 253, 5762–5766. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendation 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Idris, S.A.; Alotaibi, K.M.; Peshkur, T.A.; Anderson, P.; Morris, M.; Gibson, L.T. Adsorption kinetic study: Effect of adsorbent pore size distribution on the rate of Cr (VI) uptake. Microporous. Mesoporous. Mater. 2013, 165, 99–105. [Google Scholar] [CrossRef]
- Holland, N.; Frisby, J.; Owens, E.; Hughes, H.; Duggan, P.; McLoughlin, P. The influence of polymer morphology on the performance of molecularly imprinted polymers. Polymer 2010, 51, 1578–1584. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.; Kuang, D.; Zhang, F.; Wang, J. Exploiting β-cyclodextrin as functional monomer in molecular imprinting for achieving recognition in aqueous media. Mater. Sci. Eng. C 2008, 28, 1516–1521. [Google Scholar] [CrossRef]
- Matsui, J.; Miyoshi, Y.; Doblhoff-Dier, O.; Takeuchi, T. A molecularly imprinted synthetic polymer receptor selective for atrazine. Anal. Chem. 1995, 67, 4404–4408. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asman, S.; Mohamad, S.; Sarih, N.M. Effects of RAFT Agent on the Selective Approach of Molecularly Imprinted Polymers. Polymers 2015, 7, 484-503. https://doi.org/10.3390/polym7030484
Asman S, Mohamad S, Sarih NM. Effects of RAFT Agent on the Selective Approach of Molecularly Imprinted Polymers. Polymers. 2015; 7(3):484-503. https://doi.org/10.3390/polym7030484
Chicago/Turabian StyleAsman, Saliza, Sharifah Mohamad, and Norazilawati Muhamad Sarih. 2015. "Effects of RAFT Agent on the Selective Approach of Molecularly Imprinted Polymers" Polymers 7, no. 3: 484-503. https://doi.org/10.3390/polym7030484