Synthesis of Bio-Based Poly(lactic acid-co-10-hydroxy decanoate) Copolymers with High Thermal Stability and Ductility
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Poly(lactic acid-co-hydroxy decanoate)
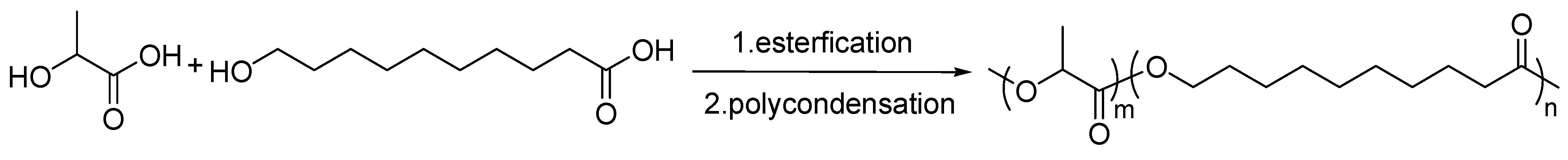
2.3. Characterization
2.4. Hydrolytic Degradation of PLH
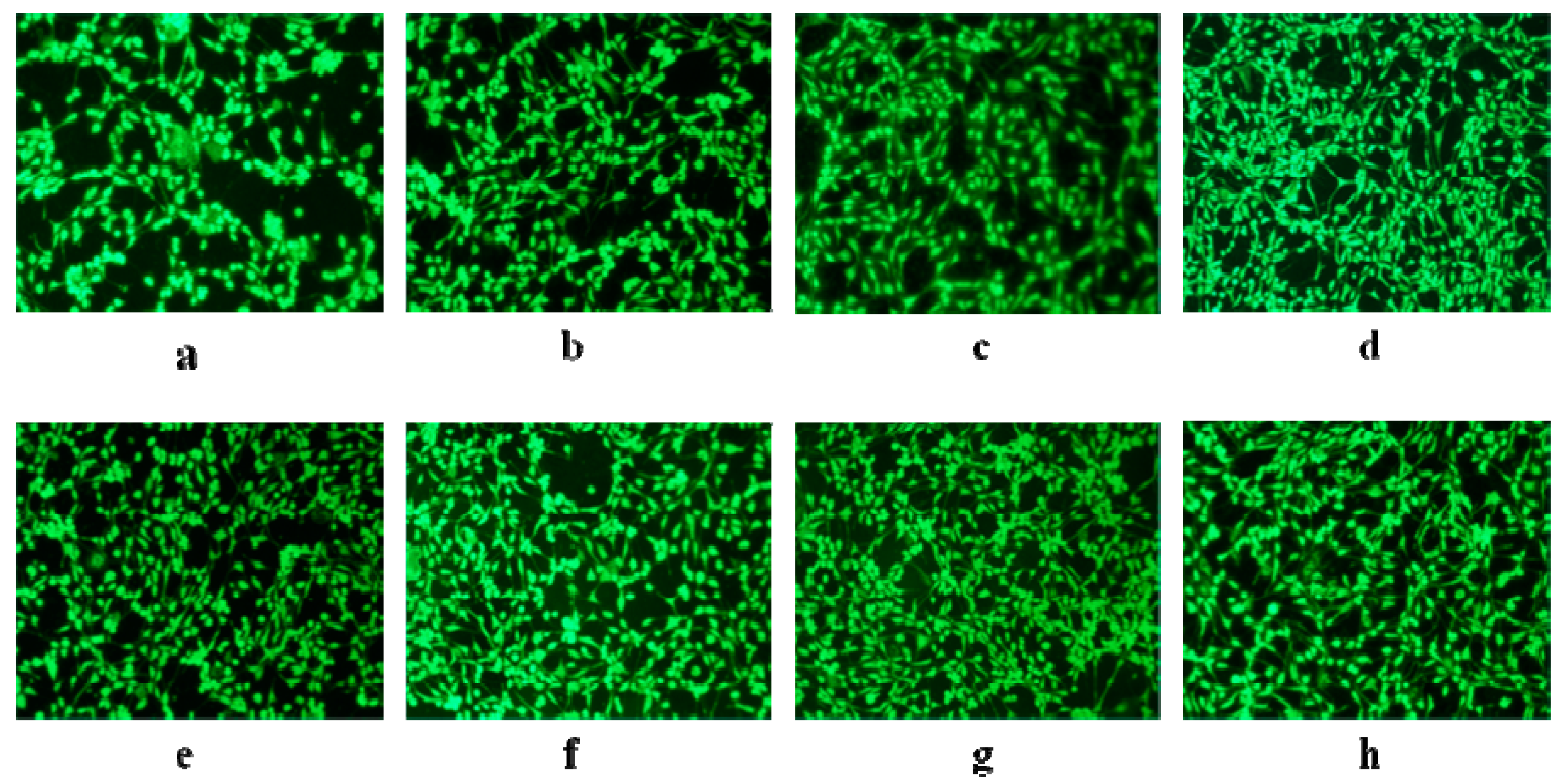
2.5. Cell Adhesion of PLH
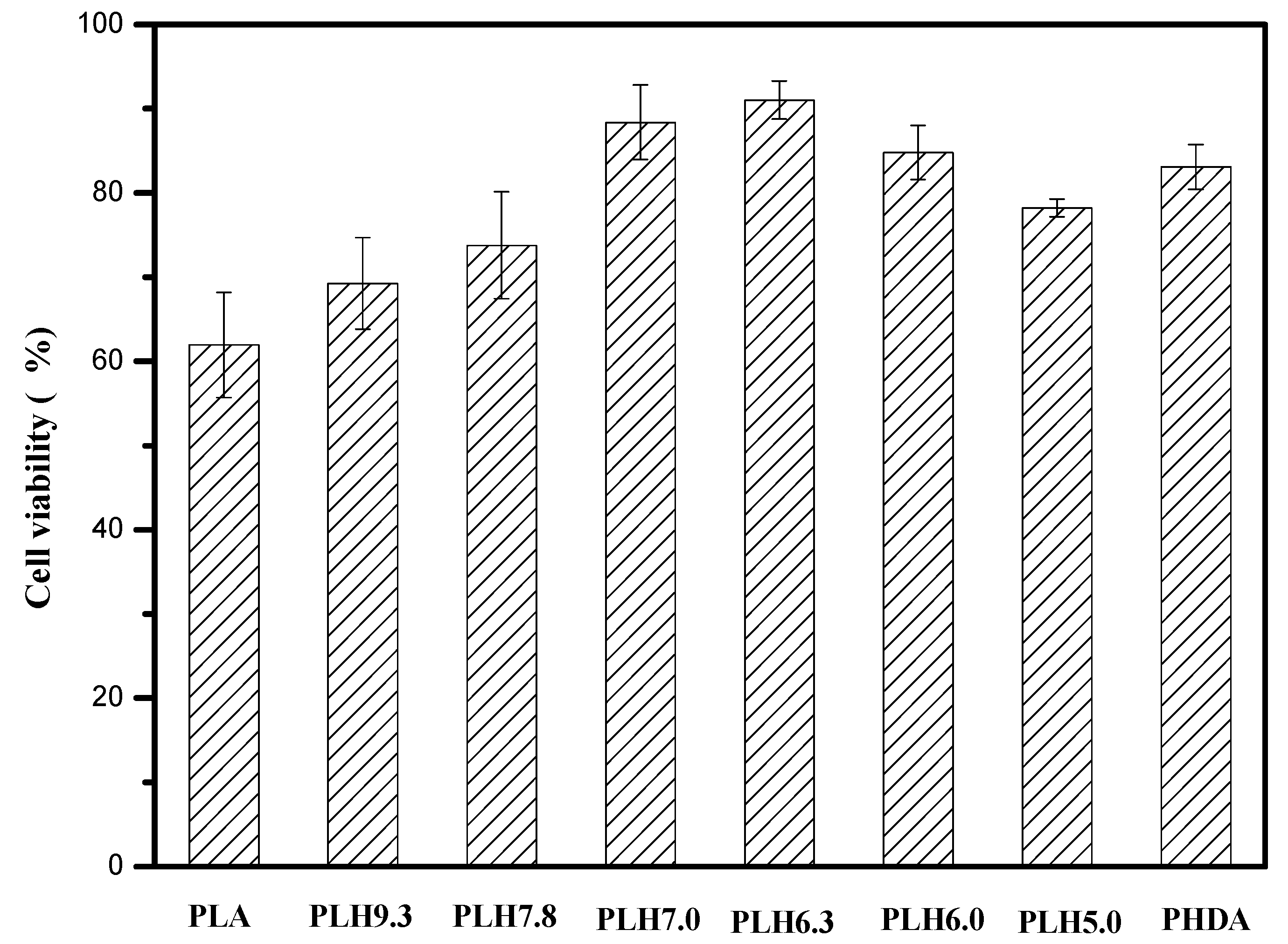
2.6. MTT Assay
3. Results and Discussion
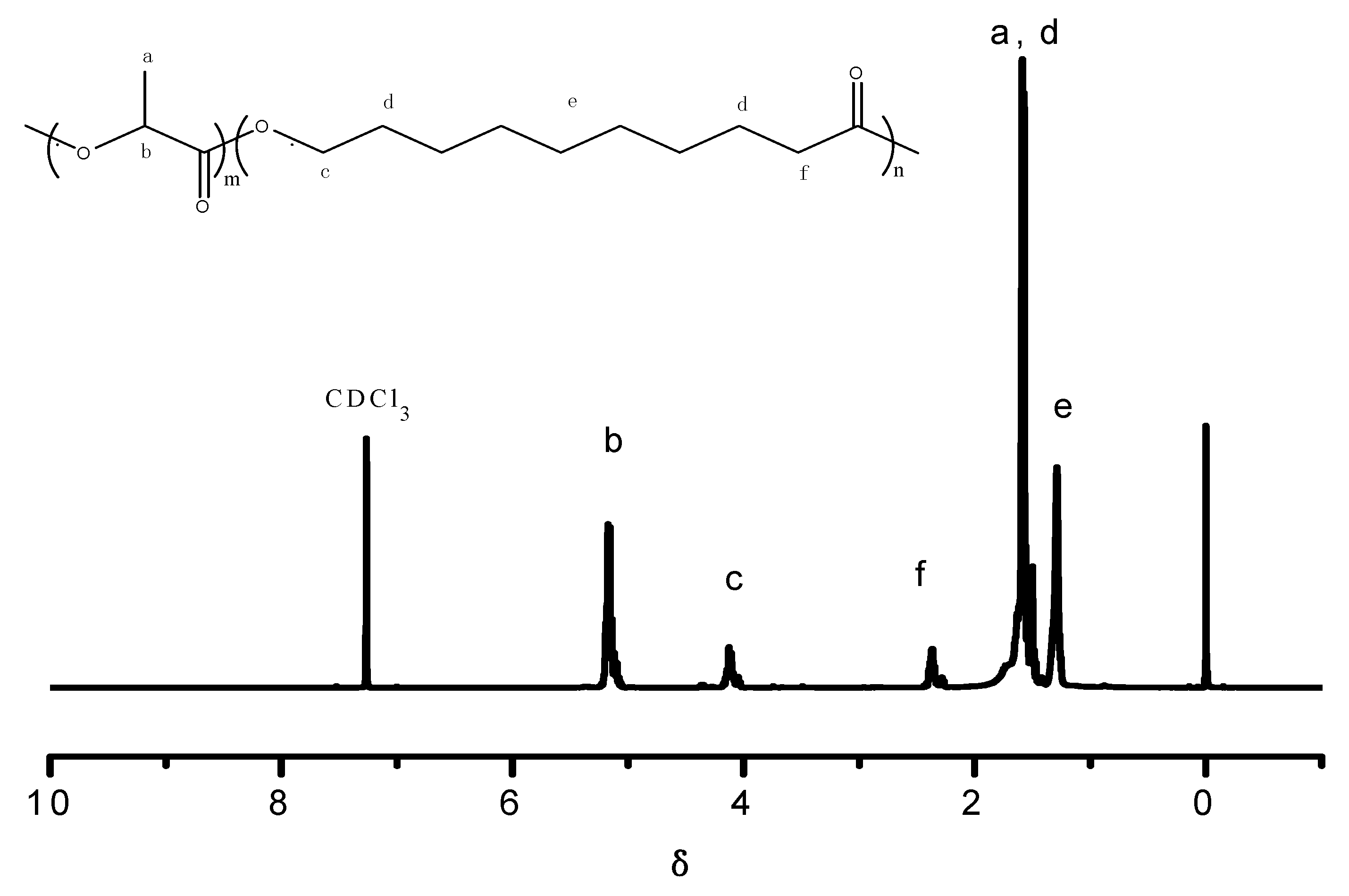
3.1. Structural Characterization and Molecular Weight
| Sample | nL-LA/nHDA a | nL-LA/nHDA b | Mn (×10-4) c | Mw (×10-4) c | PDI c |
|---|---|---|---|---|---|
| PLA | / | / | 1.75 | 2.81 | 1.61 |
| PLH9.3 | 12/1 | 9.3/1 | 1.15 | 1.85 | 1.61 |
| PLH7.8 | 11/1 | 7.8/1 | 1.48 | 2.44 | 1.65 |
| PLH7.0 | 10/1 | 7.0/1 | 2.18 | 3.79 | 1.74 |
| PLH6.3 | 9/1 | 6.3/1 | 1.82 | 3.20 | 1.76 |
| PLH6.1 | 8/1 | 6.1/1 | 1.51 | 2.48 | 1.64 |
| PLH5.0 | 7/1 | 5.0/1 | 1.17 | 2.01 | 1.72 |
| PHDA | / | / | 3.59 | 6.81 | 1.90 |
3.2. Thermal Transition Behavior
| Sample | Tg (°C) a | Tm (°C) a | Td,5% (°C) b | Td,max (°C) b | Tensile Strength (MPa) c | Tensile Modulus (Mpa) c | εb (%) c |
|---|---|---|---|---|---|---|---|
| PLA | 71.0 | 158.4 | 230 | 272 | / | / | / |
| PLH9.3 | 26.2 | 110.5 | 237 | 280 | / | / | / |
| PLH7.8 | 21.5 | 106.4 | 244 | 285 | 27.3 | 1631 | 7 |
| PLH7.0 | 19.2 | 95.0 | 253 | 288 | 20.7 | 633 | 15 |
| PLH6.3 | 18.7 | 93.3 | 257 | 290 | 5.7 | 70 | 155 |
| PLH6.1 | 18.1 | 93.0 | 259 | 293 | 3.6 | 66 | 475 |
| PLH5.0 | 11.8 | 66.4 | 262 | 295 | 1.5 | 28 | 600 |
| PHDA | / | 80.2 | 387 | 428 | / | / | / |
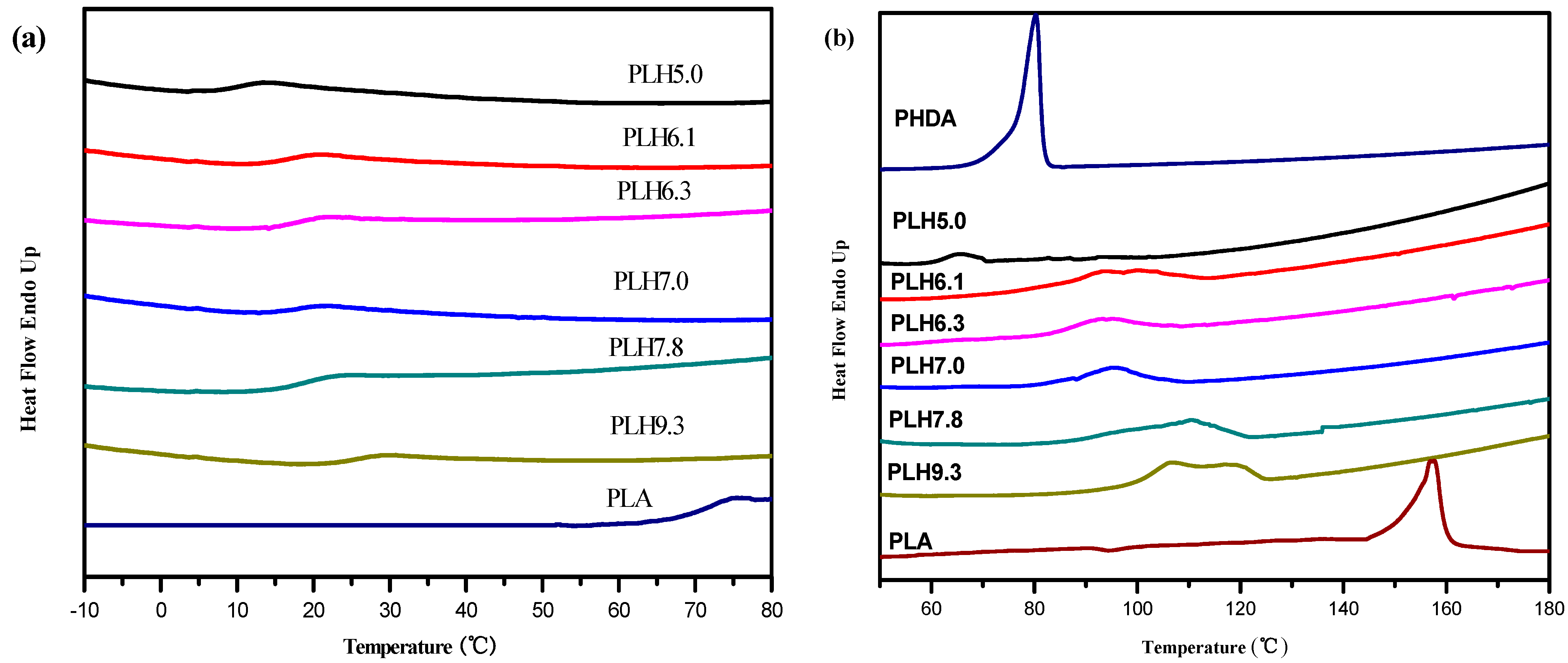
3.3. Thermal Stability
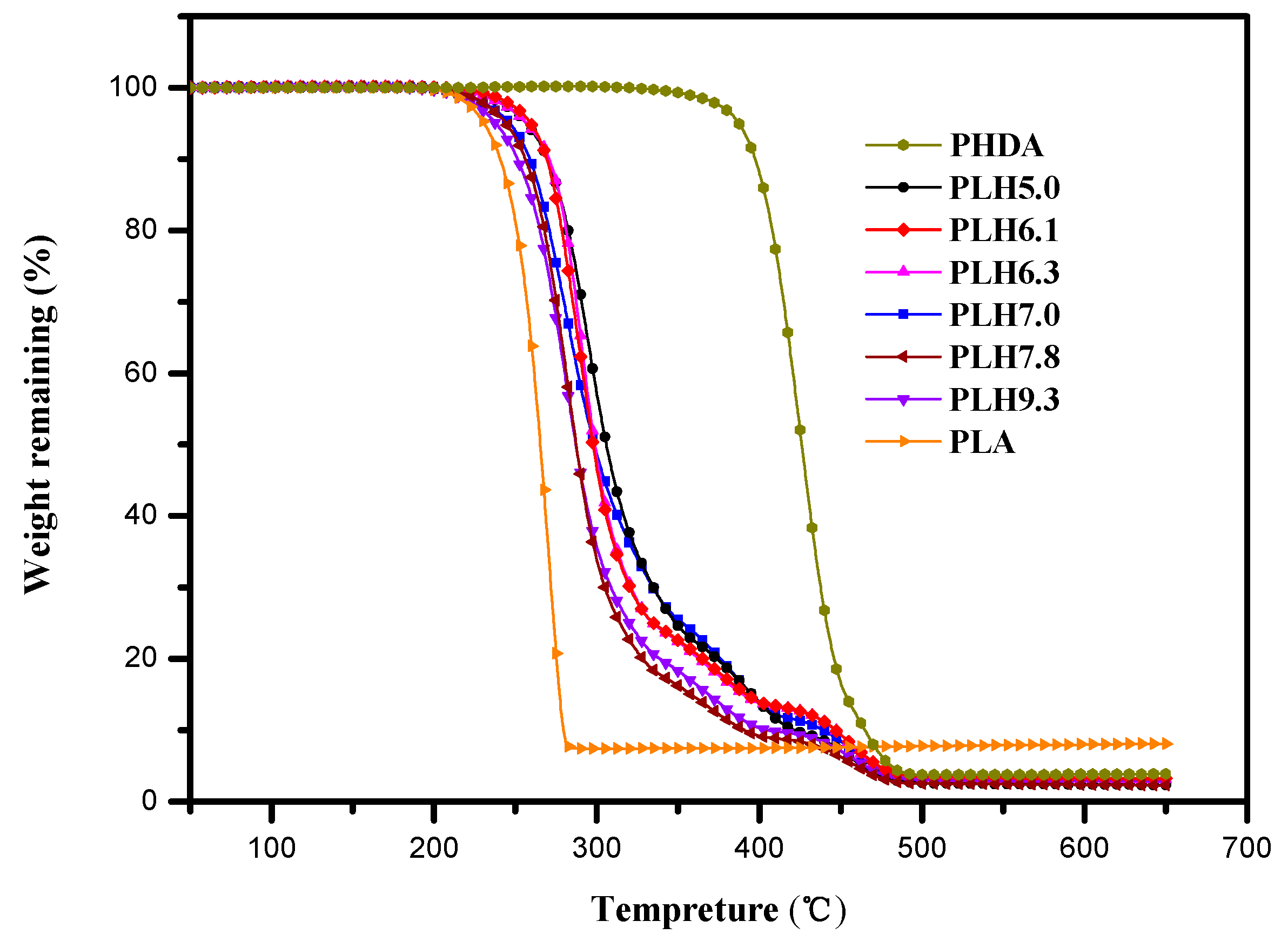
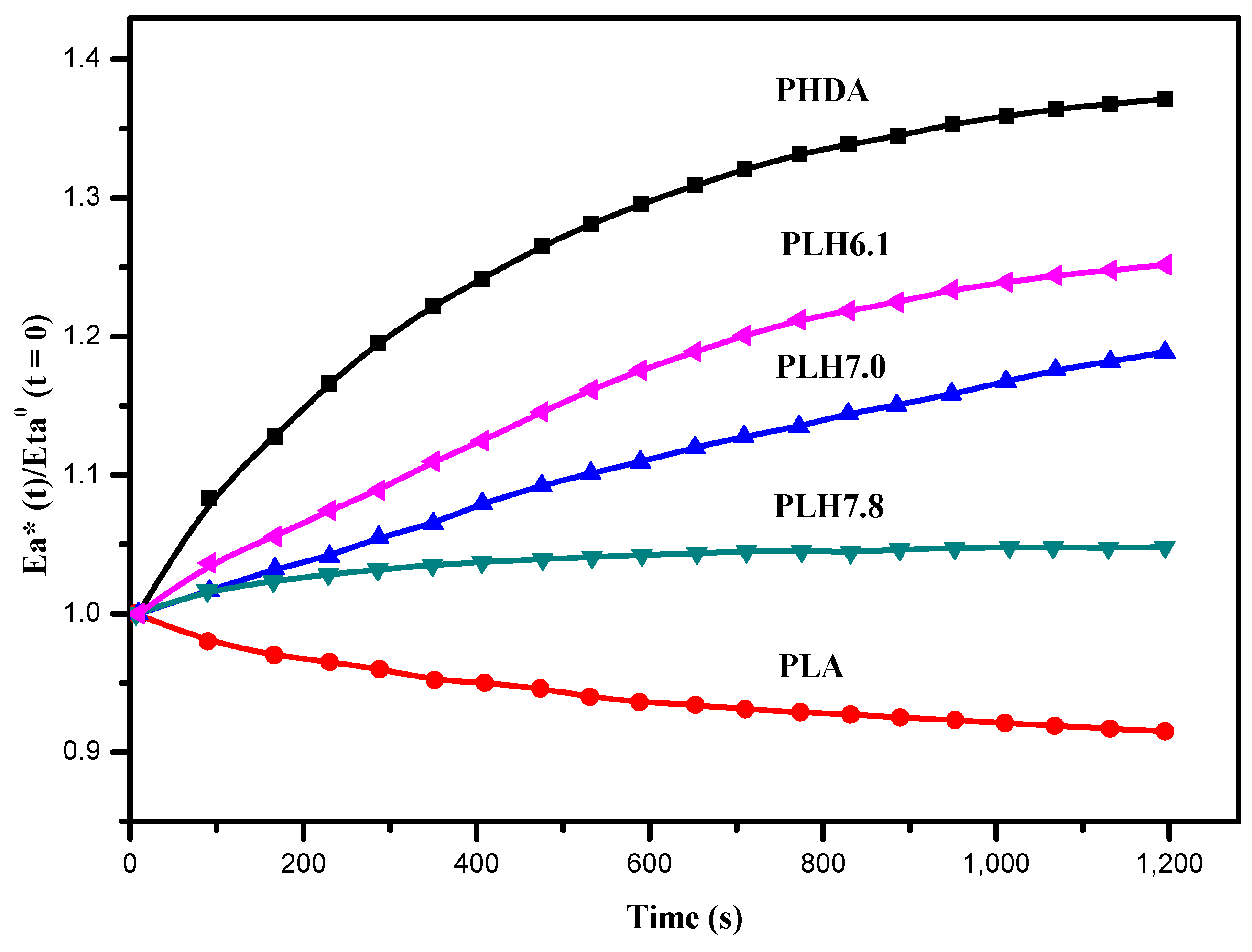
3.4. Mechanical Properties
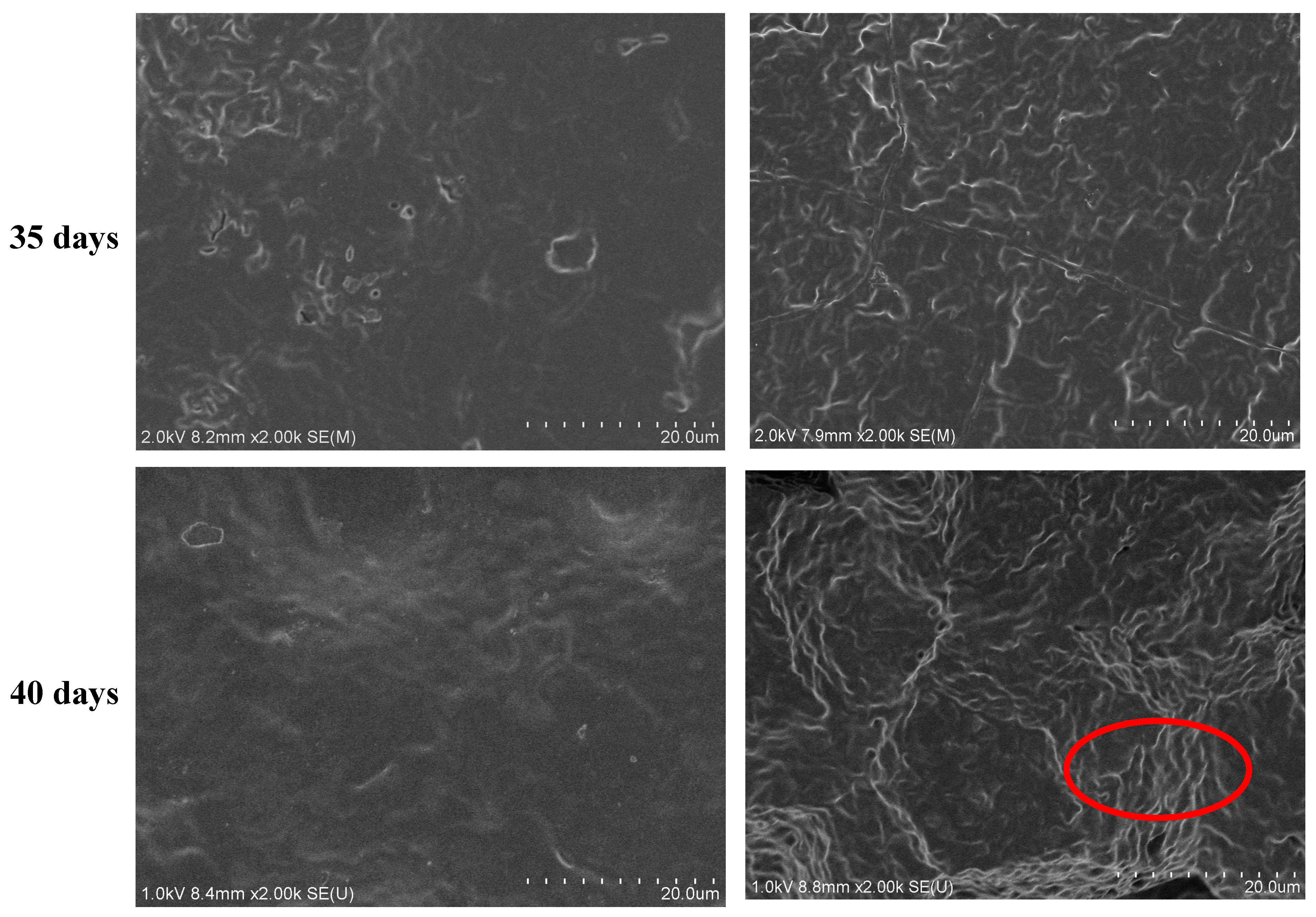
3.5. Hydrolytic Degradation
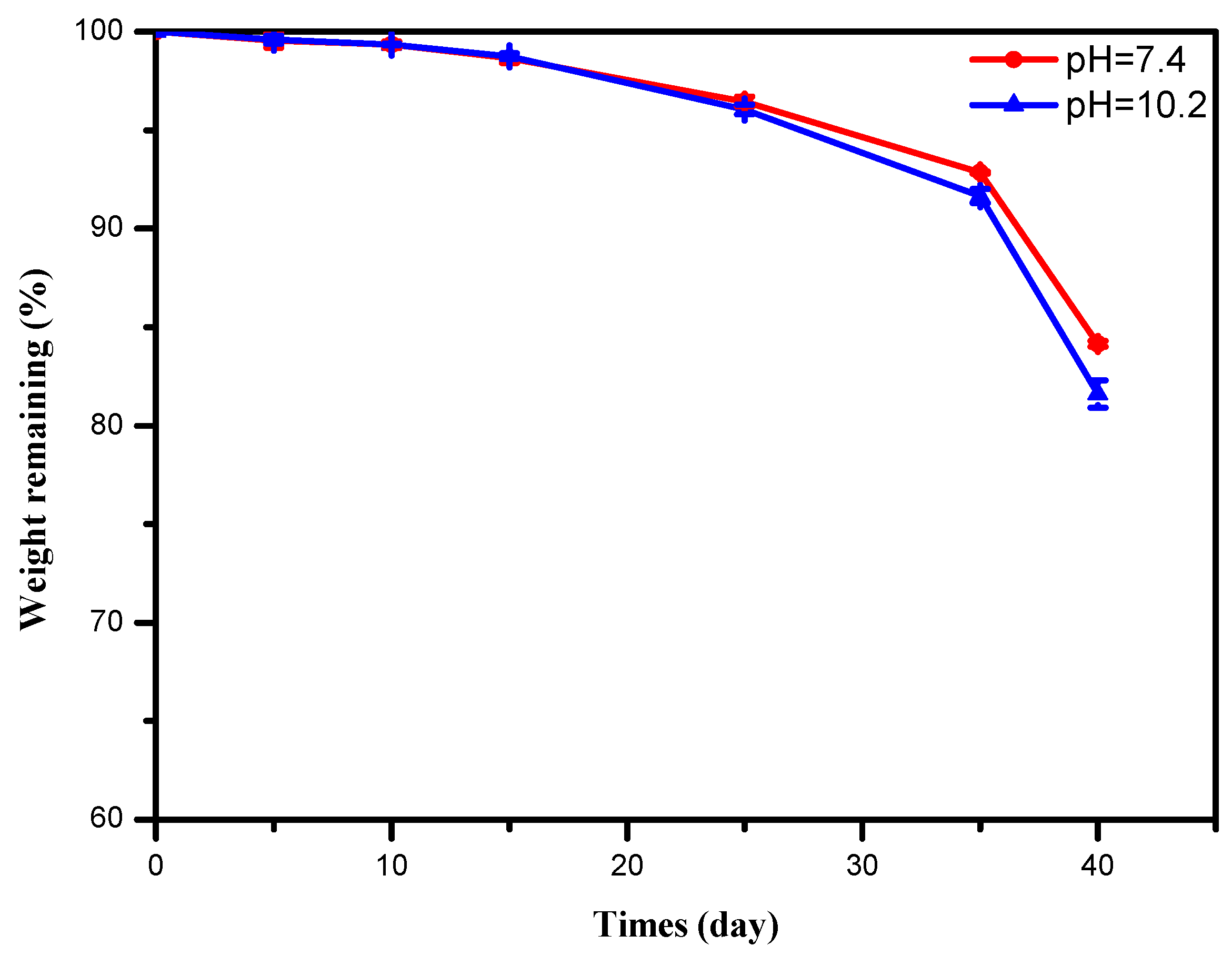
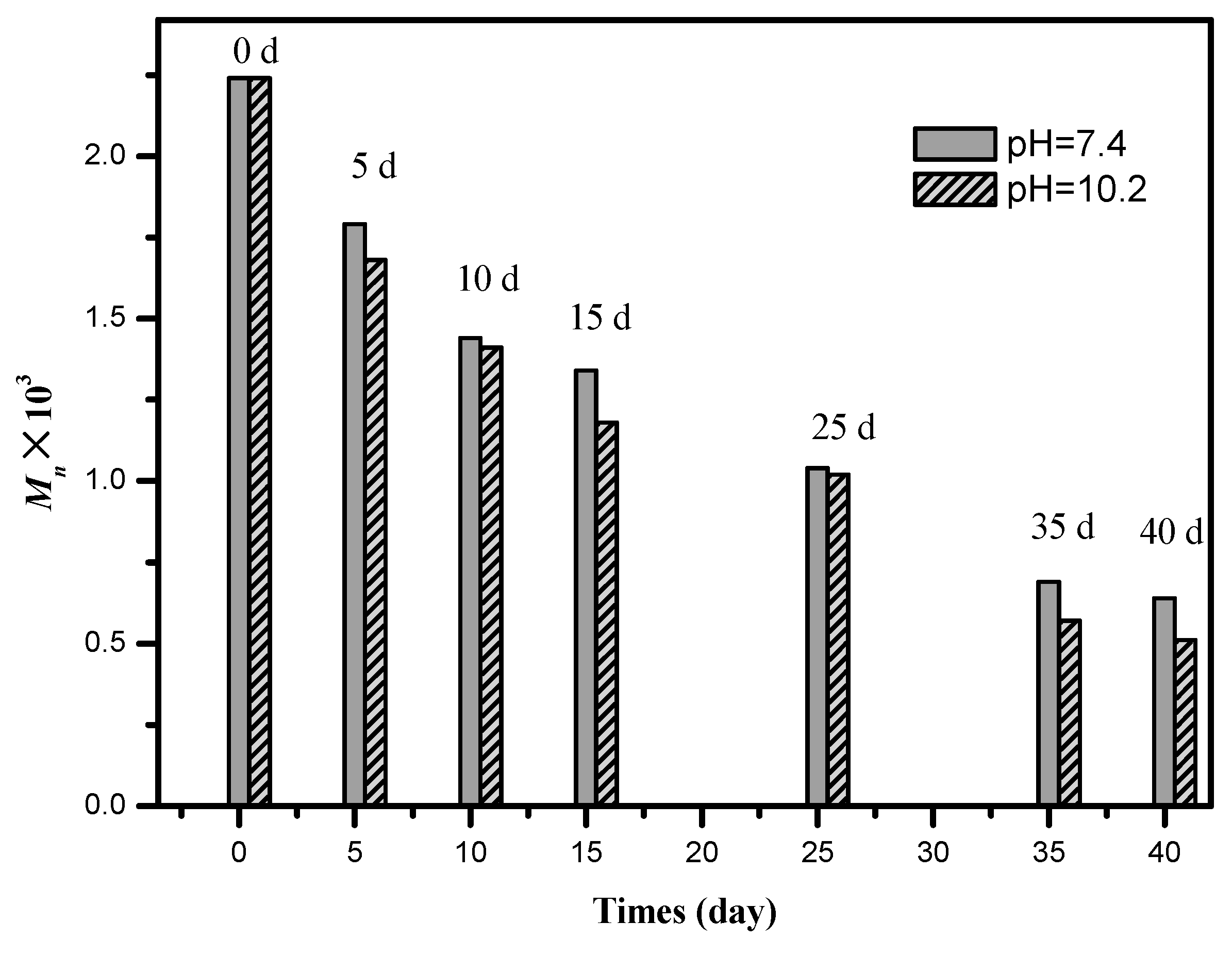
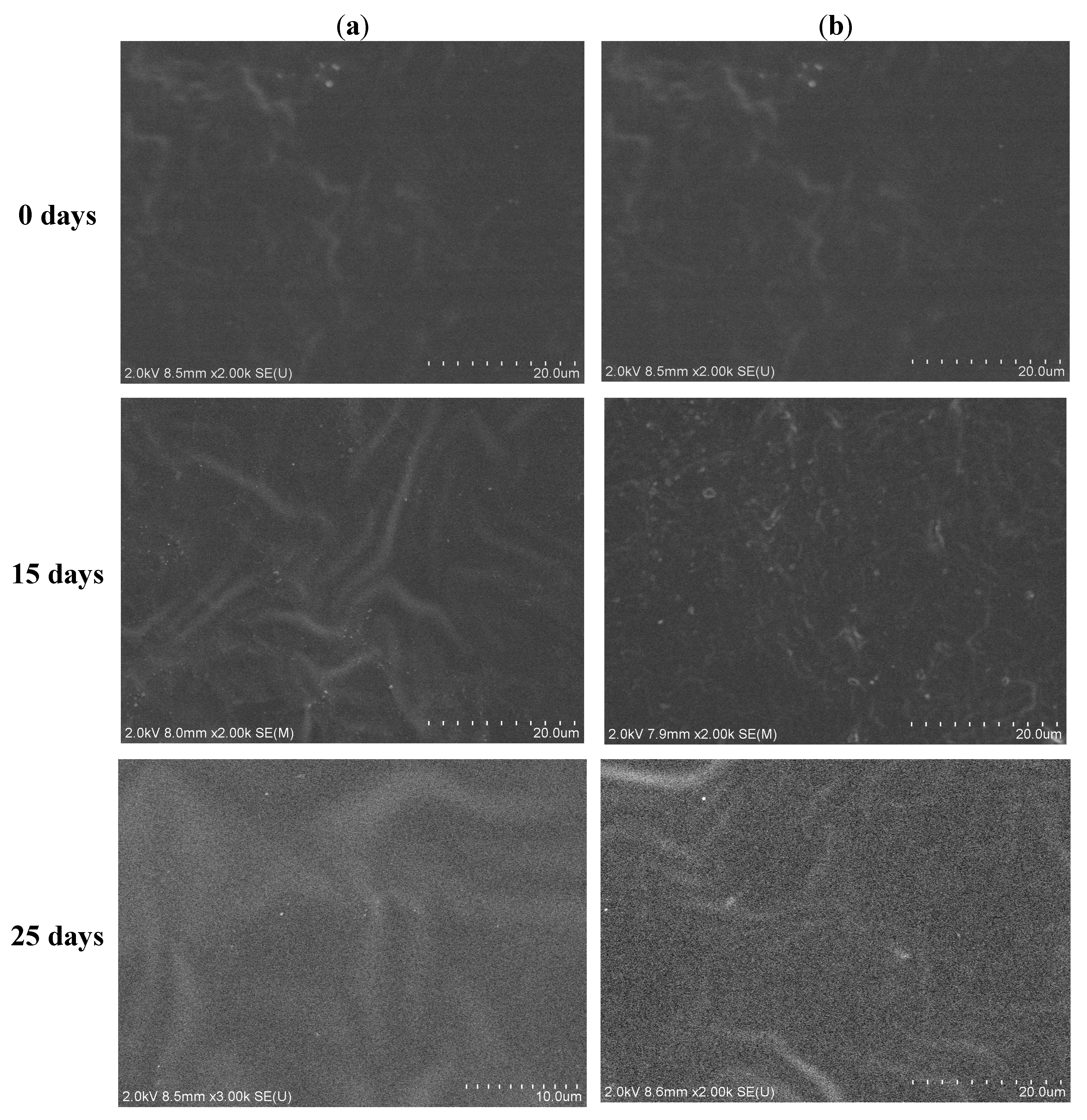
3.6. Biocompatibility
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coombs, J.; Hall, K. Chemicals and polymers from biomass. Renew. Energy 1998, 15, 54–59. [Google Scholar] [CrossRef]
- Bozell, J.J. Feedstocks for the future-biorefinery production of chemicals from renewable carbon. CLEAN Soil Air Water 2008, 36, 641–647. [Google Scholar] [CrossRef]
- Nikolau, B.J.; Perera, M.A.D.N.; Brachova, L.; Shanks, B. Platform biochemicals for a biorenewable chemical industry. Plant J. 2008, 54, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.M.; Hillmyer, M.A. Block copolymers and melt blends of polylactide with Nodax™ microbial polyesters: Preparation and mechanical properties. J. Biotechnol. 2007, 132, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Van Beilen, J.B.; Poirier, Y. Production of renewable polymers from crop plants. Plant J. 2008, 54, 684–701. [Google Scholar]
- Albertsson, A.C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. In Degradable Aliphatic Polyesters; Springer: Berlin, Germany, 2002; pp. 1–40. [Google Scholar]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2009, 43, 581–591. [Google Scholar] [CrossRef]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly(lactic-co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. Injection-molded high-density polyethylene-hydroxyapatite-aluminum oxide hybrid composites for hard-tissue replacement: Mechanical, biological, and protein adsorption behavior. J. Appl. Polym. Sci. 2012, 124, 2133–2143. [Google Scholar] [CrossRef]
- Mialon, L.; Vanderhenst, R.; Pemba, A.G.; Miller, S.A. Polyalkylenehydroxybenzoates (PAHBs): Biorenewable aromatic/aliphatic polyesters from lignin. Macromol. Rapid Commun. 2011, 32, 1386–1392. [Google Scholar] [CrossRef]
- Trzaskowski, J.; Quinzler, D.; Bährle, C.; Mecking, S. Aliphatic long-chain C20 polyesters from olefin metathesis. Macromol. Rapid Commun. 2011, 32, 1352–1356. [Google Scholar] [CrossRef]
- Barrett, D.G.; Merkel, T.J.; Christopher, L.J.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar] [CrossRef]
- Edlund, U.; Källrot, M.; Albertsson, A.C. Single-step covalent functionalization of polylactide surfaces. J. Am. Chem. Soc. 2005, 127, 8865–8871. [Google Scholar] [CrossRef] [PubMed]
- Slivniak, R.; Domb, A.J. Lactic acid and ricinoleic acid based copolyesters. Macromolecules 2005, 38, 5545–5553. [Google Scholar] [CrossRef]
- Teng, C.Q.; Yang, K.; Ji, P.; Yu, M.H. Synthesis and characterization of poly(l-lactic acid)-poly(ε-caprolactone) multiblock copolymers by melt polycondensation. J. Polym. Sci. A 2004, 42, 5045–5053. [Google Scholar] [CrossRef]
- Wanamaker, C.L.; O’Leary, L.E.; Lynd, N.A.; Hillmyer, M.A.; Tolman, W.B. Renewable-resource thermoplastic elastomers based on polylactide and polymenthide. Biomacromolecules 2007, 8, 3634–3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.X.; Kim, H.S.; Shim, J.H.; Yoon, J.S. Role of epoxy groups on clay surface in the improvement of morphology of poly(l-lactide)/clay composites. Macromolecules 2005, 38, 3738–3744. [Google Scholar] [CrossRef]
- Meaurio, E.; Zuza, E.; Sarasua, J.R. Miscibility and specific interactions in blends of poly(l-lactide) with poly(vinylphenol). Macromolecules 2005, 38, 1207–1215. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Nouvel, C.; Dubois, P.; Dellacherie, E.; Six, J.L. Controlled synthesis of amphiphilic biodegradable polylactide-grafted dextran copolymers. J. Polym. Sci. Part A 2004, 42, 2577–2588. [Google Scholar] [CrossRef]
- Hartmann, M.H. High molecular weight polylactic acid polymers. In Biopolymers from Renewable Resources; Springer: Berlin, Germany, 1998; pp. 367–411. [Google Scholar]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Xu, Y.P.; Li, J.H.; Chen, M.Q.; Ren, J.J.; Ni, Z.B.; Liu, X.Y. Synthesis and characterization of biodegradable polyester P(DHCA-co-LA). Acta Polym. Sin. 2010, 3, 300–307. [Google Scholar] [CrossRef]
- Li, F.; Li, S.M.; Ghzaoui, A.E.; Nouailhas, H.; Zhuo, R.X. Synthesis and gelation properties of PEG-PLA-PEG triblock copolymers obtained by coupling monohydroxylated PEG-PLA with adipoyl chloride. Langmuir 2007, 23, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Lee, S.H.; Kim, S.H.; Lee, Y.M.; Kim, Y.H. Synthesis and characterization of poly(l-lactide)–poly(ε-caprolactone) multiblock copolymers. Macromolecules 2003, 36, 5585–5592. [Google Scholar] [CrossRef]
- Ranganathan, S.; Kumar, R.; Maniktala, V. On the mechanism and synthetic applications of the thermal and alkaline degradation of c-18 castor oil. Tetrahedron 1984, 40, 1167–1178. [Google Scholar] [CrossRef]
- Fernándeza, J.; Etxeberriab, A.; Ugartemendiaa, J.M.; Petiscoa, S.; Sarasua, J.R. Effects of chain microstructures on mechanical behavior and aging of a poly(l-lactide-co-ε-caprolactone) biomedical thermoplastic elastomer. J. Mech. Behav. Biomed. Mater. 2012, 12, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Moon, S., II; Lee, C.W.; Miyamoto, M.; Kimura, Y. Melt polycondensation of l-lactic acid with Sn(II) catalysts activated by various proton acids: A direct manufacturing route to high molecular weight poly(l-lactic acid). J. Polym. Sci. Part A 2000, 38, 1673–1679. [Google Scholar]
- Mallet, B.; Lamnawar, K.; Maazouz, A. Improvement of blown film extrusion of poly(lactic acid): Structure–processing–properties relationships. Polym. Eng. Sci. 2014, 54, 840–857. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Hirata, M.; Masutani, K.; Kimura, Y. Synthesis of ABCBA penta stereoblock polylactide copolymers by two-step ring-opening polymerization of l-and d-lactides with poly(3-methyl-1,5-pentylene succinate) as macroinitiator (C): Development of flexible stereocomplexed polylactide materials. Biomacromolecules 2013, 14, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, L.C.; Li, C.C.; Zhu, W.X.; Zhang, D.; Guan, G.H.; Xiao, Y.N. Synthesis and properties of biodegradable multiblock poly (ester-carbonate) comprising of poly(l-lactic acid) and poly(butylene carbonate) with hexamethylene diisocyanate as chain-extender. J. Appl. Polym. Sci. 2014, 131, 39158:1–39158:9. [Google Scholar]
- Zeng, X.Q.; Wu, B.S.; Wu, L.B.; Hu, J.J.; Bu, Z.Y.; Li, B.G. Poly(l-lactic acid)-block-poly(butylene succinate-co-butylene adipate) multiblock copolymers: From synthesis to thermo-mechanical properties. Ind. Eng. Chem. Res. 2014, 53, 3550–3558. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Hua, J.; Zhang, L.; Chen, M. Synthesis of Bio-Based Poly(lactic acid-co-10-hydroxy decanoate) Copolymers with High Thermal Stability and Ductility. Polymers 2015, 7, 468-483. https://doi.org/10.3390/polym7030468
Shi D, Hua J, Zhang L, Chen M. Synthesis of Bio-Based Poly(lactic acid-co-10-hydroxy decanoate) Copolymers with High Thermal Stability and Ductility. Polymers. 2015; 7(3):468-483. https://doi.org/10.3390/polym7030468
Chicago/Turabian StyleShi, Dongjian, Jinting Hua, Li Zhang, and Mingqing Chen. 2015. "Synthesis of Bio-Based Poly(lactic acid-co-10-hydroxy decanoate) Copolymers with High Thermal Stability and Ductility" Polymers 7, no. 3: 468-483. https://doi.org/10.3390/polym7030468






